Abstract
A common topology found in many bistable genetic systems is two interacting positive feedback loops. Here we explore how this relatively simple topology can allow bistability over a large range of cellular conditions. On the basis of theoretical arguments, we predict that nonlinear interactions between two positive feedback loops can produce an ultrasensitive response that increases the range of cellular conditions at which bistability is observed. This prediction was experimentally tested by constructing a synthetic genetic circuit in Escherichia coli containing two well-characterized positive feedback loops, linked in a coherent fashion. The concerted action of both positive feedback loops resulted in bistable behavior over a broad range of inducer concentrations; when either of the feedback loops was removed, the range of inducer concentrations at which the system exhibited bistability was decreased by an order of magnitude. Furthermore, bistability of the system could be tuned by altering growth conditions that regulate the contribution of one of the feedback loops. Our theoretical and experimental work shows how linked positive feedback loops may produce the robust bistable responses required in cellular networks that regulate development, the cell cycle, and many other cellular responses.
Keywords: bistability, genetic network, synthetic biology, ultrasensitivity, hysteresis
Bistable genetic systems display a discontinuity of expression states, where two distinct stable steady states are obtained without the presence of stable intermediate steady states. The previous history of the system determines which stable steady state is occupied. One of the important problems in systems biology is to understand how genetic bistability is established and regulated. This is because bistable genetic switches play an important role in a variety of cellular processes, such as cellular oscillators, progression through the eukaryotic cell cycle, and the development of differentiated cell and tissue types in organisms ranging from the temperate bacteriophage to the human (1–7). Many previous studies have focused on whether a given circuit topology has the capacity to display bistability for some range of environmental conditions (e.g., refs. 8–10). Although the possibility of bistable behavior is important, it is also important that the range of environmental conditions at which it occurs be large enough to achieve practical control of biological processes. Here, we focus upon identification and manipulation of the parameters that control the range of environmental conditions at which bistablity is obtained for systems known to be capable of bistability. We use the methods of synthetic biology to create model experimental systems to address the functions of multiple positive feedback loops in bistability.
Theoretical studies have argued that the minimal requirements for genetic bistability are twofold. First, there must be some type of positive feedback controlling gene expression. Examples of positive feedback are when an activator protein drives its own expression or when an even number of linked negative regulatory steps are present, such as when a repressor blocks the expression of a repressor of its own expression. Second, the kinetic order or sensitivity of the system to the positive feedback element must be high (8, 10–12). For example, in the simple case where a transcriptional activator drives its own expression, the response of the transcriptional promoter to the concentration of activator protein must display a kinetic order or sensitivity greater than one (8, 10–12). If these minimal requirements are met, bistability may be anticipated to occur under some environmental conditions.
An important example of a bistable genetic system consisting of a single positive feedback loop was provided by the work of Novick and Weiner, who studied the so-called “preinduction effect” of the lacZYA operon in Escherichia coli (13). The lac operon preinduction effect is observed when the operon is induced by nonphysiological xenobiotic inducers, known as gratuitous inducers, that are not metabolized by the cell but can bind to the LacI repressor protein and cause it to release the lac operator DNA. The preinduction effect refers to the observation that a higher concentration of gratuitous inducer was required for the induction of naive (uninduced) cells than was required to maintain preinduced cells in the fully induced state. At certain concentrations of inducer, known as the maintenance concentrations, naive cells remained uninduced, and previously induced cells remained in the induced state, indefinitely (13). Even when cultures showed an intermediate level of induction, the lacZYA operon was either fully on or fully off in the individual cells (13, 14). Although the original lacZYA operon preinduction effect experiments were performed using a chemostat and with TMG as the inducer, we show in the supporting information that the effect can be demonstrated using standard flask-grown cultures and with IPTG as the inducer (Fig. S1). Mathematical modeling has confirmed the mechanism of bistability in the lacZYA operon and confirmed the role of both positive feedback and high sensitivity (14–17). This system remains the most well-characterized and widely used example of cellular bistability.
