Abstract
Accurate DNA synthesis by the replicative DNA polymerases α, δ, and ε is critical for genome stability in eukaryotes. In humans, over 20 SNPs were reported that result in amino–acid changes in Polδ or Polε. In addition, Polδ variants were found in colon–cancer cell lines and in sporadic colorectal carcinomas. Using the yeast-model system, we examined the functional consequences of two cancer-associated Polδ mutations and four polymorphisms affecting well-conserved regions of Polδ or Polε. We show that the R696W substitution in Polδ (analog of the R689W change in the human cancer-cell line DLD-1) is lethal in haploid and homozygous diploid yeast. The cell death results from a catastrophic increase in spontaneous mutagenesis attributed to low-fidelity DNA synthesis by Polδ-R696W. Heterozygotes survive, and the mutation rate depends on the relative expression level of wild-type versus mutant alleles. Based on these observations, we propose that the mutation rate in heterozygous human cells could be regulated by transient changes in gene expression leading to a temporary excess of Polδ-R689W. The similarities between the mutational spectra of the yeast strains producing Polδ-R696W and DLD-1 cells suggest that the altered Polδ could be responsible for a significant proportion of spontaneous mutations in this cancer cell line. These results suggest that the highly error-prone Polδ-R689W could contribute to cancer initiation and/or progression in humans.
Keywords: mutator, SNP, transient hypermutagenesis, DNA synthesis fidelity, colon cancer
Maintaining high-fidelity DNA replication is imperative for cells to avoid mutations that can lead to disease. In eukaryotes, chromosomal DNA is replicated by the concerted action of three DNA polymerases, Polα, Polδ, and Polε, although their exact roles at the replication fork are currently a subject of debate (1). Replication fidelity is maintained by accurate nucleotide selectivity of the three replicative enzymes and the exonucleolytic proofreading functions of Polδ and Polε. Fidelity is further increased in trans through the action of the DNA mismatch repair (MMR) pathway. Mutations affecting the fidelity of yeast Polα, Polδ, and Polε result in a spontaneous mutator phenotype (2–6). In the case of Polδ, when specific nucleotide selectivity and proofreading defects were engineered in mice, the resulting increased mutation rate was also accompanied by an accelerated tumorigenesis (7–9). This provided support for the mutator hypothesis for cancer, which states that the expression of a mutator phenotype is an early event in tumorigenesis and that it is required for the accumulation of multiple mutations typically observed in cancers (10).
The aforementioned studies examined DNA polymerase mutations expressly engineered to destroy specific polymerase functions. These are dramatic mutations, which, to our knowledge, have not been identified as naturally occurring in any human cells. In contrast, SNPs are naturally occurring DNA sequence variations that are found in most genes. Whereas some SNPs may have no functional significance, others may affect protein function and/or structure. In the frame of the National Institute of Environmental Health Sciences (NIEHS) Environmental Genome Project, 22 SNPs resulting in nonsynonymous amino acid substitutions in the catalytic subunits of Polδ and Polε have been found with frequencies ranging from 0.6% to 41% in the normal population (http://www.genome.utah.edu/genesnps/). Earlier studies also reported 11 mutations in colon–cancer cell lines and sporadic colorectal carcinomas that change the amino–acid sequence of the catalytic subunit of Polδ (11, 12). The functional consequences of these variants are not known. There is, however, evidence that variants of other DNA polymerases, such as Polγ, Polη, and Rev1, are associated with disease in humans (13). In addition, variants of Polβ found in human cancers were shown to contribute to cellular transformation in mouse cells (13, 14). These findings point to the importance of the functional analysis of naturally occurring DNA polymerase mutations.
In this study, we used the genetically tractable yeast model to examine the effects of Polδ and Polε variants found in human cells on viability, growth, mutagenesis, and DNA damage sensitivity. The results reveal that one Polδ variant (Polδ-R696W, which is analogous to the human Polδ-R689W variant) leads to a catastrophic increase in genomic instability that is incompatible with life in haploid and homozygous diploid cells. We present the genetic and biochemical data suggesting that the mutation catastrophe was caused by extremely low-fidelity DNA synthesis by the altered Polδ.
Results
Effects of SNPs and the Cancer-Associated yR511H/hR506H Mutation of Polδ on Viability, Mutagenesis, and DNA Damage Sensitivity.
The structural and functional conservation of the DNA replication machinery provides an opportunity to evaluate the consequences of human DNA polymerase variants by studying analogous mutations in yeast. A similar approach has been used previously for the identification of potentially pathogenic MMR gene variants (15). The application of this approach, by definition, is largely limited to mutations that affect evolutionarily conserved protein domains. Of the 22 Polδ and Polε SNPs identified in the NIEHS Environmental Genome Project (http://www.genome.utah.edu/genesnps/), five are located in conserved regions (Fig. 1A). Additionally, the two cancer-associated Polδ mutations (R689W and R506H substitutions), present in the colon cancer cell line DLD-1 (11, 12), are in the conserved DNA polymerase III and exonuclease III motifs, respectively (Fig. 1 A and B). Remarkably, the degree of amino–acid sequence conservation was noticeably higher for the regions affected by the cancer-associated mutations than for the regions affected by SNPs (Fig. 1A). This is consistent with the expectation that the changes found in cancers may have a more severe effect on protein function than most SNPs found in the normal population.
