Abstract
Tauopathies, characterized by the dysfunction and aggregation of the microtubule-associated protein tau (MAPT), represent some of the most devastating neurodegenerative disorders afflicting the elderly, including Alzheimer's disease and progressive supranuclear palsy. Here we review the range of Mapt knock-out and MAPT transgenic mouse models which have proven successful at providing insights into the molecular mechanisms of neurodegenerative disease. In this overview we highlight several themes, including the insights such models provide into the cellular and molecular mechanisms of tauopathy, the direct relationship between neuropathology and behaviour, and the use of mouse models to help provide a platform for testing novel therapies. Mouse models have helped clarify the relationship between pathological forms of tau, cell death, and the emergence of disease, as well as the interaction between tau and other disease-associated molecules, such as the Aβ peptide. Finally, we discuss potential future MAPT genomic DNA models to investigate the importance of alternative splicing of the MAPT locus and its role in sporadic tauopathies.
Keywords: MAPT, Tau, Tauopathies, Transgenic mouse models, Knock-out mouse models, Alzheimer's disease, Progressive supranuclear palsy
1. Introduction
With a rapidly ageing population in many developed countries, the study of neurodegenerative illness and dementia is becoming increasingly important. One group of neurodegenerative diseases, collectively referred to as tauopathies, is characterized by a dysfunction and accumulation of the microtubule-associated protein tau in selected brain regions. These include Alzheimer's disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick's disease (PiD) and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17). To gain a better understanding of the role of tau in these diseases, many knock-out and transgenic mouse models have been developed over the past decade. The present article intends to give an overview of the substantial insights such studies have provided so far, and to discuss improved models which might be useful in future.
2. The tau protein
The tau protein is widely expressed in the central nervous system, predominantly in neurons. The microtubule-associated protein tau (MAPT) gene is located on chromosome 17q21 and consists of 16 exons spanning 135 kb (Andreadis et al., 1992). Exons 2, 3 and 10 of MAPT pre-mRNA are alternatively spliced to produce six isoforms in the adult human brain which differ by the presence of a 29-amino acid repeat in the amino-terminal half of the protein (0N, 1N or 2N) and of either three or four microtubule-binding repeats in the carboxyl-terminal half (3R or 4R tau; Fig. 1). In the healthy human brain, tau is thought to play a major role in microtubule stabilization and assembly. There is also evidence that the protein might be important in signal transduction mechanisms, interactions with the actin cytoskeleton, neurite outgrowth and stabilization during brain development (Shahani and Brandt, 2002 for review).
Fig. 1.
Schematic representation of the six tau isoforms in the human CNS. Alternative splicing of exons 2 and 3 (e2 and e3, in dark and light grey) yields isoforms with two, one or no 29-amino acid inserts in the amino-terminal region of the protein (2N–0N). Alternative splicing of exon 10, which codes for the second microtubule-binding repeat (r2, with black rim) in the carboxyl-terminal part of the protein, yields isoforms with three or four microtubule-binding repeats (3R vs. 4R tau).
One way in which tau function, and in particular its interaction with microtubules, appears to be regulated is by phosphorylation (Geschwind, 2003). Broadly, phosphorylation appears to reduce the affinity between tau and microtubules (Mandelkow et al., 1995), although at certain epitopes it may also have the opposite effect (Schneider et al., 1999). In the brains of patients suffering from a tauopathic disease, tau is found in a hyperphosphorylated state, gradually forming aggregates of abnormal filaments, such as neurofibrillary tangles. Such insoluble tau fragments tend to be localized in the somatodendritic compartments of cells, in contrast to their usual localization in axons. The spatial distribution, temporal appearance and ultrastructural morphology of tangles in the brain differs according to specific tauopathies and even to specific disease-causing mutations. For instance, in AD, neurofibrillary tangles consist of both twisted hyper-phosphorylated tau filaments (paired helical filaments) and of single non-periodical tau filaments (straight filaments) (Kidd, 1963). In contrast, PSP patients and FTDP-17 patients with mutations in exon 10 tend to display neurofibrillary tangles consisting only of straight tau filaments (Lee et al., 2001).
It is still being debated as to whether abnormal aggregates of hyperphosphorylated tau are the cause of neurodegeneration or just a downstream-effect of other unknown pathogenic changes. However, study of the genetics of tauopathies clearly shows that dysfunction of the tau protein can be sufficient to cause neurodegenerative disease. At least 30 different mutations in the MAPT locus have been identified in families suffering from FTDP-17 (Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998 and Brandt et al., 2005; D'Souza and Schellenberg, 2005; Goedert and Jakes, 2005 for reviews). Moreover, a number of sporadic tauopathies, in particular PSP and CBD, have been linked to haplotypespecific sequence variations in MAPT (Baker et al., 1999; Conrad et al., 1997; Di Maria et al., 2000; Houlden et al., 2001; Pittman et al., 2005). Thus two major MAPT haplotypes appear to exist in the population, H1 and H2, with H1 being overrepresented in patients suffering from PSP and CBD. The H1 haplotype shows considerable diversity, with at least three different sub-clades having been identified, one of which (H1C) was found to be most strongly associated with PSP (Pastor et al., 2004; Pittman et al., 2004). In addition to PSP, H1 sub-haplotypes have been recently reported to be linked to Parkinson's disease (Fung et al., 2006; Healy et al., 2004; Skipper et al., 2004; Zhang et al., 2005) and AD (Laws et al., 2007; Myers et al., 2005, 2007), although the latter finding remains controversial (Mukherjee et al., 2007).
3. Mapt−/− models
As MAPT plays such a crucial role in human brain development and function, one might expect a Mapt−/− mouse to display a severe phenotype. Yet, neither of the two Mapt−/− lines generated so far has been reported to show much evidence of brain dysfunction (Dawson et al., 2001; Harada et al., 1994). Homozygous Mapt−/− mice develop normally and do not display any overt histological abnormalities. Some behavioural alterations have been reported, however, namely muscle weakness on a rod walking and a wire hanging test, as well as hyperactivity in a bright open field (Ikegami et al., 2000). Moreover, the mice displayed impaired contextual fear conditioning when administered foot shocks in an experimental chamber. While normal animals showed a characteristic freezing response to the shock as well as to the chamber itself, Mapt−/− mice displayed a less severe freezing response, both immediately after and 24 h after acquisition of the fear response. This has been explained in terms of their increased hyperactivity rather than in terms of a memory deficit, as Mapt−/− mice do not seem to be impaired on a Morris water maze, the classical test of spatial working memory (Ikegami et al., 2000). Concomitant with these mild behavioural deficits, Mapt−/− mice also show changes in neuronal maturation and morphology. Thus, delayed maturation of hippocampal neurons was observed in primary hippocampal cultures from Mapt−/− mice (Dawson et al., 2001), and microtubule number and density appeared to be reduced in the cerebellum of Mapt−/− animals (Harada et al., 1994).