The lacZYA operon preinduction effect is due to the positive feedback of the lacY product, galactoside permease, on its own expression (13, 18). This permease allows the gratuitous inducer to enter into the cell. When cells lack the galactoside permease, as in the naive state, a high concentration of gratuitous inducer is required for induction. However, upon induction of the operon, the cells come to acquire many molecules of the galactoside permease that can bring about further internalization of the inducer (positive feedback). The presence of permease protein molecules allows the induced cells to maintain a high intracellular concentration of the inducer, even when the extracellular concentration is low. As expected, the lac preinduction effect is eliminated upon mutation of the lacY gene (18). Furthermore, the preinduction effect is minimized under conditions where the function of the LacY protein is down-regulated (19). Inhibition of the LacY permease activity occurs when the PTS component and signal-transduction protein EIIAglc is present in its unphosphorylated state and binds to LacY (20). This occurs when the cell is grown in the presence of PTS sugars, such as glucose, and, to lesser extents, when the cell is grown in the presence of other substrates that exert catabolite repression (21). Thus, the lac preinduction effect was not discernible in glucose-grown cells (Fig. S1). Even in succinate-grown cells, the lac preinduction effect was a fairly weak bistability; in an experiment using IPTG as the inducer, the range of inducer concentrations at which bistability was observed was narrow [about 4-fold in flask-grown cells (Fig. S1)]. Indeed, in flask-grown cells, it was difficult to demonstrate a maintenance concentration of IPTG (Fig. S1).
In nature, simple bistable systems with a single positive feedback loop are rarely encountered; instead, natural systems are complex and contain multiple feedback loops that could be direct or indirect and using a variety of biochemical mechanisms, in combination with additional regulatory mechanisms. Fourteen examples of such systems have been noted by Brandman et al. (22). For example, in the genetic system that controls progression through the cell cycle, the mitotic trigger protein Cdc2 participates in three positive feedback loops (Cdc2 → Cdc25 → Cdc2; Cdc2-| Wee-1-| Cdc2; Cdc2-| Myt1-| Cdc2) (22). Similarly, for traversal of the start of the cell cycle in budding yeast, the Cdc28 protein participates in two positive feedback loops (Cdc28-| SicI-| Cdc28; Cdc28 → Cln → Cdc28). Other systems showing multiple positive feedback loops include those responsible for p53 regulation, Xenopus oocyte maturation, mammalian calcium signal transduction, eukaryotic chemotaxis, B cell fate specification, EGF receptor signaling, blood clotting, and platelet aggregation (noted in ref. 22). The Bcl2 apoptotic switch provides an additional example, where two independent positive feedback loops participate in producing bistability (23).
Foundational work on the behavior of biological systems containing multiple feedback loops was presented by Thomas and D’Ari (24). This work showed how the presence of multiple feedback loops could lead to an unexpectedly large number of steady states.
Two additional hypotheses for the presence of multiple positive feedback loops are that different timescales of the loops provide for resistance to noise under certain circumstances (22) or make bistability robust to certain parameter variations (23). Ferrell has noted that coherent linkage of a positive feedback loop and a double-negative feedback loop in a system of opposing enzymes could result in bistability over a wide range of conditions (10). In natural systems with complex circuit architectures, testing the roles of the multiple positive feedback loops is nontrivial and much of the work in this area to date has been purely theoretical.
We developed an experimental multiloop system by combining the “activator module” of the synthetic genetic oscillator of Atkinson et al. (12) with the galactoside permease feedback loop of the lacZYA operon. The activator module of the Atkinson et al. oscillator, when placed into cells that express LacI repressor constitutively, forms a genetic toggle switch in which an activator protein, the phosphorylated form of the glnG product (NRI or NtrC), activates the transcription of the glnG structural gene, and LacI represses transcription of the glnG gene. Because activator and repressor compete for control of transcription of the glnG gene, the system displays hysteresis, with the level of IPTG required for induction dependent on the prior history of induction (12). The hysteresis of this system was more prominent than that displayed by the galactoside permease system, in that bistability was observed over an ≈10-fold range of IPTG concentrations (12). We show that the multiloop system obtained by linking the activator module and galactoside permease feedback loops displayed extensive bistability, indicating that bistability could be built up by linkage of distinct feedback loops.