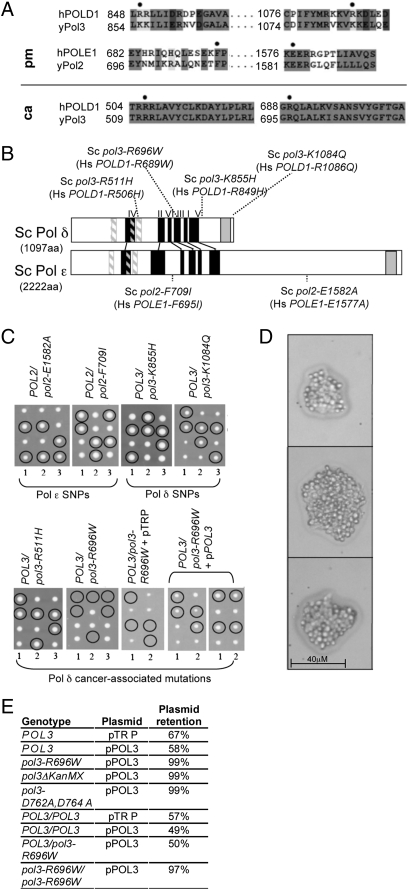
Fig. 1.
Effects of DNA polymerase mutations on viability. (A) Alignment of hPOLD1 and hPOLE1 with yPol3 and yPol2. Dots show the sites of polymorphisms (pm) and cancer-associated mutations (ca). Basic (light gray), invariant (medium gray), and acidic (dark gray) residues are highlighted. pm, polymorphisms; ca, cancer-associated mutations. (B) The catalytic subunits of yeast Polδ and ε with DNA polymerase (black), exonuclease (hatched), and zinc finger (gray) motifs indicated. Mutation locations are approximated. (C) Representative tetrad dissections of heterozygous diploids. Colonies harboring the indicated polymerase mutation are circled in black. (D) Dead cell groups formed by pol3-R696W haploid cells after tetrad dissection. (E) Frequency of plasmid retention in haploid and diploid yeast.
To assess the functional significance of Polδ and Polε variants that map to conserved regions, we constructed Saccharomyces cerevisiae strains with analogous changes in Polδ and Polε (Fig. 1B). The POL3 and POL2 genes encoding the catalytic subunits of these DNA polymerases are essential. Therefore, we began by determining the effect of the SNPs and cancer-associated mutations on cell growth and viability. Diploid strains heterozygous for the pol3 or pol2 mutations were generated, and after sporulation, tetrads were dissected to compare the colony growth of haploid cells expressing the mutant or wild-type polymerase alleles. The four SNPs examined and the cancer-associated mutation pol3-R511H had no apparent effect on viability or cell growth (Fig. 1C). We further examined these five DNA polymerase mutants with respect to spontaneous mutagenesis and DNA damage sensitivity. To reveal any subtle mutator phenotypes that may be masked by the action of MMR, we measured mutation rates in the absence of the MMR factor Msh6, which is involved in the recognition of base–base mismatches and small loops (16). The Polδ and Polε SNPs did not significantly increase the rate of mutation to canavanine resistance (Canr) in the presence or absence of MMR (Table S1). The cancer-associated mutation pol3-R511H led to a small but significant (2.5-fold) increase in the rate of the Canr mutation in the MMR-deficient strain Table S1). We observed no effects of any of the five mutations on the sensitivity of the cells to the DNA-damaging agent methyl methanesulfonate (MMS). Additionally, the pol3-R511H mutant was not appreciably sensitive to UV light, hydroxyurea, or hydrogen peroxide. Collectively, these results indicate that the pol3-K855H, pol3-K1084Q, pol2-F709I, and pol2-E1582A mutations do not significantly affect the function or fidelity of the respective polymerases. The subtle mutator effect of pol3-R511H suggests that this mutation slightly reduces the fidelity of DNA replication, which is compensated for in the wild-type cells by the action of MMR.
The pol3-R696W Mutation Is Lethal in Haploid and Homozygous Diploid Yeast.
In contrast to the other mutants examined, spores carrying the cancer-associated allele pol3-R696W failed to produce visible colonies (Fig. 1C). The lethality is rescued by the ectopic expression of POL3 (Fig. 1C), confirming that inviability is caused by the pol3-R696W mutation. The inviability of pol3-R696W could be caused by (i) severely impaired catalytic activity of Polδ or (ii) the accumulation of lethal mutations in other essential genes because of a dramatic decrease in the fidelity of Polδ. Such a “mutation catastrophe” has been previously described for yeast that are defective simultaneously in two replication error-avoidance mechanisms (exonucleolytic DNA polymerase proofreading and MMR) (17) and for E. coli carrying a defective exonuclease subunit of Pol III (18). If pol3-R696W encodes a nonfunctional polymerase, haploid cells expressing the mutant allele would not be able to replicate their DNA and divide, as observed previously (19) and in this study (Fig. S1), for cells completely lacking active Polδ. In the mutation–catastrophe scenario, lethal mutations would accumulate during replication, and pol3-R696W cells would be expected to proceed through a number of cell cycles before cell division ceases. This was previously observed for the proofreading- and MMR-deficient yeast cells (17). Microscopic examination after tetrad dissection showed that the pol3-R696W cells were able to divide and form microcolonies of varying size (estimated at ∼100 cells on average) before cell division stopped (Fig. 1D). These dead cell groups contained a mixture of cells at various stages of the cell cycle with no prevailing cell morphology. Counting cells from six microcolonies revealed on average 34% single, unbudded cells, 26% budded cells, 23% dumbbells, 11% aberrant, and 5% others (typically several cells that did not separate). The absence of a unique terminal morphology argues against a specific DNA replication defect or checkpoint activation as the cause of inviability. The capability of pol3-R696W cells to divide suggests that they can form a functional Polδ. Furthermore, the random nature of cell death is consistent with the accumulation of random mutations caused by the presence of a mutagenic DNA polymerase.
