Abstract
T-cell activation results from productive T-cell receptor (TCR) engagement by a cognate peptide–MHC (pMHC) complex on the antigen presenting cell (APC) surface, a process leading to the polarization of the T-cell secretory machinery toward the APC interface. We have previously shown that the half-life of the TCR/pMHC interaction and the density of pMHC on the APC are two parameters determining T-cell activation. However, whether the half-life of the TCR/pMHC interaction can modulate the efficiency of T-cell secretory machinery polarization toward an APC still remains unclear. Here, by using altered peptide ligands conferring different half-lives to the TCR/pMHC interaction, we have tested how this parameter can control T-cell polarization. We observed that only TCR/pMHC interactions with intermediate half-lives can promote the assembly of synapses that lead to T-cell activation. Strikingly, intermediate half-life interactions can be competed out by short half-life interactions, which can efficiently promote T-cell polarization and antagonize T-cell activation that was induced by activating intermediate half-life interactions. However, short TCR/pMHC interactions fail at promoting phosphorylation of signaling molecules at the T-cell–APC contact interface, which are needed for T-cell activation. Our data suggest that although intermediate half-life pMHC ligands promote assembly of activating synapses, this process can be inhibited by short half-life antagonistic pMHC ligands, which promote the assembly of non activating synapses.
Keywords: immunological synapse, T-cell receptor, T-cell receptor half-life, dendritic cells
T-cell activation requires an efficient engagement of the T-cell receptor (TCR) by peptide–MHC complexes (pMHC) on the surface of APCs (1, 2). The TCR/pMHC interaction defines the specificity of T-cell activation based on the recognition of a particular pMHC complex on the APC surface by the TCR (1, 2). This recognition leads to the formation of a specialized structure, known as an immunological synapse (IS), characterized by molecular rearrangements involving segregation of surface molecules, polarization of secretory machinery, and signaling components at the T-cell–APC contact interface (3–5).
Several kinetic requirements must be satisfied by the TCR/pMHC interaction to efficiently induce T-cell activation (6–13). The half-life (t1/2) of the TCR/pMHC interaction must be above certain threshold to allow productive TCR signaling, as proposed by the kinetic proofreading model (11). Several studies have provided evidence supporting this notion by showing that pMHC ligands with excessively short half-lives fail to complete the signaling cascade required for T-cell activation (14–17).
In addition, T cells need to recognize and respond to pMHC ligands, generally found only at low density on the APC surface (18, 19). This high TCR sensitivity is required to initiate an adaptive immune response against intracellular pathogens that have evolved molecular mechanisms to reduce the density of MHC molecules loaded with pathogen-derived peptides on the surface of infected cells and thus to avoid T-cell recognition (20, 21). Hence, the sustained TCR signaling needed for activation of naive T cells should be generated by few cognate pMHC complexes on the APC surface. Thus, TCR/pMHC binding half-life must be short enough to ensure that a single pMHC molecule serially engages several TCR molecules (12, 13, 22). The simultaneous fulfillment of both kinetic proofreading and TCR serial engagement implies that an optimal TCR-pMHC half-life would be required for an efficient activation of T cells in response to low density cognate pMHC (3, 6, 7, 10). Several independent studies using different experimental systems have provided evidence supporting this notion (6–10, 17, 22–25).
Although some of the binding kinetic requirements that the TCR/pMHC interaction must fulfill to induce an efficient T-cell activation have been established (1, 2, 6–9, 16, 17), the mechanisms explaining why TCR/pMHC interactions with short or long half-lives impair T-cell activation remain poorly understood. Whether T-cell polarization toward DCs, a critical event during T-cell activation, can be modulated by the TCR/pMHC binding kinetics needs to be addressed.
In addition to controlling T-cell activation in response to a particular pMHC ligand, TCR binding kinetics are critical for TCR-mediated antagonism of T-cell activation. In general, antagonist pMHC ligands confer shorter half-lives of interaction with the TCR and can inhibit T-cell activation induced by agonist pMHC ligands (14, 26–28). However, the mechanisms responsible for inhibition of T-cell activation have not been yet elucidated. It has been proposed that antagonist ligands cause partial phosphorylation of CD3ξ (29–31). Because antagonist ligands display faster off-rates, they can bind a TCR, dissociate, and bind another TCR faster than ligands with longer half-lives. As a result, short half-life antagonist pMHC ligands could be more efficient at promoting TCR serial engagement than agonist pMHCs. This could enable the antagonists to engage more TCRs than the agonists over the same period and perhaps dictate the subsequent signaling response. Most studies have analyzed TCR antagonism on a single APC bearing both antagonist and agonist pMHC ligands. However, it has also been reported that antagonist ligands on an APC can impair T-cell activation by another APC loaded with an agonist ligand (32). Whether the interaction with both APCs simultaneously can differentially polarize the T-cell secretory machinery and influence T-cell function remains unknown.
