Abstract
Probiotic formulations are widely available and have a variety of proposed beneficial effects, including promotion of gut health. The mechanisms of action of probiotic bacteria in the intestine are still unclear but are generally attributed to an antiinflammatory effect. Here, we demonstrate that the multiple probiotic formulation VSL#3 prevents the onset of intestinal inflammation by local stimulation of epithelial innate immune responses (i.e., increased production of epithelial-derived TNF-α and restoration of epithelial barrier function in vivo). We also demonstrate that probiotic bacteria stimulate epithelial production of TNF-α and activate NF-κB in vitro. Our results support the hypothesis that probiotics promote gut health through stimulation, rather than suppression, of the innate immune system. Furthermore, our findings provide the perspective that defects in innate immunity may play a critical role in the pathogenesis and progression of intestinal disorders, such as inflammatory bowel disease.
Keywords: Crohn’s disease, cytokines
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) of unknown etiology (1). The current hypothesis for CD pathogenesis suggests that it develops in response to an overly aggressive adaptive immune response to the commensal flora (2). However, it has been recently suggested that CD may result from deficits in innate immunity (3–5). In this context, the intestinal epithelium seems to play a major role, allowing sensing of the luminal environment and differential regulation of the gut immune system (6). Although previous studies have investigated the effects of probiotics on intestinal cell lines in vitro, little is known regarding their actions on the intestinal epithelium in vivo.
Probiotics are live microorganisms that seem to promote gut health and regulate intestinal homeostasis (7). Different possible mechanisms of probiotic action have been proposed, including both suppression and stimulation of host immune responses. VSL#3 is a probiotic mixture of eight different bacterial strains that has demonstrated beneficial effects in several intestinal conditions. Controlled studies have shown VSL#3 to be efficacious for patients with ulcerative colitis (UC) and pouchitis (8, 9), as well as in animal models of colitis (10, 11). However, the precise mechanisms of action are not fully understood.
In this study, we tested the hypothesis that administration of VSL#3 to SAMP1/YitFc (SAMP) mice with chronic CD-like ileitis can prevent the onset of gut inflammation through stimulation of innate immunity in the gut. The SAMP mouse model has been extensively characterized by our group and others and has unique characteristics closely resembling CD (12–14). Our results show that pretreatment of SAMP mice with VSL#3 for 6 weeks before the onset of ileitis prevents the occurrence of intestinal inflammation through a mechanism involving stimulation of TNF-α in the intestinal epithelium and restitution of normal barrier function. Therefore, we propose the concept that probiotic bacteria promote gut health and prevent chronic intestinal inflammation through stimulation of epithelial innate immunity.
Results
High-Dose VSL#3 Prevents Ileitis in SAMP Mice but Does Not Affect Established Disease.
We first investigated the effects of low-dose VSL#3 in preventing the onset of SAMP ileitis (prevention protocol). Although some amelioration of villous distortion and thickening of the muscularis mucosa was observed, a severe inflammatory infiltrate in the lamina propria was still present, with no significant differences compared with controls (Fig. 1 Bi and Bii). By comparison, SAMP mice pretreated with high-dose VSL#3 showed a marked decrease in the severity of ileitis compared with age-matched, untreated controls (total score: 3.2 ± 3.1 vs. 11.6 ± 6.1, P < 0.005); the three histologic components of the total inflammatory score (i.e., villous distortion, active inflammation, and chronic inflammation) were all significantly decreased (Fig. 1A). Interestingly, ileitis was completely prevented in 5 of 11 mice (45%; total score: 0–1), whereas no evidence of chronic infiltration (chronic score: 0) was observed in 10 of 11 of these mice (91%). Histologically, mice administered high-dose VSL#3 showed almost complete protection of mucosal integrity and virtually no inflammatory cell infiltrates in the lamina propria (Fig. 1Biii).
Fig. 1.