Because most mutations are recessive, diploid cells typically can tolerate higher levels of genome-wide mutagenesis than haploid cells. For example, the high spontaneous mutation rate resulting from a combination of defective Polδ proofreading and defective MMR is lethal in haploid but not diploid yeast cells (17). In surprising contrast, we found that, like haploids, the homozygous pol3-R696W/pol3-R696W diploids are inviable, which is indicated by their inability to lose the plasmid expressing an ectopic copy of POL3 (pPOL3) (Fig. 1E).
Mutator Effect of the pol3-R696W Allele.
To determine if both pol3-R696W haploids and pol3-R696W/pol3-R696W diploids die from a high level of mutagenesis, we examined the rate of spontaneous mutation in strains expressing the mutant allele. Because pol3-R696W is lethal when it is the sole source of Pol3, we measured the mutation rate under conditions where the expression of pol3-R696W from the natural chromosomal promoter is the primary source of Pol3 and the expression of the plasmid POL3 from the GAL1 promoter is repressed by the presence of glucose. Leaky GAL1-POL3 expression is sufficient to rescue the lethality while it diminishes competition by wild-type Pol3, which may mask pol3-R696W effects. Under these conditions, the growth of pol3-R696W-expressing cells is similar to the wild type. The rate of spontaneous mutation was increased in both haploid and diploid pol3-R696W mutants for all reporter genes tested (Table 1 and Table S2). Most remarkably, the rate of Canr mutation was increased at least 65- and 200-fold in two independently derived homozygous pol3-R696W/pol3-R696W diploids compared with wild-type diploids (Table 1). The slight difference in the mutation rate between the two diploids may reflect the differences in the genetic background of the strains used or the contribution of modifier mutations, which would likely accumulate in the mutator strains. Notably, can1 mutations are recessive, so two CAN1 alleles must be inactivated to generate a Canr mutant. Sequencing of 10 Canr mutants revealed that all resulted from simultaneous inactivation of both alleles by independent mutations rather than from the mutational inactivation of one allele followed by a loss of heterozygosity. The mutations are described in Table 2 and Table S3, and examples of sequencing electrophoregrams showing the heterozygosity at the mutation sites are shown in Fig. S2. The observed >65- and >200-fold increases in the rate of Canr mutation in the pol3-R696W/pol3-R696W diploids, therefore, translate to at least a 6,000- and 11,000-fold increase in the rate of mutation at a single CAN1 allele over the wild-type mutation rate. Even more striking, this supermutator effect is seen in the presence of wild-type Polδ that presumably reduces the contribution of mutagenic Polδ-R696W to replication. Calculations shown in the SI Text suggest that the high mutation rate in the haploid and diploid pol3-R696W mutants is expected to be incompatible with life. The spectrum of spontaneous mutations in the pol3-R696W/pol3-R696W diploid reveals a distinct mutational signature: 15 of 17 base substitutions resulted in the change to a T•A base pair (Table 2 and Table S3).
Table 1.
Mutator effects of pol3-R696W, pol3-D762A,D764A, and pol3ΔKanMX alleles in haploid and diploid cells
| Genotype | pPOL3 | Canr mutation (x 10−7)* |
| POL3 | + | 2.4 (2.2–3.2) |
| pol3-R696W | + | 67 (60–73) |
| pol3-R696W rev3 | + | 58 (45–89) |
| pol3ΔKanMX | + | 6.7 (5.4–17) |
| pol3-D762A,D764A | + | 7.6 (6.9–9.1) |
| POL3/POL3 | − | <0.3 |
| POL3/pol3-R696W | − | <0.3 |
| POL3/POL3 | + | <0.3 |
| POL3/pol3-R696W | + | <0.3 |
| pol3-R696W/pol3-R696W (1)† | + | 69 (56–90) |
| pol3-R696W/pol3-R696W (2)† | + | 20 (13–28) |
| pol3-R696W/pol3-R696W rev3/rev3 | + | 41 (36–51) |
| pol3ΔKanMX/pol3-R696W | + | 5.4 (4.2–6.0) |
*The values are medians for at least nine cultures. The 95% confidence limits are given in parentheses.
†pol3-R696W/pol3-R696W (1) and (2) were constructed using PSD93 and E134×1B-D770 strains, respectively.
Table 2.
Spontaneous can1 mutations in pol3-R696W/pol3-R696W diploids
| can1 isolate | Nucleotide changes |
| 1 | C684A |
| 2 | G1018A, C1598A |
| 3 | T649C, G670A, C1240A, T1676C |
| 4 | G687A, G1261A |
| 5 | G1018A |
| 6 | G1018A |
| 7 | G671A, G718A |
| 8 | C648A |
| 9 | G671A, C732A |
| 10 | C1426T |
All recovered mutations were in the heterozygous state (see Fig. S2). Only nucleotides 618–1773 of CAN1 were analyzed, so the isolates could contain additional undetected mutations.