Here, we have evaluated T-cell activation and polarization toward DCs in response to stimulation with pMHC ligands that bind with different half-lives to the TCR. By using T-cell Golgi polarization as a parameter of IS assembly (33), we have tested whether the TCR/pMHC half-life can influence the dynamics of T-cell polarization. Our data suggest that efficient T-cell polarization and activation require an intermediate half-life of TCR/pMHC interaction. Furthermore, pMHC ligands with short half-lives that fail to induce T-cell activation can efficiently cause cross-antagonism and abolish T-cell activation. However, we observed that short half-life antagonist ligands remained capable of efficiently promoting T-cell polarization. These data suggest that T-cell polarization toward DCs can be efficiently induced by short half-lives of TCR/pMHC interaction without T-cell activation, underscoring a possible novel mechanism for T-cell antagonism.
Results
Efficient T-Cell Activation Is Promoted Only by TCR/pMHC Interactions with Intermediate Half-Lives.
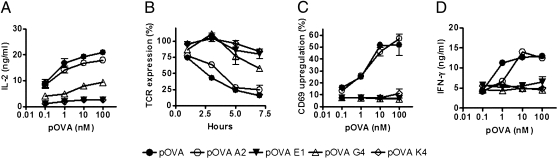
To extend our previous observations suggesting that efficient T-cell activation requires an optimal half-life of TCR/pMHC interaction (6–8, 10), we have used OT-I T cells, which express a TCR that binds to H-2Kb/SIINFEKL complex. Several altered peptide ligands (APLs) for the SIINFEKL sequence have been described (27), which show different half-lives of TCR/pMHC interaction (Table 1) (14, 27). To evaluate how the half-life of TCR/pMHC interaction influences T-cell activation, IL-2 secretion and TCR internalization by OT-I T cells stimulated with DCs pulsed with pOVA-derived APLs was measured. As shown in Fig. 1 A and B and Fig. S1, only intermediate half-lives of TCR/pMHC interaction were capable of inducing efficient T-cell activation. Similarly, expression of the early activation marker CD69 and IFN-γ secretion, were only observed on OT-I T cells when stimulated by APCs pulsed with pOVA variants that conferred intermediate half-lives for the TCR/pMHC interaction (Fig. 1 C and D). Unpulsed DCs and DCs pulsed with the OVA protein were included in all experiments as positive and negative controls, respectively (Fig. S1). These data are consistent with previous observations made in different TCR/pMHC systems (6–8, 10).
Table 1.
Binding kinetics of OT-I TCR/H-2Kb for OVA-derived peptides
| Peptide | Peptide sequence | t1/2 (25°C) (s)* |
| pOVA | SIINFEKL | 33 |
| pOVA-A2 | SAINFEKL | 34.7 |
| pOVA-G4 | SIIGFEKL | 77 |
| pOVA-E1 | EIINFEKL | 10.3 |
| pOVA-R4 | SIIRFEKL | 5.4 |
| pOVA-K4 | SIIKFEKL | <3.5 |
Fig. 1.
Intermediate half-lives of TCR/pMHC interaction are required to induce efficient T-cell activation and effector function. pOVA APLs–pulsed DCs were cocultured with OT-I T cells, and IL-2 secretion (A) and TCR internalization (B) were determined. Activated OT-I T cells were cocultured with pOVA APLs–pulsed DCs cells, and CD69 expression (C) and IFN-γ secretion (D) were determined. Graphs represent average of three to five independent experiments; error bars represent SE.
DCs Loaded with Short Half-Life pMHC Ligands Can Antagonize T-Cell Activation Induced by DCs Loaded with Intermediate Half-Life Ligands.
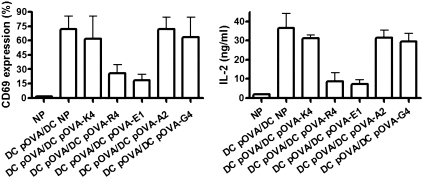
The pOVA-E1, pOVA-R4, and pOVA-G4 peptides were previously described as antagonists for the OT-I TCR (27, 28). TCR antagonism was originally shown to work on single APC loaded simultaneously with agonist and antagonist peptides (14, 26–28). Here, we have evaluated whether antagonism could be observed when T cells are exposed to APCs loaded separately with agonists and antagonists ligands, a process that we refer to as cross-antagonism. When DCs pulsed with pOVA and DCs pulsed with either pOVA-E1 or pOVA-R4 were simultaneously cocultured with OT-I T cells, activation of T cells was almost completely abolished (Fig. 2). In contrast, no significant inhibition was observed when T cells were simultaneously stimulated with DCs pulsed with pOVA and DCs pulsed with any other pOVA variant (Fig. 2). These data suggest that DCs loaded with short half-life pMHC ligands can efficiently antagonize T-cell responses by other DCs loaded with optimal agonist ligands.