VSL#3 administration prevents the onset of ileitis in SAMP mice. Three-week-old mice were administered high- or low-dose VSL#3 for 6 weeks. (A) The total inflammatory score was markedly decreased in SAMP mice administered high-dose VSL#3. (B) Representative photomicrographs of H&E-stained sections, 10× and 20× original magnification: (i) control untreated mice (n = 12) display severe distortion of the villi, with intense leukocyte infiltration of the lamina propria and thickening of the muscularis mucosa; (ii) SAMP mice administered low-dose VSL#3 (n = 12) show less distortion of tissue architecture but a high level of cellular infiltration and thickening of the muscularis mucosa; and (iii) SAMP mice administered high-dose VSL#3 (n = 11) show almost complete prevention of mucosal damage, with preservation of the normal villi morphology and minimal inflammatory infiltrate. Data are expressed as mean ± SEM. *P < 0.01; **P < 0.005.
The efficacy of high-dose VSL#3 was then evaluated in 30-week-old SAMP mice with established ileitis (treatment protocol). No beneficial effects were observed between high-dose VSL#3 treated and untreated mice (Fig. S1).
VSL#3 Administration Changes the Composition of Probiotic DNA Found in Fecal Material and the Terminal Ileum.
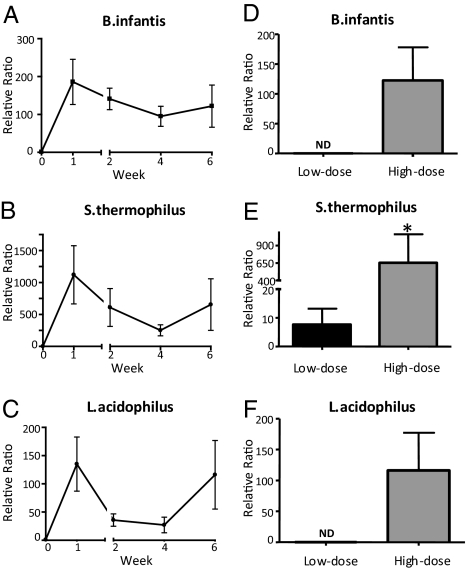
We next performed RT-PCR on total fecal DNA with primers specific for three of the probiotic bacteria contained in VSL#3 (Streptococcus thermophilus, Bifidobacterium infantis, and Lactobacillus acidophilus). We found that administration of high-dose VSL#3 markedly increased the concentration of each of these bacteria, starting from the first week after probiotic administration. The amount of bacterial DNA for the three strains was elevated throughout the 6 weeks of treatment (Fig. 2 A–C). As expected, S. thermophilus, which is present at the highest concentration in VSL#3, had the highest relative increase.
Fig. 2.
VSL#3 alters the fecal probiotic DNA composition of SAMP mice. RT-PCR with primers specific for VSL#3 probiotic bacteria was performed weekly on fecal extracts to assess the extent of bacterial colonization. Mice administered high-dose VSL#3 (n = 6) showed a relative increase in probiotic DNA for all three strains at each posttreatment time point compared with baseline (A–C). At week 6, mice given high-dose VSL#3 had significantly increased relative increases in probiotic DNA for each of the three strains, compared with mice given low-dose VSL#3 (E–G). Data are expressed as mean ± SEM. *P < 0.01 vs. low-dose VSL#3.
We next compared the bacterial composition in the feces and terminal ileum of mice treated with low- and high-dose VSL#3 after 6 weeks of treatment. In SAMP mice treated with low-dose VSL#3, only S. thermophilus was detected in the stool, whereas all three strains were significantly elevated in the high-dose treatment group (Fig. 2 D–F). In the ileum, increased trends were found for B. infantis and S. thermophilus in mice administered low-dose VSL#3 (3.7-fold for B. infantis, 10.8-fold for S. thermophilus) compared with untreated control mice. Conversely, mice administered high-dose VSL#3 showed a consistent and abundant increase for the two bacteria (265-fold for B. infantis; 639-fold for S. thermophilus) compared with untreated mice (P = 0.005 for both; Fig. 3 A–C). No detectable levels of L. acidophilus DNA were found in the ileum of untreated or VSL#3-treated mice.