Previous studies illustrate that a low level of functional Polδ can promote mutagenesis (20). The R696W substitution could potentially reduce the stability or the catalytic activity of Polδ to the extent that the pPOL3-rescued pol3-R696W cells contain a low total level of functional Polδ under our experimental conditions. The normal growth rate and insensitivity of the cells to hydroxyurea argue that replication is not severely affected under these conditions. We, however, tested the possibility that the mutator effect of pol3-R696W was caused by a decreased level of Polδ rather than the low fidelity of the Polδ-R696W. We measured mutagenesis in pPOL3-rescued pol3Δ and pol3-D762A,D764A mutants that carry no chromosomally encoded Pol3 and the catalytically inactive Pol3, respectively. Similar to the experiments with the pol3-R696W mutants, the leaky expression of the plasmid GAL1-POL3 on glucose-containing medium provided functional Polδ to rescue the viability of these strains. If the strong mutator phenotype of pol3-R696W cells was caused by a low level of active Polδ, then the same strong mutator effect should be seen when no functional Pol3 is encoded at the chromosomal locus. In contrast, we observed only a 3-fold increase in the rate of Canr mutation in pPOL3-rescued pol3-D762A,D764A or pol3Δ mutants (Table 1). This nominal increase (compared with the 30-fold increase in pol3-R696W) suggests that the mutator effect in pol3-R696W cells is specifically attributed to the presence of the Polδ-R696W.
We have shown previously that many mutations in replicative DNA polymerases can lead to an increased spontaneous mutation rate indirectly through the recruitment of the error-prone Polζ (21). To determine if any of the spontaneous mutagenesis in pol3-R696W cells is caused by the activity of Polζ, we measured the mutator effect of pol3-R696W in a rev3 strain, which lacks the catalytic subunit of Polζ. The Polζ− derivative still showed the same high level of mutagenesis (Table 1). This is consistent with the idea that the mutator effect of pol3-R696W results from inaccurate DNA synthesis by Polδ-R696W itself rather than from the recruitment of a specialized polymerase.
Polδ-R696W Is an Error-Prone DNA Polymerase.
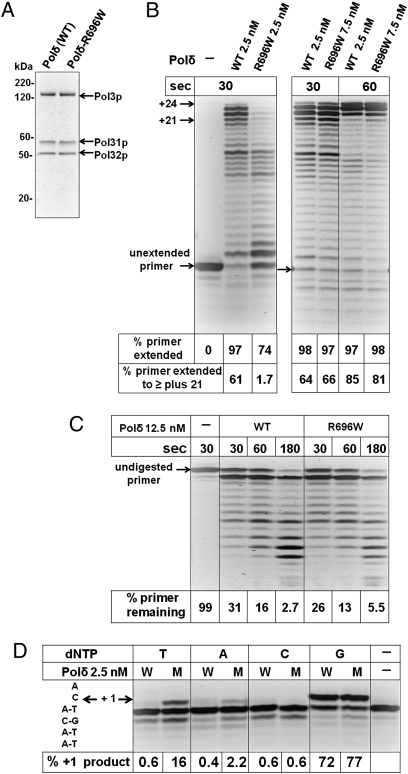
We used a biochemical approach to determine whether or not the pol3-R696W allele does indeed encode for an error-prone DNA polymerase. We purified recombinant three-subunit yeast Polδ and its R696W variant, and then, we compared their catalytic activity and their ability to misincorporate nucleotides using oligonucleotide DNA substrates (Fig. 2). The R696W substitution did not affect the ability of the catalytic subunit (Pol3p) to associate with the accessory subunits Pol31p and Pol32p, as indicated by the similar subunit stoichiometry in the Polδ and Polδ-R696W preparations (Fig. 2A). In a primer extension assay using an oligonucleotide template, Polδ-R696W showed a reduced DNA polymerase activity in comparison with the wild-type Polδ. When equal concentrations of the enzymes were used, the Polδ-R696W reaction products contained significantly more unextended and less fully extended substrate (Fig. 2B Left). However, increasing the concentration of Polδ-R696W by 3-fold resulted in a comparable amount of primer extension (Fig. 2B Right). Multiple rounds of DNA synthesis initiation are not prohibited in these experiments. Thus, the decreased activity of Polδ-R696W could reflect less efficient nucleotide incorporation, reduced enzyme cycling, or both. Unlike the DNA polymerase activity, 3′-exonuclease activity of Polδ was not significantly affected by the R696W substitution (Fig. 2C).
Fig. 2.
Polδ-R696W is a functional but highly error-prone DNA polymerase. (A) SDS/PAGE analysis of purified recombinant Polδ and Polδ-R696W. The proteins were visualized by Coomassie staining. WT, wild-type Polδ. (B) DNA polymerase activity of Polδ and Polδ-R696W was assayed on an oligonucleotide substrate as described in Materials and Methods. Incubation time and enzyme concentration is indicated. The percentage of primer extended (+1 products and longer) and the percentage of primer extended nearly to the end of the template (+21 and longer) are shown below each lane. (C) Analysis of exonuclease activity of Polδ and Polδ-R696W. The percentage of substrate remaining is shown below each lane. (D) Specificity of nucleotide incorporation by Polδ and Polδ-R696W. Nucleotide sequence of the DNA substrate in the vicinity of the primer–template junction is shown to the left of the gel image. Wild-type Polδ or Polδ-R696W were incubated with this substrate in the presence of a single dNTP (T, A, C, G) as indicated for 30 s. The percentage of primer extension (+1 product) is shown below each lane. W, wild-type Polδ; M, Polδ-R696W.