Fig. 2.
DCs loaded with short half-life pMHC ligands can antagonize T-cell activation induced by DCs loaded with intermediate half-life ligands. OT-I T cells were simultaneously stimulated with DCs pulsed with pOVA and DCs pulsed with pOVA variants, and then CD69 upregulation and IL-2 release were determined. Graphs show means of three independent experiments; error bars represent SE.
Antagonist pMHC Ligands Induce T-cell Golgi Apparatus Polarization toward DCs with Efficiency Equivalent to that of Agonist pMHCs.
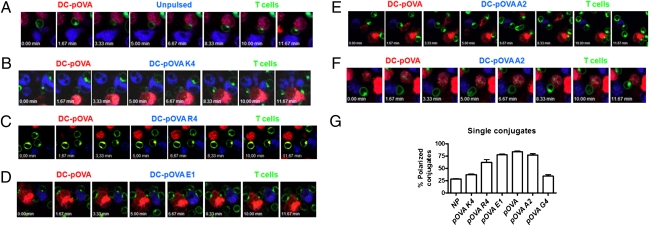
Although early studies have suggested that IS assembly requires a minimum or threshold TCR/pMHC interaction half-life (4), the role of TCR/pMHC binding kinetics on IS assembly has not been yet defined. To approach this question, we have evaluated T-cell Golgi apparatus polarization toward DCs on OT-I T cells stimulated with DCs pulsed with either pOVA or pOVA APLs. Polarization of Golgi apparatus was used as an indicator of IS assembly, because this process associates directly with other markers of IS formation, such as TCR segregation and microtubule polarization (33). Using time-lapse video microscopy, we analyzed Golgi apparatus polarization of living T cells when cocultured with DCs presenting either the WT pOVA or APLs. When OT-I T cells were cocultured with pOVA-pulsed DCs and either unpulsed DCs or pOVA-K4–pulsed DCs (null), significant Golgi apparatus polarization was observed only toward pOVA-pulsed DCs (Fig. 3 A and B and Movies S1–S4). Strikingly, although pOVA-R4 and pOVA-E1–pulsed DCs impaired T-cell activation (Fig. 1 and Fig. S1), OT-I T cells polarized with equal frequency toward either pOVA-pulsed DCs, pOVA-E1– or pOVA-R4–pulsed DCs (Fig. 3 C and D and Movies S5–S7). These data suggest an explanation for the inhibition of T-cell activation observed in the cross-antagonism assays (Fig. 2).
Fig. 3.
T cells polarize toward DCs presenting pMHC ligands that bind the TCR either with short or intermediate half-lives. Peptide-pulsed DCs were stained with BODIPY 630 (blue) or CMTMR orange (red) and cocultured with OT-I T cells stained for Golgi apparatus (green). Golgi apparatus polarization was determined by time-lapse video microscopy. Data shown are representative sequences of snapshot images for pOVA-pulsed DCs vs. unpulsed or pOVA APL-pulsed DCs, derived from one experiment out of five. Snapshots on A, B, C, D, E, and F correspond to Movies S1, S3, S5, S6, S8, and S10, respectively. (G) Percentage of T cell–DC conjugates with T-cell Golgi apparatus polarized toward the indicated peptide-pulsed DC.
As expected, OT-I T cells polarized equally toward pOVA-pulsed DCs and pOVA-A2-pulsed DCs (intermediate half-life) (Fig. 3E and Movies S8 and S9). In contrast, we found that OT-I T cells polarized more frequently to pOVA-pulsed DCs than toward pOVA-G4–pulsed DCs (Fig. 3F and Movies S10 and S11). To quantify the confocal microscopy data, several snapshot images from different fields were randomly captured after each recording and Golgi apparatus polarization in T cell–DC conjugates was quantified. DCs pulsed with ligands conferring short or intermediate half-lives induce a high frequency of OT-I T-cell polarization (Fig. 3G and Table S1). In contrast, a very low frequency of polarization was observed for T cells incubated with DCs pulsed with either pOVA-K4 or pOVA-G4 (null or long half-life, respectively) (Fig. 3F).
To evaluate whether the observations made in the OT-I system can be extended to a different TCR system, similar experiments were performed using the N30.7 TCR, which is specific for H-2Kb loaded with the VSV peptide (RGYVYQGL) (10, 34). By using the VSV-derived APLs, such as the antagonist VSV-E6 and VSV-I1E6 and the null ligand VSV-R6 (Table S2), we evaluated Golgi polarization in N30.7 T-cell hybridomas. As shown in Fig. S2, DCs pulsed with any of VSV-derived APLs were unable to induce N30.7 T-cell activation. In addition, VSV-E6– or VSV-I1E6–pulsed DCs cross-antagonized activation of N30.7 T cells induced by WT-VSV–loaded DCs (Fig. S3). However, equivalent T-cell Golgi polarization was promoted by DCs loaded with either VSV-E6 or VSV-I1E6 peptide, as compared with WT-VSV–pulsed DCs (Fig. S4). In contrast, the VSV-R6 peptide neither cross-antagonized T-cell activation (Fig. S3) nor induced T-cell Golgi polarization (Fig. S4).