Fig. 3.
VSL#3 differentially alters the ileal probiotic DNA composition of SAMP mice. At the end of the study period (6 weeks), terminal ilea were collected from SAMP mice and DNA isolated. (A) RT-PCR with primers specific for probiotic bacteria showed a significant increase of B. infantis and S. thermophilus compared with untreated mice. (B) Visualization of PCR products resolved on a 2% agarose gel. Data are expressed as mean ± SEM. *P < 0.005 vs. control.
VSL#3 Acts on the Intestinal Epithelium by Both Restoring Epithelial Barrier Function and Stimulating Production of TNF-α by Epithelial Cells.
To test the hypothesis that the intestinal epithelium is the primary site of action of VSL#3, we studied the effects of high-dose VSL#3 (prevention protocol) on intestinal permeability and production of epithelial-derived cytokines compared with control mice. After 6 weeks, VSL#3-treated mice showed a marked decrease in small intestinal permeability compared with untreated controls (0.35 ± 0.03 vs. 0.45 ± 0.03, P < 0.01; Fig. 4) to a level similar to that measured in healthy mice (15).
Fig. 4.
VSL#3 pretreatment restores small intestinal epithelial barrier function in SAMP mice. In vivo paracellullar permeability was determined by measuring the fractional urinary excretion of orally administered, region-specific (small intestinal) sugar probes (lactulose/mannitol ratio). Three-week-old SAMP mice were treated with VSL#3 for 2 weeks (n = 4) or 6 weeks (n = 6) and then compared with age-matched SAMP mice fed a normal diet. VSL#3 administration significantly decreased small intestinal epithelial permeability by the end of the treatment period. Data are expressed as mean ± SEM. *P < 0.05 vs. control.
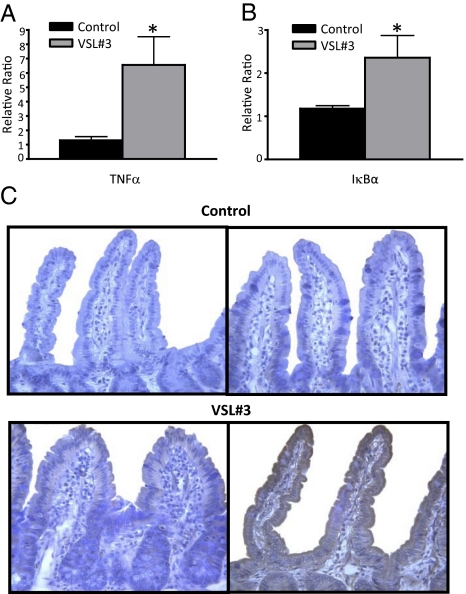
We next studied the expression of TNF-α in freshly isolated epithelial cells from different experimental groups. Administration of high-dose VSL#3 significantly increased TNF-α mRNA levels in isolated epithelial cells compared with untreated control mice (5-fold increase, P < 0.05; Fig. 5A). In addition, mRNA levels of the NF-κB inhibitory subunit IκBα were significantly increased in VSL#3-treated mice compared with untreated controls (2-fold, P < 0.05; Fig. 5B), suggesting activation of the NF-κB pathway. To confirm that TNF-α production was also increased at the protein level, immunohistochemical analysis was performed. A consistent and marked increase in TNF-α, immunolocalized to the epithelium, was observed in mice administered high-dose VSL#3 compared with low-dose and untreated control mice (Fig. 5 A–C).
Fig. 5.
VSL#3 administration stimulates epithelial cells in vivo. (A and B) Increased expression of TNF-α and IκBα mRNA in epithelial cells after VSL#3 administration. mRNA was extracted from freshly isolated epithelial cells from SAMP mice at the end of the study period. RT-PCR was performed with specific primers and data normalized to β-actin. All values are expressed as mean ± SEM. *P < 0.05. (C) Representative photomicrographs of immunohistochemical staining demonstrated that TNF-α immunolocalized to the epithelium and was markedly increased in high-dose (Bottom Right) compared with low-dose (Bottom Left) VSL#3-treated mice, whereas untreated controls (Top Right) showed no TNF-α staining. Absence of primary detecting antibody confirmed specificity of TNF-α staining (Top Left). ×40 original magnification.
