Abstract
Limited exposure of aminophospholipids on the outer leaflet of the plasma membrane is a fundamental feature of eukaryotic cells and is maintained by the action of inward-directed P-type ATPases (“flippases”). Yeast S. cerevisiae has five flippases (Dnf1, Dnf2, Dnf3, Drs2, and Neo1), but their regulation is poorly understood. Two paralogous plasma membrane-associated protein kinases, Pkh1 and Pkh2 (orthologs of mammalian PDK1), are required for viability of S. cerevisiae cells because they activate several essential downstream protein kinases by phosphorylating a critical Thr in their activation loops. Two such targets are related protein kinases Ypk1 and Ypk2 (orthologs of mammalian SGK1), which have been implicated in multiple processes, including endocytosis and coupling of membrane expansion to cell wall remodeling, but the downstream effector(s) of these kinases have been elusive. Here we show that a physiologically relevant substrate of Ypk1 is another protein kinase, Fpk1, a known flippase activator. We show that Ypk1 phosphorylates and thereby down-regulates Fpk1, and further that a complex sphingolipid counteracts the down-regulation of Fpk1 by Ypk1. Our findings delineate a unique regulatory mechanism for imposing a balance between sphingolipid content and aminophospholipid asymmetry in eukaryotic plasma membranes.
Keywords: Fpk1, plasma membrane, signal transduction, sphingolipids, Ypk1
In a eukaryotic cell, the lipid composition of the plasma membrane undergoes continuous dynamic remodeling due, in part, to the insertion of exocytic vesicles and the removal of endocytic vesicles and, in part, to other processes (such as the action of lipases). Moreover, it is well established that the outer and inner leaflets exhibit distinct lipid composition (1, 2). How a eukaryotic cell monitors such dynamic changes and adjusts the rate of the processes necessary to maintain the proper balance among the different major classes of lipids in the two leaflets of the plasma membrane (glycerophospholipids, sphingolipids, and sterols) is a question of central interest in cell biology.
Aminophospholipids (PtdEth and PtdSer) are returned to the inner leaflet via the action of particular P-type ATPases (class 4), which function as inward-directed lipid translocases, or “flippases” (3). In budding yeast (Saccharomyces cerevisiae), there are five flippases (Dnf1, Dnf2, Dnf3, Drs2, and Neo1). It has been shown recently that two related protein kinases, Fpk1 (Ynr047w) and Fpk2 (Kin82/Ycr091w), serve as flippase activators (4). However, to what signals these kinases respond was not known.
As shown here, we found that Fpk1 and Fpk2 are physiologically relevant targets of and negatively regulated by two very similar kinases, Ypk1 and Ypk2. We showed previously that Ypk1 and Ypk2 are activated, in turn, by two other paralogous protein kinases, Pkh1 and Pkh2 (5), which are associated with discrete plasma membrane-associated structures—subsequently dubbed eisosomes (6) and reportedly activated in vitro by the long-chain base phytosphingosine (PHS), a precursor to sphingolipids (7). Cells deficient in Ypk1 and Ypk2 display pleiotropic effects, including defects in membrane growth and cell wall expansion (8, 9) and endocytosis (10). The connection between Ypk1 action and Fpk1 function shown here in large measure explains these phenotypes. Moreover, given these insights, we found that, contrary to claims in the literature, the ability of Pkh1 and Pkh2 to activate Ypk1 and Ypk2 is unaffected by the level of PHS in vivo, and that, instead, a complex sphingolipid is required to counteract Ypk1-mediated down-regulation of Fpk1. Thus, these findings reveal a mechanism by which the sphingolipid pool in the plasma membrane exerts control over the amount of aminophospholipid exposed on the exocellular surface of a eukaryotic cell.
Results and Discussion
A Unique Three-Tiered Protein Kinase Cascade.
We determined the preferred phosphoacceptor site specificity of Ypk1 (-R-x-R-x-x-S/T-Φ-) quite some time ago (5), but no biologically relevant substrate of Ypk1 has yet been characterized. As a means to identify potential Ypk1 targets, we scanned the S. cerevisiae genome for gene products that contain multiple copies of this consensus sequence motif. The protein kinase Fpk1 (a member of the p70S6K family) (11) captured our attention for several reasons. First, Fpk1 contains three consensus Ypk1 phosphorylation motifs (Fig. 1A) and its paralog, Fpk2, also has one. Second, Ypk1- and Ypk2-deficient cells display plasma membrane-associated defects (8), and Fpk1 was recently shown to phosphorylate and thereby stimulate at least two aminophospholipid flippases (4), although the sites of modification were not mapped. Third, in a screen for transposon insertion mutations that significantly suppressed the temperature-sensitive growth defect of ypk1ts ypk2∆ cells, one insertion we isolated disrupted the coding sequence for flippase Dnf3 (8).
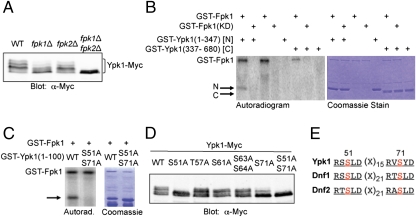
Fig. 1.