Results
A Graphic Analysis of the Problem.
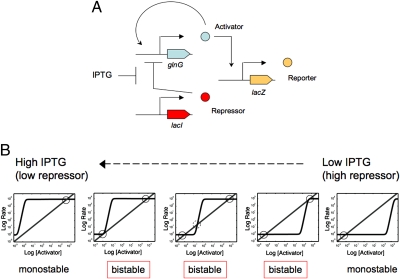
To understand the factors controlling the range of bistability of the activator module of the Atkinson et al. oscillator, we use rate-balance plots following the work of Ferrell and Xiong (10, 11). In this method, the rates of activator production and decay are plotted as a function of activator concentration. Because activator has no direct effect on its own decay, we expect activator decay to be a simple linear function of activator concentration (Fig. 1). Conversely, biochemical studies of the activation of transcription by the NRI ∼ P activator indicated a high kinetic order and an S-shaped response (25) (Fig. 1). Steady states are possible only where the curves for production rate of activator and decay rate of activator intersect (Fig. 1). A key feature of the genetic toggle switch used in our studies is that activator and repressor compete for the promoter that drives transcription of the activator structural gene (Fig. 1A). In this genetic system, activator has no direct effect on the LacI repressor, which is present at a low constitutive level. The inducer IPTG serves to decrease the concentration of functional repressor. Thus, changes in the IPTG concentration may be thought of as shifting the curve for production of activator to the right or the left, as depicted in Fig. 1. The range of IPTG concentrations at which the system displays bistability is thus limited to the extent to which the production curve may be shifted to the right or the left while still maintaining at least two points of intersection with the decay curve (Fig. 1).
Fig. 1.
Design and function of the genetic toggle switch. (A) Basic circuit design for the genetic toggle switch. The LacI repressor was produced from the natural wild-type lacI gene. The activator module containing the glnG structural gene was located in the rbs region of the E. coli chromosome. The activator and repressor competed for control of the expression of the activator module promoter; when neither activator nor repressor proteins were present, a weak promoter (not depicted) allowed for transcription of the activator gene (12). The reporter consisted of a fusion of the activator-dependent glnK promoter with the lacZ gene, located in the trp region of the chromosome. For the system as shown with a single positive feedback loop, the lacY gene was next to lacZ, but contained a null mutation. (B) Graphic representation of the factors affecting the range of IPTG concentrations at which bistability is obtained. All of the plots depict rate of activator production or destruction vs. the concentration of activator. The destruction of activator is not regulated by activator and thus is likely to have a slope of 1, as depicted. Conversely, the production of activator is known to display high kinetic order and is depicted as the S-shaped curve. The role of IPTG is to shift this S-shaped curve to the right or the left, as depicted. Steady states occur when the two curves intersect, as indicated by small circles. The dotted circle in the Center plot depicts an unstable steady state. Factors controlling the shape of the S-shaped activator production curve, such as the steepness of this curve or its absolute height, control the range of bistability of the system.
In the system considered above, activator production rate displayed a high sensitivity, whereas activator decay rate was linear, in accordance with the experimental system we use. However, it should be noted that having a nonlinear degradation rate could also help achieve bistability. This result was presented by Ferrell and Xiong, who considered a signal transduction system (26).
Simple inspection of Fig. 1 reveals two of the key parameters affecting the range of inducer concentrations at which the system will display bistability. The first of these is the steepness (sensitivity, kinetic order) of the activator production curve; the steeper this response curve is, the greater it may be shifted to the left or the right while still maintaining two intersections with the decay curve. A second key parameter affecting the range of inducer concentrations at which bistability is observed is the absolute magnitude of the activator's effect on itself. That is, the absolute height of the S-shaped activator production curve limits the distance it may be displaced to the left or the right while still maintaining at least two intersections with the decay curve. In this work, we focus on controlling the steepness of the production curve. In support of the simple graphic method used in Fig. 1, a more formal analysis of our specific system leading to the same conclusions is presented in the SI Text.