VSL#3 Conditioned Media Exerts Specific Stimulatory Effects on Epithelial Cells In Vitro.
To further explore the hypothesis that probiotics enhance the innate immune defense of the intestinal epithelium, we investigated the effects of VSL#3 conditioned media (CM) on epithelial cells in vitro. Terminal ileum organ cultures were incubated for 24 h in the presence of CM alone or concomitantly with fecal extract (FE) from SAMP mice. A significant increase was measured in TNF-α secretion for CM and CM+FE organ cultures compared with media and FE alone (2.9- and 2.5-fold, respectively, P < 0.001 for both; Fig. 6A).
Fig. 6.
VSL#3 CM stimulates TNF-α production and activates NF-κB in small intestinal organ cultures. Ilea from SAMP mice were harvested and cultured for 24 h with either VSL#3 CM, FE, or combination CM+FE and compared with controls (media alone). (A) Secreted TNF-α production was measured from resulting supernatants by ELISA and showed significantly increased levels in CM and CM+FE compared with controls. (B) Cytoplasmic and nuclear extracts analyzed by Western blot showed increased nuclear p65 (RelA) and decreased cytoplasmic IκBα levels in CM and CM+FE compared with controls, indicating activation of the NF-κB pathway by VSL#3. Data are representative of three independent experiments with similar results. *P < 0.001.
Finally, to test whether VSL#3 CM directly activates NF-κB, the NF-κB subunit p65 (RelA) and the inhibitory subunit IκBα were measured in nuclear and cytoplasm extracts, respectively, isolated from SAMP terminal ileum organ cultures stimulated with VSL#3 CM alone or in combination with FE. The levels of p65 were increased in nuclear extracts and IκBα levels reduced in cytoplasmic extracts from epithelial cells isolated from SAMP terminal ileum organ cultures stimulated with VSL#3 CM alone and CM+FE, indicating activation of the NF-κB pathway (Fig. 6B). These data confirm a stimulatory effect of VSL#3 probiotics on the intestinal epithelium, as demonstrated by the increased production of TNF-α and activation of the NF-κB pathway.
Anti–TNF-α Treatment Abrogates the Beneficial Effects of VSL#3 in SAMP Ileitis.
To further assess that the preventative effects of VSL#3 in SAMP ileitis are mediated by TNF-α, we studied the effects of TNF-α blockade administered together with VSL#3 in 3-week-old SAMP mice using the prevention protocol. SAMP mice treated with high-dose VSL#3 concomitant with anti–TNF-α monoclonal antibody administration did not show significant improvement in the severity of ileitis compared with untreated control mice (total score: 9.0 ± 1.9 vs. 9.5 ± 1.0, not significant; Fig. S2). Interestingly, 3-week old SAMP mice treated with anti–TNF-α monoclonal antibody alone also did not show any significant improvements in ileitis severity compared with untreated mice (total score: 7.9 ± 1.7 vs. 9.5 ± 1.0, not significant; Fig. S2). These data further demonstrate that the preventative effects of VSL#3 are mediated by stimulation of TNF-α.
Discussion
Although the defensive response of the intestinal epithelium against pathogenic bacteria has been extensively explored, less information is available regarding interactions with the commensal bacteria. A commonly held belief is that nonpathogenic and probiotic bacteria exert opposing effects to those induced by pathogenic bacteria, including suppression rather than stimulation of proinflammatory cytokines in epithelial cells and macrophages (16). However, several studies underscore the importance of “physiologic inflammation” induced by the commensal flora, both for the development of the immune system and for the response to pathogenic bacteria in the gut. Accordingly, the possibility that probiotic bacteria elicit the same kind of proinflammatory response is currently being explored.