The relative subtlety of the Mapt−/− phenotype is probably partly due to developmental plasticity and redundancy associated with the different microtubule-associated proteins present in the brain. This is supported by studies examining other proteins within the microtubule-associated protein family in Mapt−/− mice. For instance, MAP1A protein expression appears to be increased in some Mapt−/− animals (Dawson et al., 2001; Harada et al., 1994), and Mapt−/− Map1B−/− double knock-out mice display a severe phenotype which is lethal within 4 weeks after its onset (Takei et al., 2000). In addition to such redundancy effects, it should be noted, however, that Mapt−/− mice remain rather unstudied in the literature, making it possible that subtle deficits have as yet gone unnoticed. More extensive behavioural tests and thorough electrophysiological investigations appear to be lacking.
No conditional Mapt knock-out mouse model has so far been described. Conditional knock-out lines typically carry an allele flanked by loxP sites (a “floxed” allele) and are crossed to a second line carrying Cre recombinase under the control of a tissue-specific promoter. Offspring are produced with a gene knock-out targeted only to certain cells (Morozov et al., 2003). This technique is particularly appealing since the mice do not have a widespread allele deletion which may avoid confounding issues such as developmental compensation.
4. Tau transgenic models
4.1. Wild-type cDNA models
In contrast to Mapt−/− mice, MAPT transgenic mice have been widely generated and studied extensively. The first MAPT transgenic models used complementary DNA (cDNA) vectors to overexpress various human wild-type tau isoforms in the mouse brain (Brion et al., 1999; Gotz et al., 1995; Higuchi et al., 2002; Ishihara et al., 1999, 2001; Probst et al., 2000). All of these models resulted in hyperphosphorylation of tau, but lacked most other neuropathological changes associated with human tauopathies. In particular, neurofibrillary tangles were reported only in rare instances and at an advanced age (18–20 months).
4.2. cDNA models with MAPT mutations in exon 10
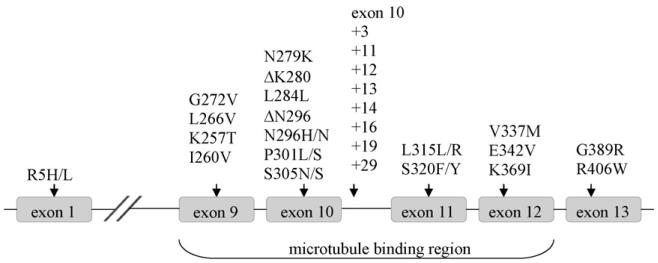
Given the lack of phenotype found when overexpressing wild-type MAPT in mice, the majority of research has now focused on overexpressing some of the more than 30 known MAPT mutations discovered in FTDP-17 patients. Most of these mutations lie within the microtubule-binding region of MAPT, spanning exons 9–12 (Fig. 2). The study of transgenic mice has consequently focused on this region, and in particular on mutations lying within exon 10 (for a summary of the most important transgenic models, cf. Table 1).
Fig. 2.
FTDP-17 mutations and their location along human MAPT. Only the exons in which mutations have been reported are shown. Point mutations are indicated by the identity and site of the amino acid change. Splice site mutations in intron 10 are indicated by their position counting away from exon 10.
Table 1.
Summary of MAPT transgenic lines
| Line | Transgene | Phenotype biology | Behaviour | References | |
|---|---|---|---|---|---|
| Mutation models | |||||
| Exon 9 | |||||
| G272V | PrP-TA mice | Mouse prion promoter | Hyperphosphorylated tau in neurons/oligodendrocytes |
Not reported | Gotz et al. (2001b) |
| G272V/P301L/R406W | VWL | 4R/2N; mouse Thy1 promoter |
Hyperphosphorylated tau; tau filaments; forebrain lysosomal abnormalities |
Not reported | Lim et al. (2001) |
| Exon 10 | |||||
| P301L | JNPL3 | 4R/0N; mouse prion promoter |
NFTs and abnormal tau filaments in neurons/oligodendrocytes; axonal degeneration in the spinal cord; loss of motor neurons; no apoptosis measured by TUNEL; NFT-bearing anterior horn motor neurons display a decrease in the density of synaptic boutons |
Motor problems (spontaneous back-paw clenching, delayed righting reflex, grasping weakness); death within 3 weeks of phenotype onset (by 10 months); no clear deficit on anxiety and cognitive tasks |
Lewis et al. (2000), Lin et al. (2003a,b, 2005), Arendash et al. (2004), Zehr et al. (2004), Katsuse et al. (2006) |
| pR5 | 4R/2N; mouse Thy1.2 promoter |
NFTs in cortex, spinal cord; mitochondrial dysfunction |
Small increase in exploratory behaviour; accelerated extinction; weight loss; intact spatial working memory, but impaired spatial reference memory |
Gotz et al. (2001a), Pennanen et al. (2004, 2006), David et al. (2005) | |
| PL-T34 | 4R/1N; mouse CNP promoter |
Tau inclusions in oligodendrocytes only; impaired axonal transport; altered myelin |
Progressive weight loss and muscle weakness |
Higuchi et al. (2005) | |
| Tg tau (P301L) 23027 |
4R/2N; hamster prion promoter |
Progressive neurofibrillary tangle pathology; independent development of glial pathology (florid glial plaques); brain atrophy in temporal lobe |
Deficits on Morris water maze; 8-arm radial maze; conditioned taste aversion task |
Murakami et al. (2006) | |
| Tau-4R-P301L | 4R/2N; Thy1 gene promoter |
Increased tau expression and changed phosphorylation patterns, but no aggregates |
Motor defect (clasping, beam walking, rotarod); improved memory recall in young mice |
Terwel et al. (2005), Boekhoorn et al. (2006) |
|
| Tg4510 | 4R/0N; CaMKII promoter under the control of the tet-operon response element |
Pretangles and NFTs, progressing with age; progressive neuronal loss; suppression of transgene by doxycycline led to reduction in neuronal loss, but continuing increase in NFTs |
Hunched posture and hind limb dysfunction at old age; increasing impairment on Morris water maze starting at 2.5 months; reduction in deficit if transgene suppressed at 5.5 months despite unchanged NFT load |
Ramsden et al. (2005), Santacruz et al. (2005), Spires et al. (2006) |
|
| 3×Tg-AD | P301L×APPswe ×PS1M146V |
Extracellular Aβ deposits and NFTs with time course and distribution resembling that of AD; synaptic dysfunction (LTP and EPSPs), not due to change in vesicle recycling |
Not reported | Oddo et al. (2003), Yao et al. (2005) | |
| TAPP | JNPL3×Tg 2576 mice (APPSWE) |
NFTs and amyloid plaques affect tangles, but not the reverse |
Not reported | (Lewis et al., 2001) | |
| N297K | N297K | 4R/0N; mouse prion promoter |
Hyperphosphorylated tau in the brain, but no NFTs |
Hyperactivity deficits in prepulse inhibition & Morris water maze |
Taniguchi et al. (2005) |