How Can the Steepness of the Activator Production Curve Be Increased?
The sensitivity of a promoter to its activator is dependent upon numerous factors, such as the oligomeric state of the activator protein, the number of molecules that are required for transcriptional activation, and the details of the interactions of activator and polymerase with each other and with other macromolecules that interact with them and therefore compete for them. In most cases, these parameters are difficult to adjust in a systematic way and thus a general solution to the problem of increasing the apparent kinetic order of an activator’s effect on itself must employ a different approach. It has long been known that ultrasensitive responses to a stimulus may be obtained in signal transduction systems consisting of linked cycles of reversible covalent modification, when the stimulus regulates multiple distinct activities in the signaling system (27) (reviewed in ref. 28). Such ultrasensitivity is referred to as “multistep ultrasensitivity” (27). Recently, Rossi and colleagues used a model experimental system to show that the apparent kinetic order of a transcriptional response to a stimulus was increased when the stimulus affected both the activation and the repression of a promoter, relative to the situations where a single function was regulated (29). We regard this result as a special class of multistep ultrasensitivity and reasoned that we could similarly increase the apparent kinetic order of activator’s response to itself by having activator influence not only the activation of its structural gene, but also the repression of its own gene. Furthermore, unlike the system of Rossi et al. (29), which requires specifically engineered proteins, we sought to have activator function to decrease repression of the system indirectly. This was accomplished by having activator drive the expression of the galactoside permease that brings about the internalization of the inducer that inactivates the repressor. In the brief formal analysis of our specific system presented in SI Text, we observed that by extending the activity of activator to inhibition of repression, the already high kinetic order of the response to activator could be significantly increased.
Combining Well-Characterized Feedback Loops to Create a Composite System with Coherent Loops.
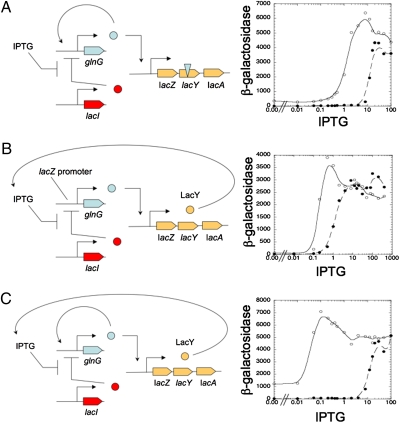
The experimental system with two positive feedback loops, and two control systems each with a single positive feedback loop, is depicted in Fig. 2. We used a previously described chromosomally integrated fusion of the E. coli glnK promoter to the structural genes of the lacZYA operon (30). Expression from the glnK promoter is dependent upon the activator of the genetic toggle switch, NRI ∼ P. The glnK promoter-lacZYA fusion was constructed such that the novel joint corresponded to the translational start codon of the lacZ gene. Thus, in the recombinant operon, the major lac operon operator element (lacO1) was not present and neither was the minor lacO3 operator. The minor lacO2 operator was present, as this element is found within the lacZ structural gene. Nevertheless, in the absence of the major lac operator (lacO1), it is anticipated that LacI repressor will have little direct effect on transcription of the lacZYA structural genes in the recombinant context, whereas expression should be tightly controlled by activator. We chose the glnK promoter and this specific operon fusion for our experiments because prior work has shown that this operon fusion has negligible basal expression in the absence of the activator protein (30). A version of the glnKp-lacZYA operon fusion containing an internal deletion within the lacY gene was generated by recombineering (31). To provide the maximum stability for our synthetic genetic system, the genetic toggle switch (activator module of the Atkinson et al. clock) was incorporated into the E. coli chromosome in the rbs region, as described previously (12). The activator of this system requires phosphorylation for activity, and we provided this function by including within the cell an altered kinase protein that brings about the phosphorylation of activator regardless of nitrogen status. To provide a control system lacking the galactoside permease positive feedback loop, we simply used the version of the system with the lacY null mutation. To provide a control system lacking the positive feedback loop of the activator module, but containing the positive feedback loop based upon galactoside permease, additional genetic manipulations were required (Fig. 2B). For this purpose, we created a system in which the lacZYA promoter region (from the upstream lacO3 site through the translational initiation codon of the lacZ gene) was fused to the activator structural gene. This was placed into the rbs region of the chromosome, analogous to the positioning of the genetic toggle switch, and combined in cells with the glnKp-lacZYA fusion, creating a system where repressor control of the lac promoter regulated activator expression, with activator then driving expression of lacZYA.