Ypk1 phosphorylates the N-terminal noncatalytic domain of Fpk1. (A) Fpk1 contains three consensus Ypk1 phosphoacceptor sites (indicated in red), and its paralog Fpk2 contains one at a similar position. (B) A wild-type strain (BY4741) or an otherwise isogenic ypk1∆ mutant, expressing from the TPI1 promoter either Fpk1-GFP (pFR150) or Fpk1(S37A T244A S481A)-GFP (pFR161), as indicated, were lysed, and the resulting extracts were resolved by SDS/PAGE and analyzed by immunoblotting with an anti-phospho-AKT-substrate antibody (Cell Signaling Technology). Phosphorylated Fpk1 (arrow); nonspecific band (asterisk) provided an internal control for equivalent loading. (C) A ypk1∆ fpk1∆ double mutant (YFR198) expressing from the GAL1 promoter either Ypk1-myc (pAM54) or catalytically inactive (“kinase dead”) Ypk1(K376A)-myc (KD) (pAM49) were lysed and the corresponding proteins recovered by immunoprecipitation with mouse ascites fluid containing anti-c-myc mAb 9E10. The resulting immunoprecipitates were then incubated with [γ-32P]ATP and with catalytically inactive GST-Fpk1(D261A) (pFR144) purified from E. coli, as described in Materials and Methods.
Using a phosphosite-specific antibody that recognizes the phosphorylated -R-x-R-x-x-S/T- motif, we readily detected a species in yeast cell extracts that represents Fpk1 phosphorylated by Ypk1 because it was present in cells expressing wild-type Fpk1 and Ypk1, but absent both in YPK1+ cells expressing Fpk1(S37A T244A S481A) as the sole source of this protein and in ypk1∆ cells expressing wild-type Fpk1 (Fig. 1B). Thus, Fpk1 was phosphorylated in vivo at one or more of the predicted sites in a Ypk1-dependent manner. This phosphorylation is likely direct because Fpk1 is also a substrate for Ypk1 in vitro. Ypk1 enriched by immunoprecipitation from yeast cell extracts phosphorylated recombinant GST-Fpk1(D261A) (a kinase-dead mutant to prevent self-phosphorylation), and did so more robustly than Ypk1 autophosphorylated itself (Fig. 1C), whereas an equal amount of catalytically deficient Ypk1 prepared in the same fashion exhibited only residual phosphorylation of Fpk1 (resulting from traces of contaminating protein kinases present in the immunoprecipitates). These findings suggested that the Pkh1- (and Pkh2-) Ypk1 (and -Ypk2) pathway may be a three-tiered cascade, and that the phenotypes of Ypk1- and Ypk2-deficient cells could arise, in part, from defective Fpk1 (and Fpk2) regulation and ensuing aberrant aminophospholipid flipping. However, because a ypk1∆ ypk2∆ double mutation is lethal, whereas an fpk1∆ fpk2∆ double mutation is not, Ypk1 (and Ypk2) must have additional targets.
Defining the Substrate Specificity of Fpk1.
We also found a reciprocal connection between Ypk1 and Fpk1. In immunoblots of whole-cell extracts resolved by SDS/PAGE, Ypk1 migrates as multiple bands that are collapsed to a single species by phosphatase treatment (12, 13). We ruled out that phosphorylation of Ypk1 on its activation loop (T504) by Pkh1 and Pkh2 contributes to this pattern because Ypk1(T504A) displayed the identical pattern (13) (Fig. S1). The Tor2-containing TORC2 complex is responsible for phosphorylation of Ypk2 at its so-called turn and hydrophobic motifs (14), and presumably phosphorylates the corresponding sites (S644 and T662) in Ypk1 (Fig. S1A). However, a Ypk1(S644A T662A) double mutant displayed the same multiple bands as wild-type Ypk1 (13) (Fig. S1B). Moreover, this pattern is not due to phosphorylation by Pkh1 or Pkh2, or Tor1 or Tor2, at any other sites because Ypk1 still migrated as the same multiple bands in pkh1ts pkh2∆ and tor1∆ tor2ts strains shifted to the nonpermissive temperature (Fig. S1C). Likewise, these species are not due to Ypk1 autophosphorylation because the same multiple species are exhibited by a kinase-dead Ypk1(K376A) mutant, and even by a Ypk1(K376A T504A S644A T662A) quadruple mutant (Fig. S1B). The kinase domain of Ypk1 alone does not run as multiple bands (Fig. S1D), suggesting that the phosphorylation sites responsible for the mobility shifts displayed by full-length Ypk1 lie in its N-terminal extension (Fig. S1A). To pinpoint the protein kinase(s) responsible for producing the multiple slower mobility species, we expressed Ypk1-myc in a genome-wide collection of deletion strains lacking each of the nonessential protein kinase genes. Strikingly, the Ypk1 mobility shift was greatly decreased in cells deleted for Fpk1, and although loss of Fpk2 had only a minor effect on its own, the multiple Ypk1 species observed in vivo were totally abolished in cells lacking both Fpk1 and Fpk2 (Fig. 2A).