In this study we report that the probiotic mixture VSL#3 completely prevents the onset of intestinal inflammation in the SAMP mouse model of CD-ileitis. These effects were associated with a local effect on the intestinal epithelium (i.e., stimulation of TNF-α production and NF-κB activation in epithelial cells, coupled with restitution of normal intestinal epithelial barrier function). In addition, blockade of TNF-α by monoclonal antibody treatment given concomitantly with VSL#3 administration completely abrogated the beneficial effects of VSL#3 in this model. Analysis of bacterial DNA in the stool and ileal mucosa of treated mice demonstrated a significant increase of probiotic strains, suggesting that effective bacterial colonization is required for the beneficial effects of probiotic treatment. Taken together, our study supports the hypothesis that probiotics promote gut health through a mechanism involving stimulation of epithelial innate responses (i.e., stimulation of TNF-α expression), rather than suppression of inflammation. In addition, our results provide important insights into the pathogenesis of IBD by supporting the concept that CD may be caused by a deficit in innate immunity.
Several studies support the concept that probiotics exert stimulatory rather than suppressive effects on the innate immune system. For example, DNA from the bacteria found in VSL#3 exerts immunostimulatory effects on macrophages, as indicated by TLR9-mediated activation of NF-κB and JNK. Despite the ameliorative effects of VSL#3 administration observed in two animal models of colitis, bone marrow–derived macrophages isolated from BALB/c mice produce the proinflammatory cytokines IL-6 and IL-12 when cultured with DNA from VSL#3 bacteria (11). In addition, several studies showed that activation of NF-κB plays a beneficial role in epithelial cells. In fact, when mice that lack epithelial expression of the NF-κB activating enzyme IκB kinase β (villin-Cre/Ikkβ F/Δ mice) were administered dextran sodium sulfate (DSS), inflammation was of equal or greater severity as that observed in control mice (17). Activation of NF-κB is similarly protective for the development of systemic inflammation, but not for the prevention of local injury to the mucosa in an animal model of intestinal ischemia–reperfusion (18). Finally, the observations that TNF-α–deficient mice or mice deficient in TNFRI signaling in the intestinal epithelium are more susceptible to DSS acute colitis strongly support the concept that TNF-α may exert protective functions in normal gut homeostasis and intestinal epithelial integrity (19, 20). Altogether, these observations suggest that the beneficial effects of probiotics are associated with immunostimulatory effects rather than immunosuppression. Our results provide direct evidence that probiotics prevent the onset of experimental ileitis by mechanisms involving stimulation of TNF-α and activation of NF-κB in the intestinal epithelium. However, stimulation of other innate immunity pathways, including the intestinal production of IL-10 and TGF-β, may also be involved.
TNF-α is a proinflammatory cytokine that plays an important role in CD pathogenesis, as evidenced by the therapeutic benefits that are observed after administration of anti–TNF-α antibodies (e.g., infliximab) (21). During the inflammatory process, TNF-α exerts its function at the apex of the inflammatory cascade. It is responsible for the recruitment and activation of lymphocytes and granulocytes, the local expression of adhesion molecules on endothelial cells at the site of inflammation, the secretion of proinflammatory mediators (IFN-γ and IL-6) and radical oxygen species, and the formation of edema and granulomata (22). Notwithstanding, TNF-α is protective for various cell types (23), in particular for the intestinal epithelium (24). Our results support the importance of TNF-α production by epithelial cells in the prevention of disease, although no beneficial effect of probiotic administration was found in established disease.
Our findings support the emerging concept that CD occurs as a progression through distinct phases (disease initiation, establishment, and maintenance), each one characterized by specific immunologic features and unique molecular mediators. This leads to a model whereby a single mediator, such as TNF-α, is fundamental in establishing the inflammatory disease yet can have an opposing, protective effect during disease initiation. Because epithelial TNF-α is an integral part of the innate immune system, one hypothesis is that TNF-α production is partly directed against luminal antigens that would otherwise threaten mucosal integrity. In this scenario, aberrant penetration of luminal molecules (i.e., bacterial or food antigens) into the lamina propria compartment could initiate a persistent inflammatory process. In a predisposed organism, this could self-amplify exponentially and lead to the chronic inflammatory state characteristic of CD.