Fig. 2.
Strong bistability was obtained by linking distinct positive feedback loops. (A) Genetic toggle switch with a single positive feedback loop. (Left) Schematic depiction of the genetic system in which the genetic toggle switch drives the expression of the lacZYA operon, but the lacY gene contains a null mutation. (Right) Result of bistability experiment, showing an ∼12-fold range of IPTG concentrations at which the system displayed bistability. Symbols:  , naive (uninduced) culture;
, naive (uninduced) culture;  , preinduced culture. (B) Genetic system with a single positive feedback loop based on galactoside permease. (Left) Schematic depiction of the genetic system where the lacZ promoter was used to drive the expression of glnG and the phosphorylated form of glnG drives the expression of lacZYA. The lacY product, galactoside permease, provides positive feedback by facilitating the uptake of IPTG, which inactivates repressor. (Right) Result of bistability experiment, showing an ∼4-fold range of inducer concentrations at which the system displayed bistability. Symbols are as in A. (C) Genetic system with two positive feedback loops. (Left) Schematic depiction of the genetic system in which the genetic toggle switch drives the expression of the lacZYA operon, as in A except with a wild-type lacY gene. (Right) Result of bistability experiment showing that bistability was obtained over an ∼480-fold range of IPTG. For A–C, cells were grown in minimal medium with succinate as the carbon source and glutamine as the nitrogen source.
, preinduced culture. (B) Genetic system with a single positive feedback loop based on galactoside permease. (Left) Schematic depiction of the genetic system where the lacZ promoter was used to drive the expression of glnG and the phosphorylated form of glnG drives the expression of lacZYA. The lacY product, galactoside permease, provides positive feedback by facilitating the uptake of IPTG, which inactivates repressor. (Right) Result of bistability experiment, showing an ∼4-fold range of inducer concentrations at which the system displayed bistability. Symbols are as in A. (C) Genetic system with two positive feedback loops. (Left) Schematic depiction of the genetic system in which the genetic toggle switch drives the expression of the lacZYA operon, as in A except with a wild-type lacY gene. (Right) Result of bistability experiment showing that bistability was obtained over an ∼480-fold range of IPTG. For A–C, cells were grown in minimal medium with succinate as the carbon source and glutamine as the nitrogen source.
Synergy of Positive Feedback Loops.
To measure the range of inducer concentrations at which bistability was obtained, cultures were incubated for 12–14 h in the absence of inducer on in the presence of saturating inducer. The cells were then washed thoroughly and diluted 1 millionfold into fresh medium containing various concentrations of inducer. These cultures were then grown to midlog phase and the level of the lacZ product, β-galactosidase was measured (32).
The greatest contribution of the positive feedback loops is expected in our system when the cells are grown on medium in which catabolite repression is minimized and thus the galactoside permease loop is operating without inhibition. For this purpose, we used succinate-based minimal medium. Under these conditions, the system with a single positive feedback loop in which activator drives its own transcription displayed bistability over a 12-fold range of inducer concentrations (Fig. 2A), consistent with earlier observations (12). The control system lacking the positive feedback of the activator on its own transcription, but containing a single positive feedback loop formed by galactoside permease, displayed bistability over an ∼4 -fold range of inducer concentrations (Fig. 2B), similar to the bistability observed for the native lacZYA system (Fig. S1). Remarkably, under these same conditions, the system with two functional positive feedback loops (strain DE1010), which we refer to as the “double-toggle switch,” displayed bistability over an ∼480-fold range of inducer concentrations (Fig. 2C). Thus, the two positive feedback loops displayed powerful synergy in increasing the range of inducer concentrations over which bistability was observed.