Furthermore, to determine T-cell Golgi polarization in response to long half-lives of TCR/pMHC interactions, a variant of the N30.7 TCR carrying a point mutation in the CDR3β (G99A) showing a longer half-life for the H-2Kb/VSV complex than the WT N30.7 TCR (Table S3) (8, 10) was used to complement the data obtained with the G4 ligand. As shown in Fig. S5, a long half-life of TCR/pMHC interaction failed to induce T-cell Golgi polarization, as compared with the intermediate half-life interaction by the N30.7 TCR.
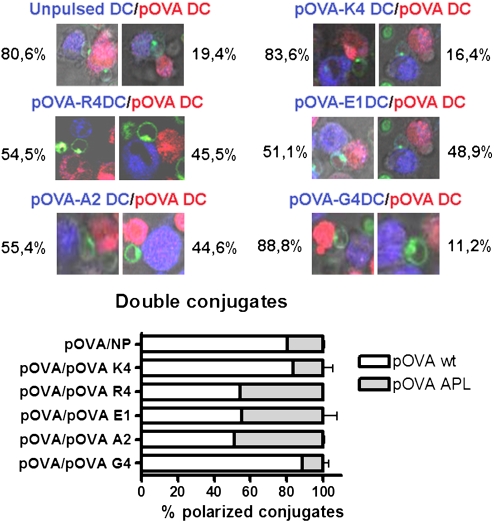
To evaluate the capability of DCs presenting different pMHC complexes to compete for the T cell, we determined the frequency of OT-I T polarization toward each DC in triple conjugates (a single T cell is in contact with two differently pulsed DCs). As shown in Fig. 4 and scored in Table S1, when incubated simultaneously with pOVA-pulsed DCs and unpulsed, pOVA-K4–pulsed or pOVA-G4–pulsed DCs, the majority of OT-I T cells polarized toward pOVA-pulsed DCs. In contrast, when incubated simultaneously with pOVA-pulsed DCs and pOVA-E1– or pOVA-R4–pulsed DCs, ≈50% of T cells polarized toward each DC type (Fig. 4). Similar results were obtained when OT-I T cells were incubated simultaneously with pOVA-pulsed DCs and pOVA-A2–pulsed DCs, as expected (Fig. 4). Interestingly, pOVA-E1 ligand is capable of inducing the repolarization of OT-I T cells already polarized toward DCs bearing pOVA ligand (Fig. S6 and Movie S12). These data suggest that DCs loaded with short half-life pMHC ligands can efficiently compete for T-cell Golgi polarization against DCs loaded with optimal pMHC ligands.
Fig. 4.
DCs loaded with antagonist ligands can compete for T-cell polarization with DCs loaded with agonist ligands. Peptide-pulsed DCs were stained with BODIPY 630 (blue) or CMTMR orange (red) and cocultured with OT-I T cells stained for Golgi apparatus (green). Triple conjugates (T cells interacting at the same time with two different types of DCs) were searched, and the polarization toward each one of the different DCs was quantified.
Antagonist pMHC-Induced T-Cell Polarization Fails to Recruit Tyrosine Phosphorylated Molecules at the Immunological Synapse.
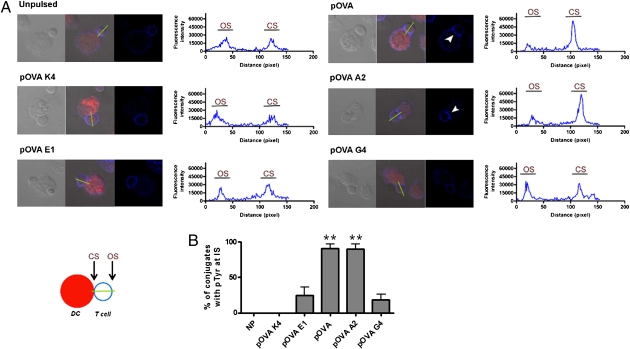
Because several signaling molecules are phosphorylated early during T-cell activation and TCR antagonism, we evaluated whether the amount and distribution of tyrosine-phosphorylated molecules at the IS can be modulated by different half-lives of TCR/pMHC interaction. It was particularly intriguing to measure this parameter for pOVA-E1–pulsed DCs, because although they antagonize T-cell activation (Fig. 2), this peptide still promotes OT-I T-cell polarization (Fig. 3). To approach this question, conjugates between OT-I T cells and pOVA- or pOVA APLs–pulsed DCs were analyzed for phosphotyrosine (pTyr) accumulation at the DC–T-cell contact site. As shown in Fig. 5, only DCs pulsed with ligands conferring intermediate half-lives were able to induce pTyr accumulation at the T-cell side of the IS. Fluorescence analyses and quantification of conjugates with pTyr accumulation showed that only intermediate half-lives were capable of inducing T-cell pTyr accumulation at the DC–T-cell contact site (Fig. 5). Remarkably, although pOVA-E1-pulsed DCs were capable of promoting an efficient polarization of OT-I T cells, they failed to induce accumulation of phosphorylated molecules at the IS.