| P301S | Line 2541 | 4R/0N; mouse Thy1.2 promoter |
Hyperphosphorylated tau in brain and spinal cord resembles half-twisted ribbons found in FTDP-17; neuroinflammation accompanies pathological changes in tau phosphorylation |
Severe paraparesis; muscle weakness; tremor by 5–6 months of age |
Allen et al. (2002), Bellucci et al. (2004) |
| Lines PS5, PS19 | 4R/1N; mouse prion promoter |
HCC synapse loss, impaired synaptic function and concomitant microglial activation by 3–6 months; later pathology: hyperphosphorylated tau accumulations; neuronal loss/atrophy of HCC; immunosuppression in young mice attenuated tau pathology and increased lifespan |
Clasping and limb retraction on tail suspension test by 3 months of age; limb weakness, hunched-back posture and progressive paralysis by 7–10 months; death by 12 months |
Yoshiyama et al. (2007) | |
| P301S/G272V | THY-tau 22 | 4R/1N; mouse Thy1.2 promoter |
Hyperphosphorylated tau in neurons; NFTs in frontal cortex, HCC, amygdale; progressive cell loss in HCC; deficits in HCC synaptic transmission (EPSPs) by 14 months |
No motor dysfunction; increased anxiety; delayed learning and reduced spatial memory |
Schindowski et al. (2006) |
| Exon 11 | |||||
| V337M | Three lines: Tg212, 214, 216 |
4R/2N; PDGF-beta promoter |
Hyperphosphorylated tau and NFTs; neurons display signs of non-apoptotic degeneration; decreased neural activity in HCC at 15 months |
Elevated plus maze: increased time spent in open arms and increased locomotion in open field; no spatial impairment (Morris water maze) |
Tanemura et al. (2001, 2002) |
| Tauv337m− APPV717I−CT100 |
V337M×APPSWE mutation |
Age-dependent increase in tau phosphorylation, significantly enhanced in double mutant compared to single MAPT mutant |
Age-dependent memory deficits | Lambourne et al. (2005) | |
| Exon 13 | |||||
| R406W | Four lines: Tg748, 502, 492, 483 |
4R/2N; CaMKII promoter |
Hyperphosphorylated tau inclusions at 18 months, mainly straight filaments |
Impaired fear conditioning; slight decrease in prepulse inhibition; no sensorimotor deficits, no increase in anxiety; significant increase in immobility time on a forced swimming test which can be reversed using SSRIs |
Tatebayashi et al. (2002), Egashira et al. (2005) |
| RW tau | 4R/2N; mouse prion promoter |
Decrease in tau phosphorylation; tau binds less efficiently to microtubules; NFT-like pathology; retardation of fast axonal transport |
Progressive motor weakness | Zhang et al. (2004) | |
| TgTau R406W | 4R/2N; hamster prion promoter |
Progressive tau hyperphosphorylation; astrogliosis; NFTs in amygdala, HCC at 14 months |
Deficit on rotarod by 10 months; memory deficit in retention phase of passive avoidance task |
Ikeda et al. (2005) | |
| Genomic models | |||||
| 8c | Genomic MAPT PAC of the H1 haplotype |
All six human tau isoforms expressed; no pathology |
Not reported | Duff et al. (2000) | |
| htau | Genomic MAPT PAC of the H1 haplotype on a Mapt−/− background |
All six human tau isoforms expressed; hyperphosphorylated tau; neurofibrillary tangles; cell loss in a spatiotemporal distribution similar to AD |
Not reported |
Andorfer et al. (2005, 2003) |
The greatest number of lines has been generated from MAPT cDNA containing the P301L mutation. The first of that kind was the JNPL3 line, with 4R/0N tau (four-repeat, no amino-terminal inserts) under the control of the mouse prion promoter (Arendash et al., 2004; Lewis et al., 2000; Lin et al., 2003a,b, 2005). The mice developed neurofibrillary tangles in the diencephalon, brainstem and cerebellar nuclei, as well as abnormal tau filaments in glial cells. Moreover, axonal degeneration could be observed in the spinal cord, leading to progressive loss of motor neurons and, correspondingly, a motor phenotype characterized by impaired grasping and righting reflexes, as well as spontaneous back-paw clenching and general motor weakness. In contrast, no clear deficits could be observed on anxiety or cognitive tasks (Arendash et al., 2004), and neuronal apoptosis was absent (Zehr et al., 2004). As the first well-studied model of its kind, the JNPL3 line has provided important insights into the relationship between tau protein and disease. However, since the mice develop such a severe phenotype in the spinal cord, leading to early morbidity, the JNPL3 line has difficulty modelling the largely cortical progression of human tauopathic diseases.
More recently, lines carrying the P301L mutation have yielded phenotypes more closely resembling human tauopathies. However, their specific nature differs quite significantly depending on which tau isoform and promoter was used (Table 1). For instance, pR5 mice (4R/2N tau under the control of the mouse Thy1.2 promoter) displayed neurofibrillary tangles predominantly in the hippocampus and cortex, and developed behavioural deficits similar to those seen in certain tauopathies, with evidence for accelerated extinction and impaired spatial reference memory (Pennanen et al., 2004, 2006). These P301L mice also showed signs of mitochondrial dysfunction leading to an impairment of mitochondrial respiration and ATP synthesis. In particular, mitochondrial complex V proteins were reduced, just as they were in post-mortem brains of four FTDP-17 patients with the same mutation (David et al., 2005). A very recent model introduced the P301L mutation onto the longest tau isoform (4R/2N) under the control of the hamster prion promoter (Murakami et al., 2006). Mice of that line (Tg23027) developed neurofibrillary tangles and glial tangle pathology in frontotemporal areas that progressed with age and culminated in neuronal loss and cerebral atrophy. At a behavioural level the mice showed no gross motor abnormalities, but were impaired on a spatial memory task and a conditioned taste aversion task which required them to associate the taste of saccharine with a transient feeling of sickness induced by an injection of lithium chloride. On the latter task, mice with high ratios of insoluble to soluble tau exhibited the most severe deficits. Such an excellent correlation between pathology and behaviour is rarely found. Similarly, age-dependent tangle pathology and neuronal loss are uncommonly found in single MAPT transgenic models. For that reason, this model might be of particular interest to future research.