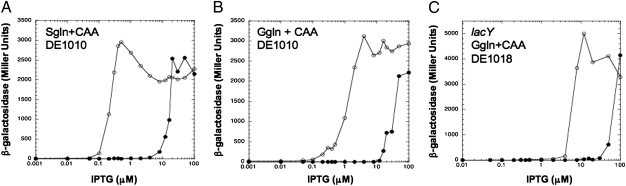
The bistability of the double-toggle switch could be controlled by increasing catabolite repression. For a very modest degree of catabolite repression, we included casein hydrolysate in the succinate growth medium. Under these conditions, the range of IPTG concentrations at which the system was bistable was ∼100-fold (Fig. 3A). Stronger catabolite repression was obtained by using medium containing both glucose and casein hydrolysate; under these conditions the range of IPTG concentrations at which the system was bistable was only ∼25-fold (Fig. 3B). Apparently, catabolite repression could be used to control the contribution of the galactoside permease feedback loop. Even though the galactoside permease feedback loop, when acting alone, did not display significant bistability in the presence of glucose (Fig. S1), in the context of the double-toggle switch this loop still increased the range of bistability about ∼2-fold in medium containing both glucose and casein hydrolysate. This can be discerned by comparison with the control strain with a null mutation in lacY grown under the same conditions (Fig. 3C).
Fig. 3.
Bistability of the double-toggle switch was tunable by growth substrates causing catabolite repression. (A and B) The double-toggle switch strain depicted in Fig. 2C was examined in medium containing succinate and casein hydrolysate (A) and in medium containing glucose and casein hydrolysate (B). (C) The experiment is as in B, but the strain contained a null mutation in lacY and thus had only a single functioning positive feedback loop.
Discussion
We observed that distinct positive feedback loops employing different biochemical mechanisms could be linked to provide powerful genetic bistability and that the functions of such systems were scalable by factors that affected one of the feedback loops. Thus, bistability could be built up piecewise, by the coherent linkage of biochemically distinct feedback mechanisms. Furthermore, because the different feedback loops interacted with each other in a nonlinear way (in our case, by causing an increase in the sensitivity of the response to activator), fairly weak feedback loops acted synergistically to produce extensive bistability. These observation provide a generally applicable foundation for the rational engineering of synthetic genetic bistable systems. The prevalence of complex natural systems with numerous interacting feedback loops, which regulate critical responses such as progression through the cell cycle, circadian clocks, or development, may reflect this capacity to build strong genetic bistability by collaboration of multiple weak elements. The sharp state transitions involved in clocks, cell cycles, and irreversible morphogenic pathways are likely to require genetic bistability over a broad range of conditions (e.g., refs. 7 and 22), which could not evolve in a single step and had to be built up piecewise.
To develop novel synthetic genetic bistable systems, the pathway followed by nature could be mimicked by developing circuitry where different feedback loops act coherently. Our work shows that there is no necessity for using highly engineered proteins; factors that indirectly affect transcriptional activation or repression are just as good as factors that directly interact with the DNA, as long as they are effective. Thus, for example, in our case we would expect that a circuit where activator brought about the repression of repressor synthesis (along with the activation of its own synthesis) would also bring about very dramatic bistability. Such a system would be closer to that used by Rossi (29) than is the system used in this paper. The flexibility of using indirect methods, such as regulation of the permease that internalizes the inducer, follows the pathway used by bacteria and simplifies the engineering of systems.
We argue that all of the cases of bistable systems with multiple interacting genetic feedback loops noted above may be demonstrating a form of the same multistep ultrasensitivity that was studied in signal transduction systems 25 years ago (27, 28). A significant and well-understood limitation to bistability in genetic systems is obtaining a sufficiently high sensitivity for the critical regulatory step, such as the activator’s effect upon its own expression in our system (8, 10–12). We expect that this type of genetic multistep ultrasensitivity may play an important role in genetic regulation requiring a high kinetic order in those cases where multiple feedback loops are focused on controlling the expression of a gene.