To investigate whether or not Polδ-R696W has a reduced DNA synthesis fidelity, we compared the abilities of Polδ and Polδ-R696W to insert an incorrect nucleotide in a primer extension assay (Fig. 2D). The oligonucleotide substrate and the reaction conditions were identical to those used for the DNA polymerase activity assay in Fig. 2B, except that only a single nucleotide was present. In the reaction containing dGTP complementary to the template C, Polδ and Polδ-R696W generated a +1 product with similar efficiency. The Polδ-R696W, however, also had a profound ability to extend the primer using a noncomplementary dTTP and to a lesser extent, dATP. The amount of misinsertion is likely to be underestimated in these experiments, because some of the +1 product could be degraded by the exonuclease activity of the enzymes. Neither enzyme showed a high rate of dCMP misinsertion in this assay. The error specificity of Polδ-R696W in vitro, thus, is in excellent agreement with the spectrum of spontaneous mutations seen in the pol3-R696W/pol3-R696W mutant in vivo (CG to AT transversions and CG to TA transitions) (Table 2 and Table S3). The sequence of the DNA substrate used in the in vitro assays mimicked the CAN1 gene sequence around position 732 where a C to A substitution was observed in the pol3-R696W/pol3-R696W diploid. The use of this particular sequence context could explain the fact that dTTP was more readily inserted at this site than dATP by Polδ-R696W (Fig. 2D).
The Mutator Effect of the pol3-R696W Allele Depends on the Relative Levels of Polδ-R696W and the Wild-Type Polδ.
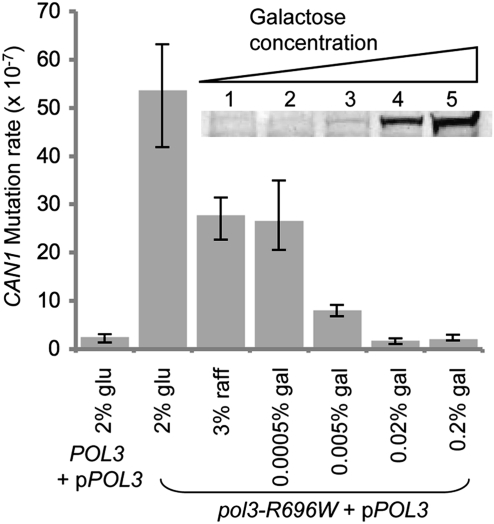
Remarkably, in contrast to the high mutagenesis in pol3-R696W strains rescued with the low expression of wild-type POL3, the mutation rate in the heterozygous pol3-R696W/POL3 diploid strains was at least two orders of magnitude lower (Table 1 and Table S2). This suggests that (i) Polδ-R696W is not able to compete efficiently with the wild-type Polδ when the latter is produced at normal endogenous levels in heterozygous diploids, and (ii) the mutagenicity of Polδ-R696W becomes a factor when the ratio of mutant to wild-type Pol3 is shifted toward the mutagenic polymerase as in the pPOL3-rescued haploids. If this hypothesis is correct, we should be able to suppress the mutator phenotype of the haploid pol3-R696W strain by increasing wild-type POL3 expression. To test this, we measured spontaneous mutagenesis in pol3-R696W cells containing pPOL3 and grown at various galactose concentrations, which would modulate the level of the wild-type POL3 expression. As expected, increasing the expression of wild-type POL3 (Fig. 3 Inset) leads to a concurrent decrease in the rate of spontaneous mutagenesis in the pol3-R696W strain (Fig. 3). By contrast, in wild-type cells containing a galactose-inducible pol3-R696W allele, mutagenesis was stimulated on galactose-containing medium (Fig. S3). Moreover, diploids with two chromosomal pol3-R696W alleles and the plasmid wild-type POL3 had a 4- to 12-fold higher mutation rate than diploids with one chromosomal pol3-R696W, a deletion of the POL3 locus on the homologous chromosome, and the plasmid wild-type POL3 (Table 1). This suggests that even small changes in the ratio of Polδ-R696W to wild-type Polδ can significantly affect mutagenesis.
Fig. 3.
Increasing the level of wild-type Polδ mitigates the mutagenic potential of Polδ-R696W. The rate of can1 mutation at different galactose concentrations was determined for haploid cells expressing the chromosomal pol3-R696W allele from the natural promoter and wild-type plasmid-borne POL3 from the galactose-inducible GAL1 promoter. The rates are medians for at least nine cultures. Error bars show the 95% confidence intervals. The inset Western blot shows increasing Pol3 production with increasing galactose concentration (lane 1, 3% raffinose; lane 2, 0.0005% galactose; lane 3, 0.005% galactose; lane 4, 0.02% galactose; lane 5, 0.2% galactose). A total of 30 μg of protein extract was analyzed for each condition.
Discussion
High-fidelity DNA replication is essential for mitigating cancer risk by reducing the DNA mutation load. Because single amino acid changes in Polα, Polδ, and Polε can dramatically affect DNA polymerase fidelity (2, 5, 6, 22–24), understanding the functional consequences of naturally occurring variants of these polymerases provides invaluable knowledge of potential cancer risk for individuals with these variants. To this end, we examined reported SNPs and cancer-associated mutations affecting Polδ and Polε. We showed that yeast analogs of the following human variants do not significantly affect the function (Fig. 1C) of the respective DNA polymerases or mutagenesis (Table S1): Polδ-K849H, Polδ-K1086Q, Polε-F695I, and Polε-E1577A. The relatively mild effect of the human cancer-associated mutation Polδ-R506H (yeast Polδ-R511H) is somewhat surprising, because the corresponding arginine residue is part of the highly conserved ExoIII motif essential for the proofreading activity of Polδ. Any proofreading defect is expected to result in increased spontaneous mutagenesis, particularly in combination with the MMR defect (17, 25). In the recently reported crystal structure of the yeast Pol3, however, Arg511 is found away from the exonuclease active site (26). This may explain why the function of the exonuclease domain is not severely compromised in the R511H variant. The nearly wild-type phenotype of the other Polδ and Polε mutants is consistent with the presence of the corresponding mutations in normal human population and in less conserved regions of the proteins.