Fig. 5.
Significant accumulation of tyrosine-phosphorylated molecules at the IS requires intermediate half-life for the TCR/pMHC interaction. (A) Distribution of pTyr (blue) was determined by confocal microscopy on T-cell conjugates with DCs (red), either unpulsed or pulsed with pOVA APLs. Arrowheads indicate pTyr accumulation at the DC–T-cell interface. pTyr accumulation at the IS was quantified by using a linescan tool from Image ProPlus software. OS and CS represent fluorescence intensity at opposite site and contact site of T cell–DC synapse, respectively, as described by the diagram. Representative data from one of three experiments are shown. (B) Percentage of conjugates with pTyr accumulation for each pOVA APL-pulsed DC of all of the conjugates analyzed in three experiments. **P < 0.01, ANOVA two-way test.
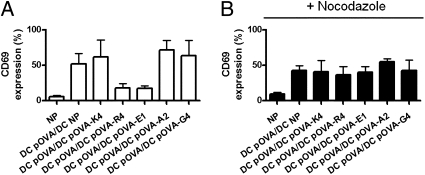
Finally, to evaluate whether cross-antagonism requires T-cell Golgi polarization promoted by antagonist ligands, OT-I T cells were treated with the microtubule organizing center (MTOC) disruptor nocodazole (35). As recently shown by Martín-Cófreces et al. (36), although disruption of MTOC translocation inhibited T-cell Golgi polarization and IL-2 secretion, it only partially blocked CD69 upregulation (Fig. S7). As expected, when OT-I T cells were pretreated with nocodazole, the cross-antagonism induced by pOVA-R4 and E1 was no longer observed (Fig. 6).
Fig. 6.
T-cell cross-antagonism requires T-cell Golgi polarization. Control (A) or nocodazole-treated (B) OT-I T cells were simultaneously stimulated with DCs pulsed with pOVA and DCs pulsed with pOVA variants, and then CD69 upregulation was determined. Graphs show means of three independent experiments; error bars represent SE.
Discussion
Although several reports support the notion that the half-life of TCR/pMHC interaction is an important parameter controlling T-cell activation (6–10, 13, 24), the mechanisms behind this process remain poorly understood. We and others have previously shown that efficient T-cell activation requires an optimal (intermediate) half-life for the TCR/pMHC interaction (1, 6, 8–10, 17, 37, 38).
Although the notion that IS assembly is necessary (12, 39–41) for initiation and maintenance of TCR signaling and T-cell activation remains controversial (37, 42, 43), it has been suggested that IS assembly could work as a platform to promote continuous TCR signaling and polarized secretion of cytokines and effector molecules at the T cell–APC interface (44–47).
Whether modulation of T-cell activation by TCR/pMHC half-life involves IS assembly has not been evaluated. Here, by using OT-I and N30.7 T cells and an extensive panel of pOVA and pVSV-derived APLs, we have analyzed T-cell Golgi polarization as a parameter for IS assembly, T-cell cross-antagonism, and T-cell activation in response to different half-lives of TCR/pMHC interaction. Consistent with our previous studies (6–8, 10, 48), only when half-life of TCR/pMHC is intermediate (peptides pOVA and pOVA–A2) were OT-I T cells fully activated (Fig. 1). Because T-cell Golgi polarization is closely related to T-cell activation, we evaluated whether the TCR/pMHC half-life can modulate this process. Although short half-lives could not induce T-cell activation, we observed that they were effective at promoting T-cell Golgi polarization toward DCs (Figs. 2 and 3). Remarkably, induction of T-cell polarization by short half-life ligands was required for their capacity to antagonize T-cell activation, because a disruption of T-cell Golgi polarization impaired T-cell antagonism by short half-life ligands (Fig. 6).