Fig. 2.
Fpk1 phosphorylates the N-terminal noncatalytic domain of Ypk1. (A) A wild-type strain (BY4741) and otherwise isogenic fpk1∆ and fpk2∆ single mutants (see Table S1), and an fpk1∆ fpk2∆ double mutant (YFR205) expressing Ypk1-myc from the GAL1 promoter (pAM76) were lysed, and the resulting extracts resolved by SDS/PAGE and analyzed by immunoblotting with anti-c-myc mAb 9E10. (B) GST-Fpk1 (pFR143) or catalytically inactive GST-Fpk1(D621A) (pFR144) were purified from E. coli and incubated with [γ-32P]ATP and either GST-Ypk1(1-347) [N] (pFR142) or GST-Ypk1(337-680) [C] (pJH1), which were also purified from E. coli, as described in Materials and Methods. (C) As in (B), except that recombinant GST-Fpk1 was incubated with [γ-32P]ATP and either GST-Ypk1(1-100) or GST-Ypk1(1-100) S51A S71A, as indicated. (D) As in (A), except that Ypk1-myc contained the indicated site-directed mutations. (E) Deduced consensus phosphoacceptor site motif for Fpk1.
Recombinant Fpk1 phosphorylated the N-terminal, but not the C-terminal, half of recombinant Ypk1 (each fused to GST), whereas a catalytically dead Fpk1 mutant did not (Fig. 2B), confirming that Fpk1-mediated phosphorylation of Ypk1 is direct and occurs on its N-terminal noncatalytic domain. (Fpk1 also autophosphorylated, but the Ypk1 kinase domain did not because Ypk1 activity depends strictly on activation loop phosphorylation by Pkh1 and/or Pkh2, and obviously these kinases were absent in the Ypk1 preparation purified from E. coli.) Using various fragments of the Ypk1 N terminus, the sites of Fpk1-mediated phosphorylation were localized within its first 100 residues and then pinpointed to Ser51 and Ser71 by site-directed mutagenesis; recombinant GST-Ypk1(1-100) S51A S71A was not phosphorylated by GST-Fpk1 in vitro (Fig. 2C), and full-length Ypk1(S51A S71A) expressed in vivo no longer migrated in SDS/PAGE as multiple lower-mobility species (Fig. 2D). S51 and S71 in Ypk1 share a remarkably similar sequence context (RxSLD) (Fig. 2E). We further confirmed that this motif represents the preferred phosphoacceptor consensus for Fpk1 by showing that two such sites (S1526 and S1552) that lie in the C-terminal cytoplasmic tail of one aminophospholipid flippase (Dnf1) (Fig. 2E) are major Fpk1 phosphorylation sites (Fig. S2).
A Complex Sphingolipid Stimulates Fpk1 Function.
Collectively, our findings show that Ypk1 phosphorylates Fpk1 and that Fpk1, in turn, is able to phosphorylate Ypk1. A somewhat analogous situation is that the MAPKK Ste7 both phosphorylates (15) and is a substrate (16) of its downstream MAPK Fus3. Thus, we could use the mobility shift of Ypk1 as a direct measure of the activity state of Fpk1 in vivo. In this regard, it had been noted previously (12), and we confirmed, that in yeast cells treated with a drug (myriocin) that inhibits the first enzyme (serine palmitoyltransferase) in sphingolipid biosynthesis (Fig. 3A), Ypk1 no longer migrated as multiple bands on SDS/PAGE (Fig. 3B), similar to what we observed in fpk1∆ fpk2∆ cells (Fig. 3B), suggesting that sphingolipid production is required for maintenance of Fpk1 (and Fpk2) activity.
Fig. 3.
The complex sphingolipid MIPC is required for optimal Fpk1 and Fkp2 activity. (A) Simplified schematic depiction of the sphingolipid biosynthetic pathway in S. cerevisiae, indicating the intermediate phytosphingosine (PHS), the reactions inhibited by myriocin (Myr) and aureobasidin A (AbA), and the steps catalyzed by the products of the genes indicated beside the corresponding arrow. (B) A wild-type strain (BY4741) or an otherwise isogenic fpk1∆ fpk2∆ double mutant (YFR205) expressing Ypk1-myc from the GAL1 promoter (pAM76) were untreated or treated with myriocin (1.25 μM), as described in Materials and Methods, and lysed, and the resulting extracts were resolved by SDS/PAGE and analyzed by immunoblotting with anti-c-myc mAb 9E10. (C) As in (B), except that the cells (BY4741) carried both a plasmid expressing Ypk1-myc from the GAL1 promoter (pAM54) and either an empty vector (YEp352GAL) or the same vector expressing Fpk1 from the GAL1 promoter (pFpk1-zz), as indicated. Because the amount of Ypk1 was reduced in the cells overexpressing Fpk1, twice the amount of total protein was loaded in those lanes, compared with the vector control lanes. (D) Wild-type cells (BY4741) expressing Ypk1-myc from the GAL1 promoter (pAM76) were untreated or treated with AbA (0.7 μM) alone, or AbA (0.7 μM) plus PHS (5 μM), or Myr (1.25 μM) alone, or Myr (1.25 μM) plus PHS (5 μM), or PHS (5 μM) alone, as described in Materials and Methods, and cell lysates were analyzed as in (B). (E) As in (D), except that wild-type cells (BY4741) and otherwise isogenic csg2∆ and ipt1∆ single mutants were untreated or treated with either Myr (1.25 μM) or PHS (5 μM).