More support for a unique role of TNF-α in maintaining the integrity of the intestinal epithelium in the early phase of disease development comes from our demonstration that there was reduced small-intestinal permeability in mice treated with probiotics. Mice treated with VSL#3 in the prevention protocol demonstrated a complete restitution of intestinal barrier function to a level comparable to that of healthy mice. Several studies have outlined the important role played by impaired epithelial barrier function in CD and demonstrated that increased permeability is an early, and perhaps initiating, feature of IBD (15, 25). Our results were unexpected given that several studies have described a direct effect of TNF-α in producing leakage of the intestinal epithelium (26). We observed an increase in TNF-α production that corresponded to decreased epithelial permeability in the VSL#3-treated group. Because decreased intestinal permeability by probiotic bacteria has previously been described (10), it is likely that the improvement we observed is directly caused by the probiotic bacteria, although an indirect effect of reduced mucosal inflammation in the VSL#3-treated group should not be completely excluded.
Interestingly, in the present study we found that chronic ileitis was effectively prevented by innate immune stimulation in the early phase of the disease. This supports the emerging theory that attributes CD susceptibility to an initial defect of innate immunity (3–5). This may, in turn, cause recruitment and dysregulated activation of the acquired immune system, leading to the establishment of the typical inflammatory features characteristic of CD. Our findings raise the attractive hypothesis that probiotic bacteria can restore the breach of the innate immunity system and thereby prevent the onset of the inflammatory disease.
One of the open issues regarding probiotics is whether the bacteria exert their effects through modification of the resident flora or whether they act as antiinflammatory agents. Our findings indicate that probiotic bacteria do indeed exert a pharmacologic effect upon epithelial cells, albeit stimulatory rather than suppressive, that requires the presence of temporary probiotic colonization of the intestinal lumen. Indeed, mice administered low-dose VSL#3 did not show significant increases in bacterial concentration in the feces or ileum, and disease was not prevented or attenuated in these mice despite the fact that this dose was comparable to that previously used to treat experimental colitis. The dose used for the prevention of ileitis is therefore an important consideration.
To assess the efficacy of bacterial colonization, we developed a culture-independent technique that used specific primers for three representative bacterial strains (L. infantis, L. acidophilus, and S. thermophilus) in the VSL#3 preparation. It is worth noting that, even though the different species displayed a similar time-course pattern for colonization, the fecal and/or ileal probiotic DNA content differed for each species, as well as with respect to the dose of VSL#3 administered. Detection of only S. thermophilus in the feces and intestinal mucosa of SAMP mice treated with low-dose VSL#3 likely reflects insufficient colonization of the other bacterial species. It would be interesting to evaluate the beneficial contribution of each separate VSL#3 bacterial species and how this relates to the concentration of DNA in the fecal matter and the ileum.
In conclusion, we have demonstrated that administration of high-dose VSL#3 effectively prevents the development of CD-like ileitis in SAMP mice. This preventive effect was mediated by increased epithelial innate immunity. As a result, cells of the acquired immune system are not recruited, and the onset of the inflammatory cascade is prevented. Our results support the hypothesis that probiotics promote gut health through a mechanism involving stimulation rather than suppression of the epithelial innate immune system.
Experimental Procedures
VSL#3 Administration Protocols.
Mice were maintained in an institutional animal facility under specific pathogen-free conditions. All protocols were approved by the UVA Institutional Animal Care and Use Committee. VSL#3 administration was initiated in either 3-week-old SAMP mice before the onset of ileitis for the prevention protocol, or in 30-week-old mice for the treatment protocol. For the former, mice were administered either low-dose VSL#3 (17 × 108 cfu/day) by gavage (10, 11), or high-dose VSL#3 (50 × 109 cfu/day) mixed in their food. Age-matched control mice were fed unsupplemented chow. For the treatment protocol, 30-week-old mice were fed high-dose VSL#3 daily in their food. For both protocols, VSL#3 was administered for 6 weeks, after which mice were killed and ilea and MLNs collected for further analysis. In some prevention protocol experiments, SAMP mice were administered a monoclonal antibody against murine TNF-α (0.5 mg weekly) together with high-dose VSL#3 (27). Control mice were either administered anti–TNF-α monoclonal antibody alone or vehicle.