Besides the P301L mutation, two other exon 10 mutations have been studied in transgenic mouse models, namely P301S and N297K. P301S mice (4R/0N tau under the control of the Thy1.2 promoter) showed hyperphosphorylated tau in brain and spinal cord. The tangles were accompanied by neuroinflammation, and resembled the aggregates found in FTDP-17. Phenotypically, the animals displayed paraparesis, muscle weakness and developed a tremor by five to six months of age (Allen et al., 2002; Bellucci et al., 2004). However, the P301S mutation does not necessarily lead to such a severe motor deficit: Schinkdowski and colleagues (Schindowski et al., 2006) created a mouse line containing two MAPT mutations, P301S and G272V. The animals showed no motor dysfunction, but increased anxiety and spatial memory impairments. Fittingly, extensive neurofibrillary tangle pathology was found in the amygdala and the hippocampus. The latter area was also shown to undergo progressive cell loss and changes in synaptic transmission by 14 months of age.
Only one mouse model has been created so far using the N297K mutation. The mutated transgene (4R/0N under the control of the mouse prion promoter) led to the development of hyperphosphorylated tau in the absence of neurofibrillary tangles. The mice displayed hyperactivity and deficits in pre-pulse inhibition and on the Morris water maze (Taniguchi et al., 2005).
4.3. cDNA models with MAPT mutations in exons 9, 12 or 13
Outside of exon 10, MAPT transgenic research has so far focused on three FTDP-17 mutations: G272V in exon 9, V337M in exon 11 and R406W in exon 13. G272V was one of the earliest mutations to be used for MAPT transgenic models (Gotz et al., 2001b; Lim et al., 2001), and on its own, seems to result in hyperphosphorylated tau not only in neurons, but also in oligodendrocytes (Gotz et al., 2001b).
The V337M mutation has been studied more extensively, with research looking not only at brain abnormalities, but also behavioural phenotype. Thus, it has been suggested that V337M mice (4R/2N tau under the control of the platelet-derived growth factor-beta promoter) have difficulty associating environmental context with their present emotional state (Tanemura et al., 2002). Mice were tested on an elevated plus maze, where open arms confer anxiety while closed arms signal safety and tend to be preferred by normal animals. Despite being as anxious as their non-transgenic littermates (as measured by their rate of defecation), transgenic mice still spent an abnormal amount of time in the open arms. This finding could not be explained in terms of a spatial memory deficit, and has therefore been interpreted by the authors to be a problem of associating the open arms with the feeling of anxiety. It is also one of the few instances (cf. also Pennanen et al., 2006; Taniguchi et al., 2005) in which a behavioural phenotype found in MAPT transgenic mice is distantly reminiscent of the disinhibition commonly observed in FTDP-17 patients. In terms of pathology, the mice displayed a variety of abnormalities in the hippocampus, including the presence of neurofibrillary tangles, decreases in neuronal activity in the CA region and evidence for non-apoptotic neuronal degeneration.
The greatest number of lines from any mutation outside exon 10 was generated from the R406W mutation in exon 13. All of the lines are based on 4R/2N tau which, however, was put under the control of different promoters (Egashira et al., 2005; Ikeda et al., 2005; Tatebayashi et al., 2002; Zhang et al., 2004). The resulting phenotypes were quite diverse, but the presence of hyperphosphorylated tau and neurofibrillary tangle-like pathology was a universal feature. In terms of behaviour, memory and motor-related deficits were reported, just as with other MAPT transgenic mouse models. However, there was also the unusual report of increased immobility time on a forced swimming test (Egashira et al., 2005). In this paradigm, which has been used as a model for depression, mice are put into a small container filled with water from which they cannot escape for several minutes. The proportion of time the mice remain immobile as opposed to swim and try to escape is used as an indicator of depression. Accordingly, the increased immobility (“depression”) observed in R406W transgenic mice could be reversed by the administration of selective serotonin re-uptake inhibitors (Egashira et al., 2005). Depression is a symptom that commonly accompanies or precedes dementia, and may even be falsely diagnosed with the underlying neurodegenerative disease remaining unrecognised (Karnik et al., 2006). The R406W mouse line developed by Egashira and colleagues (Egashira et al., 2005) is the first that could help model the relationship between depression and dementia.
4.4. cDNA models with combined mutations
The cDNA lines described in the previous two sections have yielded many interesting insights into the development of tau pathology and the effect of MAPT mutation on phenotype. In addition to these studies, it has also been possible to create combined transgenic models which can provide information about the relationship between tau and other proteins thought to play a major role in tauopathic disease. For instance, Oddo and colleagues (Oddo et al., 2003) developed a triple transgenic line (3×Tg-AD) for the study of AD. The mice not only carried the P301L MAPT mutation, but also mutations in two other genes known to be associated with AD: the PS1M146V mutation in presenilin 1 (PS1) and the so-called Swedish mutation (APPSWE: KM670/671NL double mutation) in the amyloid precursor protein gene (APP). 3×Tg-AD mice, in contrast to single MAPT or single APP transgenic mice, display both neurofibrillary tangles and extracellular Aβ deposits in the brain, just as observed in AD patients. Moreover, the regional distribution of tangles and Aβ deposits, as well as the progressive time course of their appearance, mirrored that found in AD patients. Finally, the mice were shown to exhibit deficits in synaptic transmission and synaptic plasticity in the hippocampus even before the appearance of tangle and plaque pathology.
4.5. cDNA models with inducible promoters
Another novel and advanced transgenic model makes use of cDNA driven by an inducible promoter. In contrast to transgenes expressed under a constitutive promoter, those expressed under inducible promoters can be switched on and off in the adult mouse, allowing for a more direct study of the effect of the transgene. The Tg4510 line expresses the P301L MAPT mutation (4R/0N tau) under the control of a tet-inducible CaMKII promoter (Ramsden et al., 2005; Santacruz et al., 2005; Spires et al., 2006). In these mice transgene expression is mostly restricted to forebrain areas, and can be suppressed by treatment of the animals with doxycycline. As with other P301L models, the mice developed progressive pretangle and neurofibrillary tangle pathology with age, concomitant with neuronal cell loss. Moreover, a behavioural phenotype could also be observed, characterized by a hunched posture, development of hind limb dysfunction with increasing age and an increasing impairment on the Morris water maze task. The most important findings of this model, however, were made when transgene expression was inhibited through the administration of doxycycline. This led to a reduction in neuronal loss, but a continuing increase in neurofibrillary tangle load. Similarly, in terms of behaviour, the mice showed an improvement of performance on the Morris water maze, despite unchanged neurofibrillary tangle pathology. These results seem to suggest that neurofibrillary tangles do not necessarily lead to cell death or behavioural problems. Indeed, even with the transgene switched on, loss of neurons was reported to be dissociated from tangle pathology in specific brain areas, with cell death occurring before neurofibrillary tangles in the dentate gyrus, while neurofibrillary tangles could be observed without cell loss in the striatum.