Sphingolipids are important structural components of the plasma membrane, but also have been implicated in growth regulation, response to heat stress, endocytosis, and actin cytoskeleton dynamics, although the mechanism by which they do so has remained obscure (17, 18). Consistent with the idea that blocking sphingolipid biosynthesis decreases Fpk1 and Fpk2 activity, we found that high-level overexpression of Fpk1 in myriocin-treated cells restored the normal pattern of slower-migrating Ypk1 species (Fig. 3C). In this regard, it was reported some time ago that Pkh1 and Pkh2, the essential upstream activators of Ypk1 (and Ypk2), can be stimulated by the long-chain base PHS in vitro (7), and others claim to see the same effect (19, 20), suggesting that PHS is the critical second messenger emanating from the sphinogolipid pathway (17, 18). However, using a phosphosite-specific antibody that we showed only recognizes Ypk1 phosphorylated at its Pkh1- and Pkh2-dependent site (T504) (5), we found that neither blocking production of endogenous PHS by myriocin treatment nor elevating intercellular PHS by supplying exogenous PHS had any effect on the state of T504 phosphorylation in vivo (Fig. S3). Thus, activation loop phosphorylation of Ypk1 by Pkh1 and Pkh2 is not regulated by PHS or any derived sphingolipid in vivo, contrary to the conclusion propagated in the literature that Pkh1 and Pkh2 are sphingolipid-dependent protein kinases.
By contrast, we found that treatment of cells with aureobasidin A, a drug that blocks the sphingolipid biosynthetic pathway after PHS (Fig. 3A), also inhibited Fpk1 (and Fpk2) activity, as judged by absence of the slower mobility species of Ypk1 (Fig. 3D). Furthermore, addition of exogenous PHS was unable to reverse the effect of aureobasidin A, whereas the same treatment was able to efficiently overcome the effect of myriocin (Fig. 3D). Given that aureobasidin A inhibits an enzyme (inositol phosphorylceramide synthase) needed to produce the complex inositol-containing sphingolipids found in yeast, our findings suggested that one or more of the complex sphingolipids, and not PHS per se, is necessary to support Fpk1 and Fpk2 activity. Therefore, we used mutants to pinpoint which complex sphingolipid is necessary to preserve Fpk1 and Fpk2 activity. Inositol phosphorylceramide (IPC) is converted to mannosyl-inositolphosphorylceramide (MIPC) by the action of two redundant mannosyltransferases (Csg1 and Sur1) whose membrane delivery and stability require a shared Ca2+-binding subunit (Csg2) (21). MIPC is then converted to mannosyldi(inositolphosphoryl)ceramide [M(IP)2C] by subsequent action of Ipt1. We found that Fpk1-mediated phosphorylation of Ypk1 was greatly reduced in csg2∆ cells, but not in ipt1∆ cells, indicating that it is MIPC that is important for promoting Fpk1 and Fpk2 activity in vivo (Fig. 3E).
Ypk1-Mediated Phosphorylation Down-Regulates Fpk1 Activity.
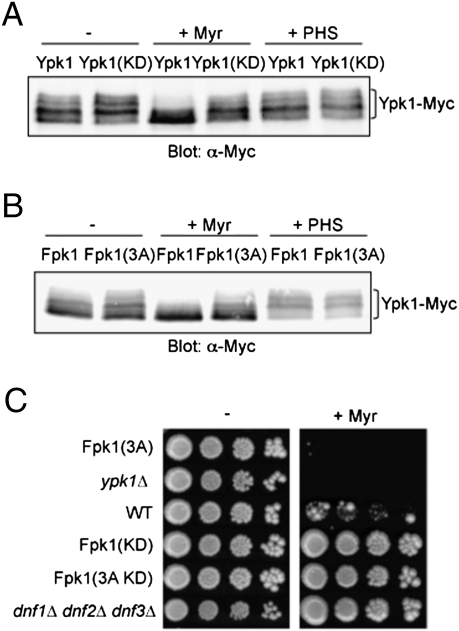
By monitoring Fpk1-mediated phosphorylation of Ypk1, we were able to show that Ypk1 action negatively regulates Fpk1 (and presumably Fpk2). When Fpk1 was not phosphorylated by Ypk1, either in cells expressing catalytically inactive Ypk1 (Fig. 4A) or in cells expressing Fpk1(S37A T244A S481A) (Fig. 4B), Ypk1 migrated as the series of slowing migrating bands diagnostic of its Fpk1-mediated phosphorylation, even in the presence of myriocin, indicating that Fpk1 remains more active under these conditions. In contrast, when active Ypk1 is present or when Fpk1 is susceptible to Ypk1-mediated phosphorylation, the population of slowing migrating Ypk1 species was markedly reduced, especially in the presence of myriocin (which prevents MIPC production). Thus, Ypk1-mediated phosphorylation inhibits Fpk1 activity and, conversely, MIPC alleviates that inhibition.