T-cell Golgi polarization has been shown to be a highly dynamic process. T cells can reorganize a polarized Golgi from an APC with low density cognate ligand into an APC presenting a higher density of cognate ligand (33). We decided to test whether Golgi apparatus repolarization could be induced by APCs presenting pMHCs with different half-lives for the TCR. Our data show that efficient IS assembly required a lower half-life threshold than did T-cell activation. However, although short TCR/pMHC half-lives could promote T-cell Golgi polarization, they failed to induce the accumulation of pTyr molecules at the IS (Fig. 5). Although it has been reported that tyrosine phosphorylation is required for MTOC reorientation in T cells (49, 50) we could not detect pTyr accumulation for antagonist ligands in our assays. However, we cannot rule out the possibility that fluorescence intensities may be below the detection level of our assays. Furthermore, because pTyr signaling initiates before IS assembly, it is possible that certain phosphorylated molecules that do not accumulate at the synapse might contribute to the efficient T-cell Golgi polarization induced by antagonist ligands.
In contrast, long half-life interactions are unable to induce T-cell activation or T-cell Golgi polarization. Although G4 behaves as a long half-life ligand at 25°C and as a short half-life ligand at 37°C (16), it is thought that its behavior as a short half-life ligand may be due to TCR dimerization induced by pOVA, which increases its relative half-life. However the first-order binding of the pMHC to the TCR is still longer for the G4 peptide than for the pOVA as measured by Biacore (14, 27). This notion is consistent with the partial T-cell activation observed in response to high pOVA-G4 peptide concentrations, because as previously shown, long half-life pMHC ligands can induce T-cell activation when they are at high density on the APC surface (8). Nevertheless, data from tetramer dissociation and potency in thymic selection assays suggest that the G4 ligand is intermediate in half-life and potency between OVA and E1 (51). The ligands that induce the novel mode of antagonism reported here have shorter half-lives than pOVA or G4 and induced only weak positive selection in thymic organ cultures (51).
Our data suggest that T-cell Golgi apparatus polarization at the T-cell–APC interface is dependent upon TCR serial engagement but independent of TCR kinetic proofreading. According to this notion, intermediate TCR/pMHC half-lives would allow fulfillment of both kinetic proofreading and TCR serial engagement and would lead to IS assembly (polarization of Golgi and pTyr accumulation) and T-cell activation. In contrast, long half-lives would not be able to fulfill the serial engagement requirement and therefore would impair IS assembly and T-cell activation. Surprisingly, antagonists with short half-lives for the TCR/pMHC interaction were able to fulfill TCR serial engagement and induce an efficient T-cell polarization in the absence of T-cell activation, probably because of a failure in the kinetic proofreading.
Taken together, our data support the notion that T-cell polarization and T-cell activation are functionally dissociated and differentially modulated by the half-life of TCR/pMHC interaction. In addition, T-cell preference for polarizing toward a particular APC seems to be defined by the TCR serial engagement efficiency. Thus, APCs with pMHC ligands that bind to TCR with shorter half-lives would be as efficient at inducing T-cell polarization as APCs loaded with pMHC with optimal half-lives, even if these latter ligands allow T-cell activation. Thus, it is possible that by having a selective advantage for T-cell polarization, pMHC ligands with shorter half-lives could antagonize T-cell activation by occupying the T-cell IS and preventing the establishment of synapses with optimal pMHC ligands. Competition for T-cell polarization by antagonist ligands and their capacity to induce repolarization of T cells previously engaged with DCs loaded with agonist ligands could work as a mechanism to prevent T-cell immunity against pathogens. Such a phenomenon could contribute to the immune evasion capacity shown by virulent bacteria and viruses that undergo epitope variation during infection.
Materials and Methods
Mice.
C57BL/6 and OT-I mice (52) were originally purchased from Jackson Laboratory and maintained at the germ-free animal facility of the Pontificia Universidad Católica de Chile. All animal work was performed according to institutional guidelines.
Cells and Peptides.
OT-I CD8+ T cells were purified by negative selection using magnetic beads (Miltenyi Biotec). DCs were produced as previously described (52). Peptides, pOVA (SIINFEKL), pOVA-K4 (SIIKFEKL), pOVA-E1 (SEINFEKL), pOVA-R4 (SIIRFEKL), pOVA-A2 (SAINFEKL), pOVA-G4 (SIIGFEKL), VSV (RGYVYQGL), VSV-E6 (RGYVYEGL), VSV-R6 (RGYVYRGL), and VSV I1E6 (IGYVYEGL) were purchased from GenScript Corporation or obtained from the Peptide Synthesis Facility at the Albert Einstein College of Medicine.
T-Cell Activation Assays.
DCs were pulsed for 16 h with 10 ng/mL of the corresponding peptide and then cocultured with OT-I T cells in 96-well U-bottom plates (105 T cells and 105 DCs per well), as previously described (6). After 24 h, IL-2 release was measured by cytokine ELISA. Alternatively, DCs were pulsed with 10 ng/mL of pOVA or pOVA APLs and cocultured with activated OT-I T cells (by 48 h stimulation on 96-well plates coated with 50 ng/mL anti-CD3) in 96-well U-bottom plates. After 24 h, surface expression of CD69 on OT-I T cells was analyzed by FACS, and IFN-γ secretion was measured by ELISA.