Histologic Assessment.
Histologic evaluation was performed on H&E-stained ileal sections by a single blinded pathologist using a validated scoring system (28). Briefly, inflammation was measured using a total inflammatory index composed of three components: active and chronic inflammation, as well as villous distortion.
Isolation of Fecal Extract and Intestinal Epithelial Cells.
Fecal extract was prepared using a method previously described (29), and total protein concentration was evaluated by the Bradford assay. Samples were stored at −80°C until further analysis. In the organ culture stimulation assay, fecal extracts were diluted to 400 μL/mL, of which 14 μg/mL per reaction well were used. Freshly isolated epithelial cells were harvested from ileal segments of experimental mice as previously described (23) and processed for real-time RT-PCR as described below.
DNA Isolation and RT-PCR.
Total DNA was isolated and purified from feces and terminal ileum specimens using the QIAamp DNA Stool Mini Kit and QIAamp DNA Mini Kit (Qiagen). Fresh feces were collected weekly and stored at −80°C until processed. Terminal ilea were washed in PBS containing 1% penicillin/streptomycin, and specimens were stored at −20°C until assay. Samples were diluted to a concentration of 20 pg/μL (feces) or 60 pg/μL (ileal tissue) and amplified using SYBR Green PCR master mix (Applied Biosystems) with primers specific for VSL#3 probiotic bacteria (B. infantis, L. acidophilus, and S. thermophilus) to determine levels of probiotic bacterial DNA (30, 31).
To measure expression of epithelial-derived mRNAs, RNA was extracted from epithelial cells using the RNAeasy Miniprep Kit (Qiagen). Reverse transcription was performed using the GeneAmp RNA PCR kit (Applied Biosystems). IκBα (forward: 5′-AGACTCGTTCCTGCACTTG-3′; reverse: 5′-CCTGGCTGGTTGGTGATC-3′) and TNF-α (forward: 5′-GCGGTGCCTATGTCTCAG-3′; reverse: 5′-GCCATTTGGGAACTTCTCATC-3′) were designed using Beacon Designer 2 software (Premier Biosoft International).
PCR reactions were performed in a total volume of 20 μL (16 μL of SYBR Green PCR master mix and 4 μL of DNA) in an iCycler iQ detection system (Bio-Rad Laboratories). Reactions were run under the following conditions: 2 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 60°C, and 15 s at 72°C. Samples were then run on a 2% agarose gel with ethidium bromide for visualization. Target mRNA was normalized to β-actin for each sample, and all samples were run in duplicate. Results were expressed as a ratio relative to the lowest control sample.
Measurement of Intestinal Permeability.
In vivo epithelial paracellular permeability was measured as previously described (15), before and after VSL#3 therapy. Permeability was evaluated after administration of high-dose VSL#3 for 2 (n = 4) and 6 weeks (n = 6) in comparison with age-matched, untreated mice (n = 4). Mice were fasted for 2 h then fed a sugar-probe mixture (60 mg/mL lactulose, 40 mg/mL mannitol, and 30 mg/mL sucralose) via orogastric gavage, and urine collected after 22 h. Urine volume was measured, and the concentration of each of the sugar probes was determined by HPLC. Fractional excretion of each probe was determined as a ratio of the amount of probe in the urine to the amount administered.
Immunohistochemical Analysis.