Fig. 4.
Ypk1 action down-regulates Fpk1 activity. (A) Wild-type cells (BY4741) expressing either Ypk1-myc (pAM54) or catalytically inactive Ypk1(K376A)-myc (pAM49) from the GAL1 promoter were untreated or treated with either myriocin (1.25 μM) or PHS (5 μM), as described in Materials and Methods, and lysed, and the resulting extracts resolved by SDS/PAGE and analyzed by immunoblotting with anti-c-myc mAb 9E10. (B) As in (A), except that Ypk1-myc was expressed from the GAL1 promoter (pAM76) in an fpk1∆ fpk2∆ double-mutant strain (YFR205) coexpressing either Fpk1 (pFR150) or Fpk1(S37A T244A S481A) (pFR161). (C) Serial 10-fold dilutions of wild-type cells (BY4741), isogenic strains expressing from the FPK1 locus Fpk1(S37A T244A S481A) [3A] (YFR235), Fpk1(D621A) [KD] (YFR236), or Fpk1(S37A T244A S481A D621A) [3A KD] (YFR237), isogenic ypk1∆ cells, or isogenic dnf1∆ dnf2∆ dnf3∆ cells, were spotted on YPD or the same medium containing Myr (0.8 μM). Plates were photographed after incubation for 3 days at 30 °C.
Because Fpk1 is a positive activator of flippases, Ypk1-mediated negative regulation of Fpk1 should down-modulate the function of flippases. Thus, the defects exhibited by Ypk1-deficient cells could be manifestations of hyperactive flippase activity. If so, cells lacking Ypk1 or cells in which Fpk1 is not susceptible to Ypk1-mediated down-regulation should display the same phenotype. Indeed, both ypk1∆ cells and cells expressing Fpk1(S37A T244A S481A) are markedly more sensitive to myriocin than wild-type cells and, conversely, cells expressing catalytically inactive Fpk1 are more resistant (Fig. 4C). Thus, it appears that the increased aminophospholipid flippase activity present in cells lacking Ypk1 (or in cells where Fpk1 cannot be negatively regulated by Ypk1) becomes deleterious to cell viability when sphingolipid biosynthesis is compromised. In further agreement with a role for Ypk1 in down-modulating the ability of Fpk1 to stimulate flippase activity, we (13) and others (12) have shown previously that cells overexpressing Ypk1 are more myriocin resistant than wild-type cells. Consistent with these findings, cells lacking three of the five flippases (dnf1∆ dnf2∆ dnf3∆) are also more resistant to myriocin than wild-type cells (Fig. 4C). Moreover, we found that loss of Fpk1 and Fpk2 detectably suppressed the temperature-sensitive growth of ypk1ts ypk2∆ mutant cells (Fig. S4), consistent with the view that the growth defects observed in Ypk1- and Ypk2-deficient cells arise, at least in part, from increased aminophospholipid flippase function due to lack of proper negative regulation of the flippase activators Fpk1 and Fpk2.
The idea that the phenotypic effects of Ypk1 deficiency are due to overactive Fpk1 function leading to hyperactive flippase activity, and thus to membrane perturbations, now explains many prior findings. First, interruption of a flippase gene (DNF3) by transposon insertion rescued significantly the temperature sensitivity of ypk1ts ypk2∆ cells (8). Second, in the same screen, we also identified as effective suppressors transposon insertions in several genes required for Asn-linked protein glycosylation (8), and others have shown that defects in N-linked glycosylation compromise sphingolipid biosynthesis (22). In such mutants, the resulting reduction in MIPC level presumably decreases Fpk1 activity, thereby bypassing the need for Ypk1-mediated inhibition of Fpk1. Third, in a screen for dosage suppressors (8), we identified YPC1 as a gene that, when overexpressed, rescued significantly the temperature sensitivity of ypk1ts ypk2∆ cells. YPC1 encodes a phytoceramidase that degrades a precursor to sphingolipids. Presumably, therefore, YPC1 overexpression also leads to less MIPC and the lowering of Fpk1 activity, thereby alleviating the need for its Ypk1-mediated inhibition.
Finally, the drug duramycin, which binds to PtdEth in the outer leaflet of the plasma membrane and kills cells (23), can be used to monitor the efficiency of flippase function. The less active the flippases, the more PtdEth left at the cell surface, and the more sensitive the cells are to duramycin killing. In agreement with the role of Fpk1 in stimulating flippase activity, we found that cells expressing catalytically inactive Fpk1 were hypersensitive to duramycin (Fig. 5A). In agreement with our evidence that MIPC is needed for optimal Fpk1 activity, we found that csg2∆ mutants, but not ipt1∆ mutants, are also hypersensitive to duramycin. Wild-type cells are already rather resistant to duramycin, and it did not appear that further elevation of Fpk1 activity in ypk1∆ cells conferred increased duramycin resistance (Fig. 5A); however, consistent with the conclusion that Ypk1 phosphorylation of Fpk1 down-regulates its function and thus reduces flippase activity, cells overexpressing Ypk1 were demonstrably more sensitive to duramycin than control cells (Fig. 5B).