4.6. Genomic DNA models
All MAPT transgenic models described so far employ cDNA constructs and suffer from the fundamental problem that their phenotypic profile is highly dependent on the nature of the promoter employed, be it tissue-specific or broadly expressed, constitutive or inducible. As a result, the transgene is expressed in a non-physiological fashion, which can lead to general overexpression, expression in irrelevant brain areas and expression at inappropriate times. Promoter dependence may be one of the reasons (along with other factors such as strain differences) that account for different transgenic lines displaying such different phenotypes even when the same mutation is studied.
Transgenic models using genomic DNA constructs can circumvent such issues. In the case of MAPT, there are two which have been reported so far. The entire human MAPT locus including its endogenous promoter was introduced into a mouse as a P1-based artificial chromosome (PAC) genomic DNA insert (Duff et al., 2000). The line thus generated expressed all six human tau isoforms and did not show any pathology. However, the continuing presence of mouse Mapt was a potential confound. To create an improved model, these mice were therefore backcrossed onto a Mapt−/− knock-out background (Andorfer et al., 2005, 2003). The resulting animals displayed hyperphosphorylated tau, neurofibrillary tangles and cell loss in a spatiotemporal distribution similar to that found in AD. Moreover, ultrastructurally, the insoluble tau fragments extracted from these mice resembled the paired helical filaments found in AD patients. It is intriguing that the presence of human wild-type genomic DNA alone should lead to a tauopathic phenotype. Andorfer and colleagues (Andorfer et al., 2005, 2003) suggested that it might be related to a change in tau isoform composition also observed in these mice, away from the usual 1:1 ratio of 3R:4R tau to an increase in 3R tau over 4R tau. This explanation is particularly interesting in light of evidence that relative tau isoform levels may be implicated in sporadic tauopathies (Caffrey et al., 2006; Chambers et al., 1999; Delacourte et al., 1998; Myers et al., 2007; Takanashi et al., 2002). Specifically, the htau mice might serve as a model for Pick's disease, as patient brains have been shown to display an abundance of 3R tau over the 4R isoforms (Delacourte et al., 1998).
5. Discussion
By now, the sheer number and diversity of mouse models of tauopathies should be apparent. While the findings resulting from this research are equally diverse, it is nevertheless possible at this stage to draw some general conclusions. Mouse models have made it clear, even more so than the study of FTPD-17 patients, that MAPT mutations alone are sufficient to cause tauopathic disease. Beyond that, the study of mice has also yielded additional insights into the mechanisms of that disease process, four of which stand out in particular.
Firstly, there is now good evidence that the formation of neurofibrillary tangles is independent of cell death. Thus, Andorfer and colleagues used electron microscopy in their human tau (htau) mice, and reported that the presence of tau filaments did not correlate with the death of individual cells (Andorfer et al., 2005). And more recently, a conditional P301L transgenic model has confirmed these findings (Ramsden et al., 2005; Santacruz et al., 2005; Spires et al., 2006): suppression of the transgene by doxycycline led to a reduction in the neuronal loss and the behavioural abnormalities observed in the transgenic animals, but left neurofibrillary tangle load unaffected. Conversely, there is some evidence that the presence of hyperphosphorylated tau at least is required for the onset of a phenotype. Young P301L mice, which still possessed normal tau phosphorylation patterns, were found to be unimpaired and indeed showed superior performance on an object recognition task compared to non-transgenic littermates (Boekhoorn et al., 2006). Concomitantly, they also showed increased long-term potentiation in the dentate of the hippocampus. Once those mice had reached a certain age however, and tau hyperphosphorylation had appeared, the pattern of results was reversed, with non-transgenic littermates outperforming their transgenic counterparts. Thus it seems that toxicity might be caused somewhere in between the emergence of tau hyperphosphorylation and its endpoint, the formation of neurofibrillary tangles.
Mouse models of tauopathies have also provided some insight into the mechanisms of cell death. At least two studies looking at two different mutations have so far reported the conspicuous absence of neuronal apoptosis. Thus, in JNPL3 mice TUNEL-staining appeared mainly in cells that electron microscopy indicated to be oligodendrocytes (Zehr et al., 2004). Similarly, Tanemura and colleagues (Tanemura et al., 2002) did not observe any signs of neuronal apoptosis in a V377M transgenic mouse line.
In addition to deepening our understanding of how dysfunction of tau itself can cause disease, transgenic and knock-out mice have provided some new information about the interaction between tau and other proteins relevant to tauopathies, in particular Aβ. For instance, it has been shown that treating primary neuronal cultures of tau knock-out mice with fibrillar Aβ does not have a deleterious effect on cells, while the same treatment on wild-type cultures results in extensive degeneration of neurons (Rapoport et al., 2002). The authors concluded that tau might be a key component of the mechanism by which Aβ exerts toxicity on cells. Similarly, the TAPP line, generated by crossing P301L tau mice with Aβ-producing Tg2576 mice, demonstrates the likelihood of a link between Aβ and tau pathology, with the former exerting an influence on the latter (Lewis et al., 2001). Thus, the neurofibrillary tangle load in MAPT APP double transgenics was found to be increased by a factor of seven compared to single MAPT or APP transgenics. In contrast, plaque formation was unaffected, indicating that presence of tau lesions had no effect on Aβ pathology.
Lastly, MAPT transgenic models are beginning to provide insights into the potential of new therapeutic approaches (cf. Table 2). Zhang and colleagues (Zhang et al., 2004) found their MAPT transgenic mice to display reduced microtubule numbers resulting in impaired fast axonal transport. Treatment with microtubule-stabilising drugs had a beneficial effect on both microtubule numbers and fast axonal transport, as well as reducing pathological tau inclusions and motor deficits.
Table 2.
Potential therapeutic targets for the treatment of tauopathic disease that have been investigated via MAPT transgenic models
| Therapeutic target | Mechanism | References |
|---|---|---|
| Microtubule transport | Microtubule-stabilising drugs were shown to restore microtubule numbers and fast axonal transport affected by a MAPT mutant transgene |
Zhang et al. (2004) |
| GSK-3 (Cdk5) | Lithium inhibition of GSK-3, responsible for aberrant phosphorylation of tau, could be shown to reduce and sometimes reverse tau pathology |
Engel et al. (2006), Mudher et al. (2004), Nakashima et al. (2005), Noble et al. (2005), Perez et al. (2003), Plattner et al. (2006) |
| CHIP/Hsp90 | CHIP-mediated inhibition of Hsp90 was shown to influence the pathway involved in ubiquitination and degradation of proteins and decrease abnormally phosphorylated tau |
Dickey et al. (2007a) |
| Neuroinflammation | Use of the immunosuppressor FK506 resulted in a decrease of hyperphosphorylated tau and an increased lifespan in MAPT mice that display early neuroinflammation as a result of a mutant transgene |
Yoshiyama et al. (2007) |
Several studies utilising transgenic models have demonstrated the promising therapeutic potential of glycogen synthase kinase-3 (GSK-3) inhibition. Along with cyclin-dependent kinase 5 (Cdk5), GSK-3 is one of the main kinases thought to be involved in the aberrant phosphorylation of tau in neurodegenerative disease (e.g. Hanger et al., 1992; Mandelkow et al., 1992). Moreover, it may also affect MAPT pre-mRNA splicing (Hernandez et al., 2004), making it a particularly interesting candidate for the treatment of sporadic tauopathies. Most GSK-3 inhibition studies use lithium. The chronic administration of lithium in mice overexpressing wild-type or mutant human MAPT has been shown to reduce hyperphosphorylation of tau (Noble et al., 2005; Perez et al., 2003) and in some cases even to reverse tau pathology and behavioural abnormalities observed in aged mice (Engel et al., 2006; Nakashima et al., 2005). Similar findings have also been reported in a MAPT overexpression model in Drosophila (Mudher et al., 2004). Furthermore, it appears that the negative effect of GSK-3 on tau phosphorylation can be modulated by Cdk5 which was shown to act as an inhibitor of GSK-3 in a Cdk5 mouse model (Plattner et al., 2006). Cdk5 may therefore also be a promising candidate for medical intervention.