One Polδ mutant, R696W (analogous to human R689W), forms a functional but extremely inaccurate DNA polymerase. We present several pieces of evidence that support this conclusion. First, yeast cells containing the mutant allele as the only source of Pol3 die after multiple cell divisions (Fig. 1 C–E). Second, the pol3-R696W mutants rescued by a low-level expression of wild-type POL3 show a strong mutator phenotype (Table 1). Third, purified Polδ-R696W has only a moderately reduced DNA polymerase activity but a dramatically increased nucleotide misinsertion rate (Fig. 2). Taken together, these observations provide strong support for the idea that the death of the pol3-R696W mutants occurs because of the accumulation of random mutations during DNA replication.
These results indicate that Arg696 is a crucial residue for proper Polδ function. Defects in several DNA polymerase functions, including nucleotide selectivity, exonuclease, processivity, and protein–protein interactions, could contribute to the high mutagenicity of this polymerase. The initial biochemical characterization of Polδ-R696W reported here suggested that this Polδ mutant is severely compromised at the stage of nucleotide selectivity. Arg696 is located in the fingers domain but does not constitute a part of the nascent base pair–binding pocket that plays a primary role in the high fidelity of Polδ (26). We speculate that the replacement of the basic Arg with a neutral aromatic Trp residue alters the structure of the active site in a way that allows misinsertion. It has also been suggested that the R696W change could affect the partitioning of the primer terminus between the polymerase and exonuclease active sites (26). Future, more extensive biochemical and structural studies will help define the molecular basis for the low fidelity of Polδ-R696W as well as the possible effects of the accessory proteins, such as PCNA, on the activity and fidelity of this polymerase. Arg696 residue is conserved in some of the other B family DNA polymerases, including Polα and Polζ (Table S4), suggesting that its function in DNA synthesis fidelity likely extends to other DNA polymerases as well.
The human Polδ-R689W substitution was originally identified in the colon cancer cell line DLD-1 (12). The DLD-1 cell line is a MMR-deficient cell line that displays microsatellite instability and increased spontaneous mutagenesis (27). In addition to the mutation of the MMR gene MSH6, this heterogeneous cell line has four documented, heterozygous Polδ mutations, including the R506H and R689W mutations examined here (12, 28). Experiments with the derivatives of DLD-1 expressing exogenous MSH6 and POLD1 showed that, although the MSH6 defect is responsible for a significant fraction of the mutator phenotype of this cell line, the contribution of the POLD1 mutations could not be excluded (28–30). About half of the spontaneous hprt gene mutations in the DLD-1 cells are GC→TA transversions and GC→AT transitions (29, 31, 32), which constitute the characteristic mutational signature of the yeast pol3-R696W cells (Table 2 and Table S3). The proportion of these mutations becomes even higher when the MMR defect of DLD-1 cells is suppressed by exogenous expression of MSH6 (29). The DLD-1 cells are also known to carry homozygous, nonsilent mutations in the APC gene, as well as mutations affecting RAS, p53, and αE-catenin (12, 33–35). All of them, with the exception of the APC mutations, are GC→AT transitions or GC→TA transversions. These observations are consistent with the idea that the putative Polδ-R689W mutator could contribute to the generation of these mutations and tumorigenesis.
Interestingly, the heterozygous POL3/pol3-R696W diploid yeast strain did not show a strong mutator phenotype (Table 1 and Table S2). This result is not completely unexpected, because recessive or nearly recessive DNA polymerase mutations have been reported previously (17, 36). Titration of wild-type POL3 expression in pol3-R696W cells (Fig. 3) and the analysis of DNA polymerase activity of the purified enzymes (Fig. 2) revealed that Polδ-R696W is likely unable to compete efficiently with wild-type Polδ when the latter is produced at normal endogenous levels. The dependence of mutagenesis on the relative levels of wild-type Polδ and Polδ-R696W (human Polδ-R689W) has important implications for human cancer cells that are frequently aneuploid and/or could have other allele dosage alterations. Additionally, the dosage dependence of Polδ-R689W is relevant to the concept of transient hypermutability in cancers. This concept suggests that tumors that do not display high mutator phenotypes may have accumulated transforming mutations during a transient burst of hypermutability (37). Although largely speculative at this point, this transient hypermutability may arise because of the transient up-regulation of a stress response (38, 39), random translational or transcriptional errors that generate aberrant proteins, or deregulation of various processes (37). Along those lines, deregulation of transcription that results in a transcriptional bias for the expression of POLD1-R689W versus the wild-type POLD1 allele in heterozygous humans could tip the balance to a hypermutable state, thereby generating cancer-initiating or metastasis-promoting mutations. Thus, given the critical roles that mutations and mutator phenotypes are postulated to play in cancer development (40), our study points to the importance of future investigations of the causative relationship between the Polδ-R689W variant and cancer in humans.