Fig. 5.
Reduction of Fpk1 activity impairs plasma membrane aminophospholipid flipping. (A) Wild-type cells (BY4741), isogenic fpk1∆ fpk2∆ cells expressing Fpk1(D261A) (YFR236), and isogenic csg2∆, ipt1∆ and ypk1∆ mutants were each plated as a lawn on YPD plates, and 10 μL of a stock solution of duramycin (either 8 mM or 2 mM, as indicated) were spotted onto sterile filter paper disks, which were immediately placed onto the lawn. Plates were photographed after incubation at 30 °C for 2 days. (B) A wild-type strain (BY4741) was transformed with an empty high-copy number LEU2-marked vector or the same vector expressing Ypk1 from the ADH1 promoter (pAM23), as indicated, plated as a lawn on SCD-Leu medium, and then tested for sensitivity to duramycin, as in (A). (C) Under conditions of nutrient sufficiency, TORC2-mediated phosphorylation of the hydrophobic and turn motifs in Ypk1 make its activation loop accessible to phosphorylation by eisosome-localized Pkh1 (and Pkh2), thereby activating membrane-associated Ypk1. Activated Ypk1 phosphorylates and down-regulates membrane-associated Fpk1, thereby reducing its ability to stimulate phospholipid flippase activity; conversely, the complex sphingolipid MIPC stimulates Fpk1 and counteracts the effect of Ypk1-mediated inhibition of Fpk1. Available evidence (see legend to Fig. 3C) suggests that Fpk1 action may down-regulate the level of Ypk1. This regulatory circuit provides a homeostatic mechanism that can adjust the rate of aminophospholipid flipping in the plasma membrane to the level of sphingolipid available, thereby maintaining the appropriate membrane organization.
Analysis of the subcellular localization of the constituents of the Pkh1-Ypk1-Fpk1 cascade is consistent with their role in regulating aminophospholipid flippases. GFP-tagged flippases (Dnf1-GFP and Dnf2-GFP) localize to the plasma membrane, mainly in small buds and at the bud neck (24). We have previously shown that 3GFP-Pkh1 and 3GFP-Pkh2 are distributed around the plasma membrane (8) in static patches that subsequently have been dubbed eisosomes (6). Ypk1-GFP also localizes to the plasma membrane, enriched in small buds and at the bud neck (12). Although it was reported that N-terminally GFP-tagged Fpk1 localizes primarily to early endosomes and to the trans-Golgi network (4), we found that C-terminally GFP-tagged Fpk1 clearly localized to the plasma membrane at the site of bud growth, in small buds, and at the bud neck, in both haploid and diploid cells (Fig. S5 A and B), whether Fpk1-GFP was expressed from a low-copy-number plasmid or from its native promoter at its normal chromosomal locus (Fig. S5C), even though the latter signal was dimmer because endogenously expressed Fpk1 is estimated to be present at only ∼750 molecules/cell (25). Thus, Pkh1, Ypk1, Fpk1 and the flippases Dnf1 and Dnf2 are all situated in the plasma membrane, and Ypk1, Fpk1 and the flippases colocalize.
The regulatory circuit we have discovered (Fig. 5C) provides a mechanism to couple the rate of aminophospholipid flipping within the plasma membrane to the local concentration of a complex sphingolipid. Absence of MIPC does not affect either the level or membrane recruitment of Fpk1 (Fig. S6). Technical challenging biochemical analysis will be needed to determine whether MIPC directly stimulates Fpk1 activity, or acts indirectly to promote Fpk1 function (perhaps, e.g., MIPC inhibits Ypk1). Although cross-talk between sphingolipids and aminophospholipid flippases had been postulated before (26), no regulatory connection was known before our findings. Maintaining this balance appears to be crucial for many aspects of membrane function because defects in flippase activity have been reported to cause problems in the vesicle-mediated transport of proteins in both the endocytic and exocytic pathways (27, 28), in the nonvesicular trafficking of sterols (29), and in maintaining the axis of polarized growth through effects on membrane recruitment of Cdc42 (30). Because homologs of all of the yeast protein kinases involved are found in animal cells, it is possible that an analogous cascade is used for this purpose in mammals. Indeed, proper distribution of PtdEth (and PtdSer) between the outer and inner leaflets of the plasma membrane in animal cells is necessary for membrane protein activity, for vesicle biogenesis, and for cell signaling, morphology and movement (31). When flippase activity is not regulated properly, abnormal exposure of aminophospholipids in the extracellular leaflet can initiate phagocytosis, platelet activation, and apoptotic death (31).
Materials and Methods
Strains and Growth Conditions.