Another such candidate is the C-terminus of the Hsp70-interacting protein (CHIP) which is a crucial catalyst for chaperone-mediated protein ubiquitination and degradation of abnormally phosphorylated and folded tau proteins (Dickey et al., 2007b). Accordingly, inhibition of one of the chaperones involved in this pathway (Hsp90) in htau mice (Andorfer et al., 2003) was shown to decrease hyperphosphorylated tau levels, a process which appears to be mediated by CHIP (Dickey et al., 2007a).
Finally, there is very recent evidence indicating that immunosuppression may have beneficial effects on tau pathology. P301S transgenic mice have been shown to display synapse loss and impaired synaptic function in the hippocampus that was followed by microglial activation and neuroinflammation at an early age. Only later was the usual tau pathology and accumulation observed, concomitant with neuronal loss and hippocampal atrophy. Use of the immunosuppressor FK506 resulted in a decrease of abnormally phosphorylated tau, as well as an increased lifespan (Yoshiyama et al., 2007). These findings not only highlight a potential future therapeutic avenue, but also provide additional evidence that crucial pathogenic changes in tauopathies may occur well before the accumulation of tau and the formation of neurofibrillary tangles.
6. Future models
While Mapt−/− and MAPT transgenic models have thus yielded important insights into the development of tauopathies, many vital questions still remain unexplored. The most obvious omission in research so far is the lack of models for any tauopathy other than AD and FTDP-17. While it is true that neurofibrillary tangles are the hallmark of all tauopathies, their ultrastructural composition differs significantly between the individual syndromes. Moreover, diseases like PSP, CBD and Pick's are quite unlike AD and frontotemporal dementia in terms of symptoms, aetiology and brain pathology as a whole. In particular PSP and CBD tend to resemble Parkinson's disease more than Alzheimer's disease, and they lack amyloid plaques entirely. Moreover, all three diseases have been linked to an alteration in the splice ratio of the tau protein. Thus, in PSP and CBD, the normal 1:1 ratio of 3R to 4R tau is changed in favour of 4R tau (Chambers et al., 1999; Takanashi et al., 2002). Conversely, in Pick's disease, patient brains display an abundance of 3R tau (Delacourte et al., 1998). Despite MAPT alternative splicing thus potentially playing a causal role in the development of tauopathic disease, it has remained largely unexplored in the transgenic literature. Most transgenic models so far employ cDNA constructs, which do not allow realistic investigation of MAPT alternative splicing and, as discussed above, have additional problems of promoter dependence and non-physiological expression levels. There are only two reports so far of a genomic MAPT transgene being used to create a transgenic mouse line (Andorfer et al., 2003; Duff et al., 2000). Despite the introduction of wild-type MAPTDNA, the mice displayed a phenotype when backcrossed onto knock-out background. They also showed an overall excess of 3R tau over 4R tau, and thus might serve as a model for Pick's disease. Overall therefore, these results add support to the hypothesis that MAPT alternative splicing may play a role in the genesis of tauopathies.
The creation of more genomic mouse lines would now be of great interest. The models created so far by Duff, Andorfer and colleagues have demonstrated that genomic constructs can lead to expression of both 3R and 4R tau in mice, even though adult mice – under normal circumstances – show constitutive expression of 4R tau only. It thus appears that the presence of cis-splicing regulatory elements, in conjunction with conserved trans elements, might be sufficient to mimic the splicing pattern observed in humans. However, it still remains to be seen whether it is possible to obtain a transgenic animal in which the baseline splice ratio of 3R to 4R tau is closer to 1:1, thus reflecting the conditions present in healthy individuals. One could also attempt to model the most common isoform imbalance found in tauopathies, namely that of an increase in 4R tau over 3R tau. Furthermore, to study the effects of splicing, one might consider exploring one of the many FTDP-17 mutations that have been found to affect MAPT alternative splicing instead of or in addition to tau microtubule binding (e.g. N296K, ΔK280, S305N, exon 10 + 3).
Considering the microtubule-binding mutations, it seems that studying one mutation, and one particular transgenic line even, in great detail may be more beneficial than generating many new transgenic models incorporating previously unexplored mutations. The former method is more likely to provide insights into the mechanisms of the disease-causing process, as is exemplified by research into the JNPL3 line. With its severe motor neuron and spinal cord phenotype it is arguably not a particularly accurate model of human FTDP-17. Yet its in-depth study has resulted in valuable new evidence about the mechanism of cell death associated with tauopathies (Zehr et al., 2004), as well as the deleterious influence of neurofibrillary tangles on synaptic architecture (Katsuse et al., 2006).
In conclusion then, study of MAPT alternative splicing and further in-depth analysis of tau microtubule-binding mutations are likely to yield important new mouse models of tauopathies. Thus, they might contribute to the valuable insights into disease progression, aetiology and treatment that have already been provided by the Mapt−/− and MAPT transgenic models to date.
Acknowledgements
Franziska Denk is a Wellcome Trust Prize Student in Neuroscience. Richard Wade-Martins is a Wellcome Trust Research Career Development Fellow.
Footnotes
Disclosure statement The authors declare no conflicts of interest.