TCR Downmodulation Assays.
OT-I T cells (105) were stimulated during 1, 3, 5, or 7 h with DCs pulsed with 10 ng/mL pOVA or pOVA APLs at 37°C. Subsequently, T cells were harvested, stained with anti-CD8α and anti-Vα2 antibodies (BD PharMingen), and analyzed by FACS.
T-Cell Antagonism Assays.
OT-I T cells (105) were stimulated simultaneously with 105 DCs pulsed with 10 ng/mL pOVA and 105 DCs unpulsed or pulsed with 10 ng/mL of pOVA variants. After 24 h of coculture, IL-2 release was measured by ELISA. Alternatively, T cells were harvested and stained with anti-CD8α and anti-CD69 antibodies (BD PharMingen) and analyzed by FACS. For Golgi disruptions experiments, T cells were pretreated during 6 h with 30 μM of Nocodazole (Sigma) at 37°C, washed twice, and then cocultured with DCs, as described above.
Dynamics of Golgi Apparatus in Living Cells.
DCs were pulsed with one of pOVA APLs (10 ng/mL) for 16 h. After peptide pulse, DCs pulsed with pOVA were stained with 0.5 μM CMTMR-Orange (Molecular Probes). Unpulsed DCs or pOVA APLs–pulsed DCs were stained with 0.5 μM BODIPY 630 (Molecular Probes). Golgi apparatus of OT-I T cell was labeled with 5 μM BODIPY FL C5-Ceramide (Molecular Probes) at 37°C for 30 min. After staining, DCs were seeded on microchambers (Nalge Nunc International) previously coated with poly-D-lysine (Sigma-Aldrich). Time-lapse recordings were performed as described in SIText. Quantification of T-cell Golgi polarization, T-cell/DC single conjugates, and double conjugates was scored by visual inspection in a blind study, considering as polarized to those T cells in contact with DCs showing Golgi staining at the T-cell–DC contact site.
Detection of Tyrosine Phosphorylation on T Cells.
DCs were pulsed with one of the pOVA APLs peptides described above (10 ng/mL) during 16 h and then stained with 0.5 μM of CMTMR-Orange. Subsequently, conjugates of stained-DCs and OT-I T cells were formed, as previously described (53). Cells were incubated for 15 min at 37°C, then fixed with 1% paraphormaldehyde and permeabilized with 0.1% triton X-100, as previously described (41). Conjugates were stained with antiphosphotyrosine (Santa Cruz Biotechnology) followed by anti-IgG2b-Cy5 (Santa Cruz Biotechnology). Finally, the samples were mounted and analyzed by using a Carl Zeiss LSM 510 confocal microscope (Carl Zeiss). Fluorescence intensity analyses were performed using Image Pro Plus software (Media Cybernetics).
Supplementary Material
Acknowledgments
This work was supported by Grants Fondo Nacional de Desarrollo Científico Tecnológico (FONDECYT) 1030557, 1050979, 1070352, and 3100090; Fondo de Fomento al Desarrollo Científico y Tecnológico (FONDEF) D04I1075; INCO-CT-2006-032296; and the CONICYT-INSERM collaboration program and Millennium Nucleus on Immunology and Immunotherapy (P04/030-F). L.C. and E.R. are Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) fellows.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911258107/DCSupplemental.