Yeast strains used in this study are listed in Table S1 and were grown routinely at 30 °C. Standard rich (YP) and defined minimal (SC) media (32) containing either 2% glucose (Glc); 2% raffinose and 0.2% sucrose (Raf-Suc); or 2% galactose (Gal) as the carbon source and supplemented with appropriate nutrients to maintain selection for plasmids were used for yeast cultivation. Conditions for gene induction by Gal and for treatment with drugs are described in detail in SI Text. Standard yeast genetic techniques were performed according to Sherman et al. (32).
Plasmids and Recombinant DNA Methods.
Plasmids used in this study are listed in Table S2. Plasmids were constructed using standard procedures (33) in E. coli strain DH5α. Fidelity of all constructs was verified by nucleotide sequence analysis. All PCR reactions were performed using Phusion DNA polymerase (Finnzymes, Inc.). Site-directed mutagenesis using appropriate mismatch oligonucleotide primers was conducted using the QuikChange method and Pfu Turbo DNA polymerase (Stratagene Division, Agilent Technologies, Inc.).
Cell Extracts and Immunoblotting.
The cells in samples (1.5 mL) of an exponentially growing culture (A600 nm = 0.6) were collected by brief centrifugation, immediately frozen in liquid N2, and then lyzed by resuspension in 150 μL of 1.85 M NaOH, 7.4% β-mercaptoethanol. Protein in the resulting lysate was precipitated by the addition of 150 μL of 50% trichloroacetic acid on ice for 10 min. The resulting denatured protein was collected by centrifugation, washed twice with acetone, solubilized by resuspension in 80 μL of 0.1 M Tris, 5% SDS, and then 20 μL of a 5×stock of SDS/PAGE sample buffer was added. After boiling for 5 min, portions (15 μL) of these samples were resolved by SDS/PAGE (8% acrylamide, 75/1 monomer to cross-linker), transferred to nitrocellulose, incubated with appropriate primary antibodies in Odyssey buffer (Li-Cor Biosciences), washed, incubated with appropriate secondary antibodies conjugated to infrared fluorophores, and visualized using an Odyssey infrared imaging system (Li-Cor). The antibodies used in this work are described in Table S3.
Immune-Complex Protein Kinase Assay.
Cultures (40 mL) of yeast strain ypk1∆ fpk1∆ (YFR198) expressing from the GAL1 promoter either Ypk1-myc (pAM54) or catalytically inactive Ypk1(K376A)-myc (pAM49) were grown in SC+Raf-Suc to an A600nm = 0.6, induced by addition of Gal (2% final concentration), incubated with shaking at 30 °C for 3 h, collected by centrifugation, washed in ice-cold 1× PBS, resuspended in 0.2 mL of ice-cold IP buffer [20 mM Tris-HCl (pH 7.5), 125 mM K-acetate, 0.5 mM EDTA, 0.5 mM EGTA, 1 mM DTT, 0.1% Tween-20] containing protease inhibitors (Complete, EDTA-free; Roche) and phosphatase inhibitors (25 mM β-glycerol phosphate and 1 mM sodium orthovanadate), lysed and immunoprecipitated with IgG-coated Protein A/G beads (Calbiochem-Novabiochem International, Inc.), essentially as described previously (8), except that an automated device for shaking with glass microspheres (FastPrep 120; Thermo Fisher Scientific) was used for cell breakage, instead of manual vortex mixing. Bead-bound immune complexes were collected by centrifugation, washed once with IP buffer, twice with kinase assay buffer [20 mM Tris-HCl (pH 7.2), 125 mM K-acetate, 12 mM MgCl2, 0.5 mM EDTA, 0.5 mM EGTA, 2 mM DTT, 1% glycerol, 0.02% BSA, 25 mM β-glycerol phosphate, and 1 mM sodium orthovanadate], resuspended in 20 μL of kinase assay buffer containing 100 μM [γ-32P]ATP (∼5 × 105 cpm/nmole), and incubated for 30 min with 0.5 μg of GST-fused substrate proteins, which were prepared by expression in and purification from E. coli, as described below. Reactions were terminated by addition of SDS/PAGE sample buffer containing 6% SDS followed by boiling for 5 min. Labeled proteins were resolved by SDS/PAGE and analyzed by autoradiography using a PhosphorImager (Molecular Dynamics Division, Amersham Pharmacia Biotech, Inc.).
Purification of GST Fusions.
Freshly transformed BL21(DE3) cells carrying a plasmid expressing the desired GST-fusion protein were grown to A600nm= 0.6, and expression was induced by addition of isopropyl-β-D-thiogalactopyranoside (0.2 mM final concentration). After vigorous aeration for 8 h at 26°C, cells were harvested and the GST-fusion protein was purified by column chromatography on glutathione agarose using standard procedures.
Fluorescence Imaging.
The instrumentation and procedures for fluorescence microscopy, image capture, and processing are described in detail in SI Text.