Materials and Methods
All S. cerevisiae strains used in this study are isogenic to the haploid strains E134 (MATα ade5-1 lys2::InsEA14 trp-289 his7-2 leu2-3,112 ura3-52) or 1B-D770 (MATa ade5-1 lys2-Tn5-13 trp1-289 his7-2 leu2-3,112 ura3-4) (41). Heterozygous diploid pol3 or pol2 mutants were constructed by replacing one chromosomal copy of the corresponding gene with the mutant allele (confirmation primers listed in Table S5), Table S5 and haploids were obtained by tetrad dissection. The rate of forward mutation to Canr was measured by fluctuation analysis as described previously (41). Immunoblot analysis of whole-cell extracts was performed as described (21) with rabbit polyclonal antiserum to yeast Polδ (42) provided by Peter Burgers (St. Louis, MO). Polδ and Polδ-R696W were overproduced and purified as previously described (43) with minor modification (see SI Text). DNA substrates for DNA polymerase and exonuclease assays were created by annealing a Cy5-labeled primer to an oligonucleotide template, and reactions were performed at 30°C with 40 mM Tris-HCl (pH 7.8), 125 mM NaAc, 8 mM MgAc, 1 mM DTT, 0.2 mg/mL BSA, 4% polyethylene glycol 8000, 25 nM DNA substrate, and 100 μM each dNTPs. For exonuclease assays, dNTPs and polyethylene glycol were omitted. A more thorough description of methods can be found in the SI Text.
Supplementary Material
Acknowledgments
We thank Peter Burgers for POL3, POL31, and POL32 overexpression plasmids and Polδ antiserum, Victoria Liston and Corinn Grabow for technical assistance, and Tadayoshi Bessho and Youri Pavlov for critical manuscript reading. This work was supported by the Eppley Institute for Research in Cancer and Allied Diseases and by the National Institutes of Health Grant ES015869 (to P.V.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907526106/DCSupplemental.
References
- 1.Pavlov YI, Shcherbakova PV. DNA polymerases at the eukaryotic fork—20 years later. Mutat Res. 2009 doi: 10.1016/j.mrfmmm.2009.08.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nick McElhinny SA, Stith CM, Burgers PM, Kunkel TA. Inefficient proofreading and biased error rates during inaccurate DNA synthesis by a mutant derivative of Saccharomyces cerevisiae DNA polymerase δ. J Biol Chem. 2007;282:2324–2332. doi: 10.1074/jbc.M609591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon M, Giot L, Faye G. The 3′ to 5′ exonuclease activity located in the DNA polymerase δ subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J. 1991;10:2165–2170. doi: 10.1002/j.1460-2075.1991.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison A, Bell JB, Kunkel TA, Sugino A. Eukaryotic DNA polymerase amino acid sequence required for 3′—5′ exonuclease activity. Proc Natl Acad Sci USA. 1991;88:9473–9477. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pursell ZF, Isoz I, Lundström EB, Johansson E, Kunkel TA. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niimi A, et al. Palm mutants in DNA polymerases α and η alter DNA replication fidelity and translesion activity. Mol Cell Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldsby RE, et al. High incidence of epithelial cancers in mice deficient for DNA polymerase δ proofreading. Proc Natl Acad Sci USA. 2002;99:15560–15565. doi: 10.1073/pnas.232340999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan RN, et al. Mutation at the polymerase active site of mouse DNA polymerase δ increases genomic instability and accelerates tumorigenesis. Mol Cell Biol. 2007;27:7669–7682. doi: 10.1128/MCB.00002-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldsby RE, et al. Defective DNA polymerase-δ proofreading causes cancer susceptibility in mice. Nat Med. 2001;7:638–639. doi: 10.1038/88963. [DOI] [PubMed] [Google Scholar]
- 10.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 11.da Costa LT, et al. Polymerase δ variants in RER colorectal tumours. Nat Genet. 1995;9:10–11. doi: 10.1038/ng0195-10. [DOI] [PubMed] [Google Scholar]
- 12.Flohr T, et al. Detection of mutations in the DNA polymerase δ gene of human sporadic colorectal cancers and colon cancer cell lines. Int J Cancer. 1999;80:919–929. doi: 10.1002/(sici)1097-0215(19990315)80:6<919::aid-ijc19>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Sweasy JB, Lauper JM, Eckert KA. DNA polymerases and human diseases. Radiat Res. 2006;166:693–714. doi: 10.1667/RR0706.1. [DOI] [PubMed] [Google Scholar]
- 14.Lang T, Dalal S, Chikova A, DiMaio D, Sweasy JB. The E295K DNA polymerase β gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol Cell Biol. 2007;27:5587–5596. doi: 10.1128/MCB.01883-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou J, et al. Functional analysis helps to clarify the clinical importance of unclassified variants in DNA mismatch repair genes. Hum Mutat. 2007;28:1047–1054. doi: 10.1002/humu.20580. [DOI] [PubMed] [Google Scholar]