References
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 2002;22(21):9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 2005;25(22):5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86(3):582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31(43):10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Lewis J, Leighty RE, McGowan E, Cracchiolo JR, Hutton M, Garcia MF. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer's disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Res. 2004;1012(1–2):29–41. doi: 10.1016/j.brainres.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 1999;8(4):711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Bellucci A, Westwood AJ, Ingram E, Casamenti F, Goedert M, Spillantini MG. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am. J. Pathol. 2004;165(5):1643–1652. doi: 10.1016/S0002-9440(10)63421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J. Neurosci. 2006;26(13):3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta. 2005;1739(2–3):331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Brion JP, Tremp G, Octave JN. Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer's disease. Am. J. Pathol. 1999;154(1):255–270. doi: 10.1016/S0002-9440(10)65272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum. Mol. Genet. 2006;15(24):3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- Chambers CB, Lee JM, Troncoso JC, Reich S, Muma NA. Overexpression of four-repeat tau mRNA isoforms in progressive supranuclear palsy but not in Alzheimer's disease. Ann. Neurol. 1999;46(3):325–332. doi: 10.1002/1531-8249(199909)46:3<325::aid-ana8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Conrad C, Andreadis A, Trojanowski JQ, Dickson DW, Kang D, Chen X, Wiederholt W, Hansen L, Masliah E, Thal LJ, Katzman R, Xia Y, Saitoh T. Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann. Neurol. 1997;41(2):277–281. doi: 10.1002/ana.410410222. [DOI] [PubMed] [Google Scholar]
- David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Drose S, Brandt U, Muller WE, Eckert A, Gotz J. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005;280(25):23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 2001;114(Pt 6):1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Sergeant N, Wattez A, Gauvreau D, Robitaille Y. Vulnerable neuronal subsets in Alzheimer's and Pick's disease are distinguished by their tau isoform distribution and phosphorylation. Ann. Neurol. 1998;43(2):193–204. doi: 10.1002/ana.410430209. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Jr., Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007a;117(3):648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Patterson C, Dickson D, Petrucelli L. Brain CHIP: removing the culprits in neurodegenerative disease. Trends Mol. Med. 2007b;13(1):32–38. doi: 10.1016/j.molmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann. Neurol. 2000;47(3):374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- D'Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim. Biophys. Acta. 2005;1739(2–3):104–115. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, Burki K, Davies P. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol. Dis. 2000;7(2):87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- Egashira N, Iwasaki K, Takashima A, Watanabe T, Kawabe H, Matsuda T, Mishima K, Chidori S, Nishimura R, Fujiwara M. Altered depression-related behavior and neurochemical changes in serotonergic neurons in mutant R406W human tau transgenic mice. Brain Res. 2005;1059(1):7–12. doi: 10.1016/j.brainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J. Neurochem. 2006;99(6):1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- Fung HC, Xiromerisiou G, Gibbs JR, Wu YR, Eerola J, Gourbali V, Hellstrom O, Chen CM, Duckworth J, Papadimitriou A, Tienari PJ, Hadjigeorgiou GM, Hardy J, Singleton AB. Association of tau haplotype-tagging polymorphisms with Parkinson's disease in diverse ethnic Parkinson's disease cohorts. Neurodegener. Dis. 2006;3(6):327–333. doi: 10.1159/000097301. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: the chicken or the egg? Neuron. 2003;40(3):457–460. doi: 10.1016/s0896-6273(03)00681-0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta. 2005;1739(2–3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J. Biol. Chem. 2001a;276(1):529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14(7):1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Tolnay M, Barmettler R, Chen F, Probst A, Nitsch RM. Oligodendroglial tau filament formation in transgenic mice expressing G272V tau. Eur. J. Neurosci. 2001b;13(11):2131–2140. doi: 10.1046/j.0953-816x.2001.01604.x. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 1992;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369(6480):488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Lees AJ, Casas JP, Quinn N, Bhatia K, Hingorani AD, Wood NW. Tau gene and Parkinson's disease: a case-control study and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2004;75(7):962–965. doi: 10.1136/jnnp.2003.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Perez M, Lucas JJ, Mata AM, Bhat R, Avila J. Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35 Implications for Alzheimer's disease. J. Biol. Chem. 2004;279(5):3801–3806. doi: 10.1074/jbc.M311512200. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Ishihara T, Zhang B, Hong M, Andreadis A, Trojanowski J, Lee VM. Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration. Neuron. 2002;35(3):433–446. doi: 10.1016/s0896-6273(02)00789-4. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Zhang B, Forman MS, Yoshiyama Y, Trojanowski JQ, Lee VM. Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J. Neurosci. 2005;25(41):9434–9443. doi: 10.1523/JNEUROSCI.2691-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, Khan MN, Hardy J, Lantos PL, St. George-Hyslop P, Munoz DG, Mann D, Lang AE, Bergeron C, Bigio EH, Litvan I, Bhatia KP, Dickson D, Wood NW, Hutton M. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56(12):1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Shoji M, Kawarai T, Kawarabayashi T, Matsubara E, Murakami T, Sasaki A, Tomidokoro Y, Ikarashi Y, Kuribara H, Ishiguro K, Hasegawa M, Yen SH, Chishti MA, Harigaya Y, Abe K, Okamoto K, St. George-Hyslop P, Westaway D. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am. J. Pathol. 2005;166(2):521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S, Harada A, Hirokawa N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci. Lett. 2000;279(3):129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24(3):751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VM. Age-dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mice. Am. J. Pathol. 2001;158(2):555–562. doi: 10.1016/S0002-9440(10)63997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik NS, D'Apuzzo M, Greicius M. Non-fluent progressive aphasia, depression, and OCD in a woman with progressive supranuclear palsy: neuroanatomical and neuropathological correlations. Neurocase. 2006;12(6):332–338. doi: 10.1080/13554790601125957. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Lin WL, Lewis J, Hutton ML, Dickson DW. Neurofibrillary tangle-related synaptic alterations of spinal motor neurons of P301L tau transgenic mice. Neurosci. Lett. 2006;409(2):95–99. doi: 10.1016/j.neulet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Kidd M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Lambourne SL, Sellers LA, Bush TG, Choudhury SK, Emson PC, Suh YH, Wilkinson LS. Increased tau phosphorylation on mitogen-activated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer's disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities. Mol. Cell. Biol. 2005;25(1):278–293. doi: 10.1128/MCB.25.1.278-293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SM, Friedrich P, Diehl-Schmid J, Muller J, Eisele T, Bauml J, Forstl H, Kurz A, Riemenschneider M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer's disease. Mol. Psychiatry. 2007;12(5):510–517. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Mol. Cell. Neurosci. 2001;18(6):702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am. J. Pathol. 2003a;162(1):213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultra-structural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J. Neurocytol. 2003b;32(9):1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Lin WL, Zehr C, Lewis J, Hutton M, Yen SH, Dickson DW. Progressive white matter pathology in the spinal cord of transgenic mice expressing mutant (P301L) human tau. J. Neurocytol. 2005;34(6):397–410. doi: 10.1007/s11068-006-8726-0. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging. 1995;16(3):355–362. doi: 10.1016/0197-4580(95)00025-a. discussion 62–63. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Drewes G, Biernat J, Gustke N, Van Lint J, Vandenheede JR, Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992;314(3):315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Morozov A, Kellendonk C, Simpson E, Tronche F. Using conditional mutagenesis to study the brain. Biol. Psychiatry. 2003;54(11):1125–1133. doi: 10.1016/s0006-3223(03)00467-0. [DOI] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Drummond JA, Berg S, MacKay D, Asuni AA, Bhat R, Lovestone S. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry. 2004;9(5):522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- Mukherjee O, Kauwe JS, Mayo K, Morris JC, Goate AM. Haplotype-based association analysis of the MAPT locus in late onset Alzheimer's disease. BMC Genet. 2007;8:3. doi: 10.1186/1471-2156-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Paitel E, Kawarabayashi T, Ikeda M, Chishti MA, Janus C, Matsubara E, Sasaki A, Kawarai T, Phinney AL, Harigaya Y, Horne P, Egashira N, Mishima K, Hanna A, Yang J, Iwasaki K, Takahashi M, Fujiwara M, Ishiguro K, Bergeron C, Carlson GA, Abe K, Westaway D, St. George-Hyslop P, Shoji M. Cortical neuronal and glial pathology in TgTauP301L transgenic mice: neuronal degeneration, memory disturbance, and phenotypic variation. Am. J. Pathol. 2006;169(4):1365–1375. doi: 10.2353/ajpath.2006.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, Marlowe L, Pittman AM, Lees A, Fung HC, Duckworth J, Leung D, Gibson A, Morris CM, de Silva R, Hardy J. The H1c Haplotype at the MAPT locus is associated with Alzheimer's Disease. Hum. Mol. Genet. 2005;14(16):2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Pittman AM, Zhao AS, Rohrer K, Kaleem M, Marlowe L, Lees A, Leung D, McKeith IG, Perry RH, Morris CM, Trojanowski JQ, Clark C, Karlawish J, Arnold S, Forman MS, Van Deerlin V, de Silva R, Hardy J. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 2007;25(3):561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, Terada S, Hamamura T, Trojanowski JQ, Lee VM, Kuroda S. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. (Berl.) 2005;110(6):547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. U.S.A. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Pastor P, Ezquerra M, Perez JC, Chakraverty S, Norton J, Racette BA, McKeel D, Perlmutter JS, Tolosa E, Goate AM. Novel haplotypes in 17q21 are associated with progressive supranuclear palsy. Ann. Neurol. 2004;56(2):249–258. doi: 10.1002/ana.20178. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Welzl H, D'Adamo P, Nitsch RM, Gotz J. Accelerated extinction of conditioned taste aversion in P301L tau transgenic mice. Neurobiol. Dis. 2004;15(3):500–509. doi: 10.1016/j.nbd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Wolfer DP, Nitsch RM, Gotz J. Impaired spatial reference memory and increased exploratory behavior in P301L tau transgenic mice. Genes Brain Behav. 2006;5(5):369–379. doi: 10.1111/j.1601-183X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J. Alzheimers Dis. 2003;5(4):301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Abou-Sleiman P, Fung HC, Kaleem M, Marlowe L, Duckworth J, Leung D, Williams D, Kilford L, Thomas N, Morris CM, Dickson DW, Wood NW, Hardy J, Lees AJ, de Silva R. Linkage disequilibrium fine-mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J. Med. Genet. 2005;42(11):837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Duckworth J, Bryden L, Hanson M, AbouSleiman P, Wood NW, Hardy J, Lees A, de Silva R. The structure of the tau haplotype in controls and in progressive supranuclear palsy. Hum. Mol. Genet. 2004;13(12):1267–1274. doi: 10.1093/hmg/ddh138. [DOI] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006;281(35):25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998;43(6):815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. (Berl.) 2000;99(5):469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J. Neurosci. 2005;25(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol. 2006;169(2):599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262 Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38(12):3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- Shahani N, Brandt R. Functions and malfunctions of the tau proteins. Cell. Mol. Life Sci. 2002;59(10):1668–1680. doi: 10.1007/PL00012495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper L, Wilkes K, Toft M, Baker M, Lincoln S, Hulihan M, Ross OA, Hutton M, Aasly J, Farrer M. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am. J. Hum. Genet. 2004;75(4):669–677. doi: 10.1086/424492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. U.S.A. 1998;95(13):7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am. J. Pathol. 2006;168(5):1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi M, Mori H, Arima K, Mizuno Y, Hattori N. Expression patterns of tau mRNA isoforms correlate with susceptible lesions in progressive supranuclear palsy and corticobasal degeneration. Brain Res. Mol. Brain Res. 2002;104(2):210–219. doi: 10.1016/s0169-328x(02)00382-0. [DOI] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 2000;150(5):989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemura K, Akagi T, Murayama M, Kikuchi N, Murayama O, Hashikawa T, Yoshiike Y, Park JM, Matsuda K, Nakao S, Sun X, Sato S, Yamaguchi H, Takashima A. Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiol Dis. 2001;8(6):1036–1045. doi: 10.1006/nbdi.2001.0439. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J. Neurosci. 2002;22(1):133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Doe N, Matsuyama S, Kitamura Y, Mori H, Saito N, Tanaka C. Transgenic mice expressing mutant (N279K) human tau show mutation dependent cognitive deficits without neurofibrillary tangle formation. FEBS Lett. 2005;579(25):5704–5712. doi: 10.1016/j.febslet.2005.09.047. [DOI] [PubMed] [Google Scholar]
- Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima K, Iwasaki K, Fujiwara M, Tanemura K, Murayama M, Ishiguro K, Planel E, Sato S, Hashikawa T, Takashima A. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc. Natl. Acad. Sci. U.S.A. 2002;99(21):13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, Van Leuven F. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J. Biol. Chem. 2005;280(5):3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Bushlin I, Furukawa K. Preserved synaptic vesicle recycling in hippocampal neurons in a mouse Alzheimer's disease model. Biochem. Biophys. Res. Commun. 2005;330(1):34–38. doi: 10.1016/j.bbrc.2005.02.121. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zehr C, Lewis J, McGowan E, Crook J, Lin WL, Godwin K, Knight J, Dickson DW, Hutton M. Apoptosis in oligodendrocytes is associated with axonal degeneration in P301L tau mice. Neurobiol. Dis. 2004;15(3):553–562. doi: 10.1016/j.nbd.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang B, Higuchi M, Yoshiyama Y, Ishihara T, Forman MS, Martinez D, Joyce S, Trojanowski JQ, Lee VM. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy. J. Neurosci. 2004;24(19):4657–4667. doi: 10.1523/JNEUROSCI.0797-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Song Y, Chen H, Fan D. The tau gene haplotype h1 confers a susceptibility to Parkinson's disease. Eur. Neurol. 2005;53(1):15–21. doi: 10.1159/000082956. [DOI] [PubMed] [Google Scholar]