Supplementary Material
Acknowledgments
We thank members of the Thorner Laboratory, especially Pamela D. Torrance, Raymond E. Chen, Michael A. McMurray, and Jesse C. Patterson, for reagents, plasmids, and many helpful discussions, and Todd R. Graham for the gift of the dnf1∆ dnf2∆ dnf3∆ strain. This work was supported by National Institutes of Health Research Grant GM21841 (to J.T.) and by Kirschstein National Institutes of Health National Research Service Award Postdoctoral Fellowship GM77886 (to A.E.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912497106/DCSupplemental.
References
- 1.Gordesky SE, Marinetti GV. The asymetric arrangement of phospholipids in the human erythrocyte membrane. Biochem Biophys Res Commun. 1973;50:1027–1031. doi: 10.1016/0006-291x(73)91509-x. [DOI] [PubMed] [Google Scholar]
- 2.Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 3.Graham TR. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Nakano K, Yamamoto T, Kishimoto T, Noji T, Tanaka K. Protein kinases Fpk1p and Fpk2p are novel regulators of phospholipid asymmetry. Mol Biol Cell. 2008;19:1783–1797. doi: 10.1091/mbc.E07-07-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casamayor A, Torrance PD, Kobayashi T, Thorner J, Alessi DR. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr Biol. 1999;9:186–197. doi: 10.1016/s0960-9822(99)80088-8. [DOI] [PubMed] [Google Scholar]
- 6.Walther TC, et al. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 7.Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 2001;20:6783–6792. doi: 10.1093/emboj/20.23.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roelants FM, Torrance PD, Bezman N, Thorner J. Pkh1 and pkh2 differentially phosphorylate and activate ypk1 and ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell. 2002;13:3005–3028. doi: 10.1091/mbc.E02-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmelzle T, Helliwell SB, Hall MN. Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol Cell Biol. 2002;22:1329–1339. doi: 10.1128/mcb.22.5.1329-1339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deHart AK, Schnell JD, Allen DA, Hicke L. The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J Cell Biol. 2002;156:241–248. doi: 10.1083/jcb.200107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter T, Plowman GD. The protein kinases of budding yeast: Six score and more. Trends Biochem Sci. 1997;22:18–22. doi: 10.1016/s0968-0004(96)10068-2. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, et al. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol. 2000;20:4411–4419. doi: 10.1128/mcb.20.12.4411-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roelants FM, Torrance PD, Thorner J. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology. 2004;150:3289–3304. doi: 10.1099/mic.0.27286-0. [DOI] [PubMed] [Google Scholar]
- 14.Kamada Y, et al. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol. 2005;25:7239–7248. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardwell L, Cook JG, Chang EC, Cairns BR, Thorner J. Signaling in the yeast pheromone response pathway: Specific and high-affinity interaction of the mitogen-activated protein (MAP) kinases Kss1 and Fus3 with the upstream MAP kinase kinase Ste7. Mol Cell Biol. 1996;16:3637–3650. doi: 10.1128/mcb.16.7.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson BJ, Rhodes N, Errede B, Sprague GF., Jr Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- 17.Obeid LM, Okamoto Y, Mao C. Yeast sphingolipids: Metabolism and biology. Biochim Biophys Acta. 2002;1585:163–171. doi: 10.1016/s1388-1981(02)00337-2. [DOI] [PubMed] [Google Scholar]
- 18.Dickson RC, Sumanasekera C, Lester RL. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog Lipid Res. 2006;45:447–465. doi: 10.1016/j.plipres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Zhang X, Lester RL, Dickson RC. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J Biol Chem. 2005;280:22679–22687. doi: 10.1074/jbc.M502972200. [DOI] [PubMed] [Google Scholar]
- 20.Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC. The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J Biol Chem. 2008;283:10433–10444. doi: 10.1074/jbc.M709972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura S, Kihara A, Iwaki S, Inokuchi J, Igarashi Y. Regulation of the transport and protein levels of the inositol phosphorylceramide mannosyltransferases Csg1 and Csh1 by the Ca2+-binding protein Csg2. J Biol Chem. 2007;282:8613–8621. doi: 10.1074/jbc.M606649200. [DOI] [PubMed] [Google Scholar]
- 22.Pittet M, Uldry D, Aebi M, Conzelmann A. The N-glycosylation defect of cwh8Delta yeast cells causes a distinct defect in sphingolipid biosynthesis. Glycobiology. 2006;16:155–164. doi: 10.1093/glycob/cwj043. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto KHT, et al. Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys J. 2007;93:1608–1619. doi: 10.1529/biophysj.106.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomorski T, et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 26.Kihara A, Igarashi Y. Cross talk between sphingolipids and glycerophospholipids in the establishment of plasma membrane asymmetry. Mol Biol Cell. 2004;15:4949–4959. doi: 10.1091/mbc.E04-06-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthusamy BP, Natarajan P, Zhou X, Graham TR. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim Biophys Acta. 2009;1791:612–619. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthusamy BP, et al. Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol Biol Cell. 2009;20:2920–2931. doi: 10.1091/mbc.E08-10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito K, et al. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev Cell. 2007;13:743–751. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Folmer DE, Elferink RP, Paulusma CC. P4 ATPases-lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Sherman F, Fink GR, Hicks JB. Laboratory Course Manual for Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1986. [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.