References
- 1.Carreño LJ, González PA, Kalergis AM. Modulation of T cell function by TCR/pMHC binding kinetics. Immunobiology. 2006;211:47–64. doi: 10.1016/j.imbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Kalergis AM. Modulation of T cell immunity by TCR/pMHC dwell time and activating/inhibitory receptor pairs on the antigen-presenting cell. Curr Pharm Des. 2003;9:233–244. doi: 10.2174/1381612033392062. [DOI] [PubMed] [Google Scholar]
- 3.González PA, Carreño LJ, Figueroa CA, Kalergis AM. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18:19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Grakoui A, et al. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 5.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 6.Carreño LJ, Bueno SM, Bull P, Nathenson SG, Kalergis AM. The half-life of the T-cell receptor/peptide-major histocompatibility complex interaction can modulate T-cell activation in response to bacterial challenge. Immunology. 2007;121:227–237. doi: 10.1111/j.1365-2567.2007.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombs D, Kalergis AM, Nathenson SG, Wofsy C, Goldstein B. Activated TCRs remain marked for internalization after dissociation from pMHC. Nat Immunol. 2002;3:926–931. doi: 10.1038/ni838. [DOI] [PubMed] [Google Scholar]
- 8.González PA, et al. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc Natl Acad Sci USA. 2005;102:4824–4829. doi: 10.1073/pnas.0500922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudrisier D, et al. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J Immunol. 1998;161:553–562. [PubMed] [Google Scholar]
- 10.Kalergis AM, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2:229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 11.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 14.Alam SM, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 15.Kessler BM, Bassanini P, Cerottini JC, Luescher IF. Effects of epitope modification on T cell receptor-ligand binding and antigen recognition by seven H-2Kd-restricted cytotoxic T lymphocyte clones specific for a photoreactive peptide derivative. J Exp Med. 1997;185:629–640. doi: 10.1084/jem.185.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: A revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 17.Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh HL, Eisen HN. Peptide antagonism and T cell receptor interactions with peptide-MHC complexes. Immunity. 1998;9:475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- 18.Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 19.Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 20.Gewurz BE, et al. Antigen presentation subverted: Structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc Natl Acad Sci USA. 2001;98:6794–6799. doi: 10.1073/pnas.121172898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo ME, Ploegh HL, Tirabassi RS. Viral immune evasion strategies and the underlying cell biology. Semin Immunol. 2001;13:1–9. doi: 10.1006/smim.2000.0290. [DOI] [PubMed] [Google Scholar]
- 22.Rachmilewitz J, Lanzavecchia A. A temporal and spatial summation model for T-cell activation: Signal integration and antigen decoding. Trends Immunol. 2002;23:592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
- 23.McMahan RH, et al. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116:2543–2551. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 25.Ueno T, Tomiyama H, Fujiwara M, Oka S, Takiguchi M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR-ligand interactions. J Immunol. 2004;173:5451–5457. doi: 10.4049/jimmunol.173.9.5451. [DOI] [PubMed] [Google Scholar]
- 26.Alam SM, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 27.Gascoigne NR, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. [DOI] [PubMed] [Google Scholar]
- 28.Koniaras C, Carbone FR, Heath WR, Lew AM. Inhibition of naive class I-restricted T cells by altered peptide ligands. Immunol Cell Biol. 1999;77:318–323. doi: 10.1046/j.1440-1711.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- 29.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dittel BN. Mechanisms of T cell receptor antagonism: Implications in the treatment of disease. Curr Mol Med. 2001;1:339–355. doi: 10.2174/1566524013363799. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Grey HM. Study of the mechanism of TCR antagonism using dual-TCR-expressing T cells. J Immunol. 2003;170:4532–4538. doi: 10.4049/jimmunol.170.9.4532. [DOI] [PubMed] [Google Scholar]
- 32.Stotz SH, Bolliger L, Carbone FR, Palmer E. T cell receptor (TCR) antagonism without a negative signal: Evidence from T cell hybridomas expressing two independent TCRs. J Exp Med. 1999;189:253–264. doi: 10.1084/jem.189.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depoil D, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Kalergis AM, Nathenson SG. Altered peptide ligand-mediated TCR antagonism can be modulated by a change in a single amino acid residue within the CDR3 beta of an MHC class I-restricted TCR. J Immunol. 2000;165:280–285. doi: 10.4049/jimmunol.165.1.280. [DOI] [PubMed] [Google Scholar]
- 35.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 36.Martín-Cófreces NB, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Hori Y, Groves JT, Dustin ML, Chakraborty AK. Correlation of a dynamic model for immunological synapse formation with effector functions: Two pathways to synapse formation. Trends Immunol. 2002;23:492–499. doi: 10.1016/s1471-4906(02)02285-8. [DOI] [PubMed] [Google Scholar]
- 39.Berg NN, Puente LG, Dawicki W, Ostergaard HL. Sustained TCR signaling is required for mitogen-activated protein kinase activation and degranulation by cytotoxic T lymphocytes. J Immunol. 1998;161:2919–2924. [PubMed] [Google Scholar]
- 40.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 41.Zaru R, Cameron TO, Stern LJ, Müller S, Valitutti S. Cutting edge: TCR engagement and triggering in the absence of large-scale molecular segregation at the T cell-APC contact site. J Immunol. 2002;168:4287–4291. doi: 10.4049/jimmunol.168.9.4287. [DOI] [PubMed] [Google Scholar]
- 42.O’Keefe JP, Blaine K, Alegre ML, Gajewski TF. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:9351–9356. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 44.Cemerski S, et al. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark RH, et al. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 46.Faroudi M, et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: Manifestation of a dual activation threshold. Proc Natl Acad Sci USA. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valitutti S. Immunological synapse: Center of attention again. Immunity. 2008;29:384–386. doi: 10.1016/j.immuni.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Riquelme E, Carreño LJ, González PA, Kalergis AM. The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity. Eur J Immunol. 2009;39:2259–2269. doi: 10.1002/eji.200939341. [DOI] [PubMed] [Google Scholar]
- 49.Kuhné MR, et al. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 50.Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 52.Clarke SR, et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110–117. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 53.Leupin O, Zaru R, Laroche T, Müller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–280. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.