- 16.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 17.Morrison A, Johnson AL, Johnston LH, Sugino A. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 1993;12:1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fijalkowska IJ, Schaaper RM. Mutants in the Exo I motif of Escherichia coli dnaQ: Defective proofreading and inviability due to error catastrophe. Proc Natl Acad Sci USA. 1996;93:2856–2861. doi: 10.1073/pnas.93.7.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokoska RJ, Stefanovic L, DeMai J, Petes TD. Increased rates of genomic deletions generated by mutations in the yeast gene encoding DNA polymerase δ or by decreases in the cellular levels of DNA polymerase δ. Mol Cell Biol. 2000;20:7490–7504. doi: 10.1128/mcb.20.20.7490-7504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northam MR, Garg P, Baitin DM, Burgers PM, Shcherbakova PV. A novel function of DNA polymerase ζ regulated by PCNA. EMBO J. 2006;25:4316–4325. doi: 10.1038/sj.emboj.7601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Q, Copeland WC, Wang TS. Mutational studies of human DNA polymerase α. Identification of residues critical for deoxynucleotide binding and misinsertion fidelity of DNA synthesis. J Biol Chem. 1993;268:24163–24174. [PubMed] [Google Scholar]
- 23.Dong Q, Copeland WC, Wang TS. Mutational studies of human DNA polymerase α. Serine 867 in the second most conserved region among α-like DNA polymerases is involved in primer binding and mispair primer extension. J Biol Chem. 1993;268:24175–24182. [PubMed] [Google Scholar]
- 24.Limsirichaikul S, et al. The Gly-952 residue of Saccharomyces cerevisiae DNA polymerase α is important in discriminating correct deoxyribonucleotides from incorrect ones. J Biol Chem. 2003;278:19079–19086. doi: 10.1074/jbc.M208604200. [DOI] [PubMed] [Google Scholar]
- 25.Morrison A, Sugino A. The 3′—>5′ exonucleases of both DNA polymerases δ and ε participate in correcting errors of DNA replication in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 26.Swan MK, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase δ. Nat Struct Mol Biol. 2009;16:979–986. doi: 10.1038/nsmb.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya NP, Skandalis A, Ganesh A, Groden J, Meuth M. Mutator phenotypes in human colorectal carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:6319–6323. doi: 10.1073/pnas.91.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yabuta T, et al. Effect of exogenous MSH6 and POLD1 expression on the mutation rate of the HPRT locus in a human colon cancer cell line with mutator phenotype, DLD-1. Int J Oncol. 2004;24:697–702. [PubMed] [Google Scholar]
- 29.Lettieri T, et al. Effect of hMSH6 cDNA expression on the phenotype of mismatch repair-deficient colon cancer cell line HCT15. Carcinogenesis. 1999;20:373–382. doi: 10.1093/carcin/20.3.373. [DOI] [PubMed] [Google Scholar]
- 30.Umar A, et al. Correction of hypermutability, N-methyl-N’-nitro-N-nitrosoguanidine resistance, and defective DNA mismatch repair by introducing chromosome 2 into human tumor cells with mutations in MSH2 and MSH6. Cancer Res. 1997;57:3949–3955. [PubMed] [Google Scholar]
- 31.Malkhosyan S, McCarty A, Sawai H, Perucho M. Differences in the spectrum of spontaneous mutations in the hprt gene between tumor cells of the microsatellite mutator phenotype. Mutat Res. 1996;316:249–259. doi: 10.1016/s0921-8734(96)90007-7. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya NP, et al. Molecular analysis of mutations in mutator colorectal carcinoma cell lines. Hum Mol Genet. 1995;4:2057–2064. doi: 10.1093/hmg/4.11.2057. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues NR, et al. p53 mutations in colorectal cancer. Proc Natl Acad Sci USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen SJ, et al. The αE-catenin gene (CTNNA1) acts as an invasion-suppressor gene in human colon cancer cells. Oncogene. 1999;18:905–915. doi: 10.1038/sj.onc.1202348. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov YI, Maki S, Maki H, Kunkel TA. Evidence for interplay among yeast replicative DNA polymerases alpha, delta and epsilon from studies of exonuclease and polymerase active site mutations. BMC Biol. 2004;2:11. doi: 10.1186/1741-7007-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake JW, Bebenek A, Kissling GE, Peddada S. Clusters of mutations from transient hypermutability. Proc Natl Acad Sci USA. 2005;102:12849–12854. doi: 10.1073/pnas.0503009102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards B, Zhang H, Phear G, Meuth M. Conditional mutator phenotypes in hMSH2-deficient tumor cell lines. Science. 1997;277:1523–1526. doi: 10.1126/science.277.5331.1523. [DOI] [PubMed] [Google Scholar]
- 39.Meuth M, Richards B, Schneider B. The conditional mutator phenotype in human tumor cells: Correction. Science. 1999;283:641. doi: 10.1126/science.283.5402.639d. [DOI] [PubMed] [Google Scholar]
- 40.Loeb LA, Bielas JH, Beckman RA. Cancers exhibit a mutator phenotype: Clinical implications. Cancer Res. 2008;68:3551–3557. doi: 10.1158/0008-5472.CAN-07-5835. [DOI] [PubMed] [Google Scholar]
- 41.Shcherbakova PV, Kunkel TA. Mutator phenotypes conferred by MLH1 overexpression and by heterozygosity for mlh1 mutations. Mol Cell Biol. 1999;19:3177–3183. doi: 10.1128/mcb.19.4.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer GA, Heller HM, Burgers PM. DNA polymerase III from Saccharomyces cerevisiae. I. Purification and characterization. J Biol Chem. 1988;263:917–924. [PubMed] [Google Scholar]
- 43.Fortune JM, Stith CM, Kissling GE, Burgers PM, Kunkel TA. RPA and PCNA suppress formation of large deletion errors by yeast DNA polymerase δ. Nucleic Acids Res. 2006;34:4335–4341. doi: 10.1093/nar/gkl403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.