Tissue samples were prepared in Bouin’s fixative (LabChem), processed as previously described (15), cut into 3-μm-thick sections, and mounted on superfrost/PLUS glass slides (Fisher Scientific). Sections were deparaffinized in xylene (twice for 10 min) and dehydrated in 100% ethanol (twice for 5 min) and 95% ethanol (twice for 5 min). Sections were then blocked for endogenous peroxidase activity using 1% hydrogen peroxide in methanol for 30 min, washed twice with PBS, then blocked with 3% BSA/PBS (Sigma Chemical) for 20 min. Tissue sections were incubated overnight at 4°C with mouse anti-TNF (R&D Systems) primary antibody, followed by a biotinylated horse anti-goat antibody, and then incubated with an avidin/biotin complex (Vectastain Elite ABC Kit; Vector Laboratories) for 30 min. Immunoreactive cells were visualized by addition of diaminobenzidine substrate and counterstained with hematoxylin. All incubations were conducted at room temperature unless otherwise noted. Negative controls were prepared using the same conditions as described above, but replacing the primary antibody with an equivalent volume of PBS.
Organ Culture Stimulation Assay.
VSL#3 CM was prepared as previously described (32), and the CM diluted 1:10 in unconditioned media for organ culture stimulation assays. The production of cytokines from epithelial cells was measured in an in vitro assay as described by Rakoff-Nahoum et al. (24), and the concentration of TNF-α was evaluated by ELISA. TNF-α levels were corrected for total protein concentration measured by the Bradford assay.
Western Blot.
Nuclear and cytoplasmic extracts were prepared using the NE-PER kit and protein concentrations measured using BCA protein assay reagent (Pierce). Equal amounts of proteins were electrophoresed using precast NuPAGE Triacetate gels and separated proteins transferred using 0.2-μm PVDF membrane (Invitrogen). Proteins were then probed with primary antibody, followed by incubation with HRP-conjugated secondary antibody (Santa Cruz Biotechnology). Proteins were visualized using the ECL detection system (Amersham Pharmacia). Densitometry was performed using Imagequant software, and band intensity ratios were measured.
Statistical Analysis.
Statistical analysis was performed using a Mann-Whitney U test. Data were expressed as mean ± SEM. An α level of 0.05 was considered significant (P < 0.05).
Supplementary Material
Acknowledgments
This work was supported by US Public Health Service/National Institutes of Health Grants DK-42191, DK-44540, DK-55812 (to F.C.), and DK-56762 (to T.T.P.) and by the University of Virginia Digestive Health Research Center (UVA DHRC) (DK-67629). We thank Sharon B. Hoang and James R. Mize of the Morphology/Imaging Core of the UVA DHRC for histologic evaluation and scoring of samples; William G. Ross for FACS analysis; Dr. Claudio De Simone for providing the VSL#3 preparation; Dr. John Meddings for the permeability assay; and Dr. Robert Schreiber for kindly providing the monoclonal antibody against TNF-α.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910307107/DCSupplemental.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 3.Bamias G, Nyce MR, De La Rue SA, Cominelli F American College of Physicians; American Physiological Society. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Marks DJ, et al. Defective acute inflammation in Crohn’s disease: A clinical investigation. Lancet. 2006;367:668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 6.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 7.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 8.Bibiloni R, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 9.Gionchetti P, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 10.Madsen K, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 11.Rachmilewitz D, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Kosiewicz MM, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto S, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera-Nieves J, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–982. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 15.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jijon H, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Chen LW, et al. The two faces of IKK and NF-kappaB inhibition: Prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 19.Naito Y, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi E, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 21.Hanauer SB, et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–1157. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- 23.Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278:36688–36698. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 24.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Hollander D, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 26.Söderholm JD, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–1824. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- 28.Burns RC, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 29.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 30.Furet JP, Quénée P, Tailliez P. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol. 2004;97:197–207. doi: 10.1016/j.ijfoodmicro.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Vitali B, Candela M, Matteuzzi D, Brigidi P. Quantitative detection of probiotic Bifidobacterium strains in bacterial mixtures by using real-time PCR. Syst Appl Microbiol. 2003;26:269–276. doi: 10.1078/072320203322346128. [DOI] [PubMed] [Google Scholar]
- 32.Petrof EO, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.