Methods
Genetic Elements.
The activator module of the NC12 synthetic genetic clock and the fusion of the glnK promoter to the lacZYA structural genes were described previously (12, 30). The fusion of the lacZ promoter to the glnG structural gene was constructed in several steps, as described in SI Text, and the primers used for construction of the lacY null mutation by recombineering are listed in SI Text. All molecular cloning, PCR, P1vir transduction, and plasmid transformation used standard techniques (33, 34). The bacterial strains used and their relevant genotypes are listed in Table S1.
Physiology Experiments.
Growth medium for bistability experiments used W-salts (12), with added vitamin B1 (0.004% wt/vol), tryptophan (0.04% wt/vol), and glutamine (0.2% wt/vol), and contained succinate at 0.4% wt/vol, casein hydrolysate at 0.5% wt/vol, and glucose at 0.4% wt/vol, as indicated. IPTG was used at 0.4 mM for overnight induction of the cultures. β-Galactosidase activity was measured using the method of Miller (32).
Supplementary Material
Acknowledgments
We thank Patrick O’Brien for reviewing an early version of this manuscript. This work was supported by Grant GM63642 (to A.J.N.) from the National Institutes of Health–National Institute of General Medical Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908314107/DCSupplemental.
References
- 1.Pomerening JR, Sontag ED, Ferrell JE., Jr Building a cell cycle oscillator: Hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 2.Sha W, et al. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci USA. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Hao N, Dohlman HG, Elston TC. Bistability, stochasticity, and oscillations in the mitogen-activated protein kinase cascade. Biophys J. 2006;90:1961–1978. doi: 10.1529/biophysj.105.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrell JE., Jr Self-perpetuating states in signal transduction: Positive feedback, double-negative feedback, and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 5.Bagowski CP, Ferrell JE., Jr Bistability in the JNK cascade. Curr Biol. 2001;11:1176–1182. doi: 10.1016/s0960-9822(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 6.Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 7.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: Dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15:221–231. doi: 10.1016/s0955-0674(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 8.Angeli D, Ferrell JE, Jr, Sontag ED. Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc Natl Acad Sci USA. 2004;101:1822–1827. doi: 10.1073/pnas.0308265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craciun G, Tang Y, Feinberg M. Understanding bistability in complex enzyme-driven reaction networks. Proc Natl Acad Sci USA. 2006;103:8697–8702. doi: 10.1073/pnas.0602767103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrell JE., Jr Feedback regulation of opposing enzymes generates robust, all-or-none bistable responses. Curr Biol. 2008;18:R244–R245. doi: 10.1016/j.cub.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong W, Ferrell JE., Jr A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson MR, Savageau MA, Myers JT, Ninfa AJ. Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell. 2003;113:597–607. doi: 10.1016/s0092-8674(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 13.Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 15.Santillán M, Mackey MC. Influence of catabolite repression and inducer exclusion on the bistable behavior of the lac operon. Biophys J. 2004;86:1282–1292. doi: 10.1016/S0006-3495(04)74202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong P, Gladney S, Keasling JD. Mathematical model of the lac operon: Inducer exclusion, catabolite repression, and diauxic growth on glucose and lactose. Biotechnol Prog. 1997;13:132–143. doi: 10.1021/bp970003o. [DOI] [PubMed] [Google Scholar]
- 17.Yildirim N, Mackey MC. Feedback regulation in the lactose operon: A mathematical modeling study and comparison with experimental data. Biophys J. 2003;84:2841–2851. doi: 10.1016/S0006-3495(03)70013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzenberg LA. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959;31:525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- 19.Cohn M, Horibata K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inada T, Kimata K, Aiba H. Mechanism responsible for glucose-lactose diauxie in Escherichia coli: Challenge to the cAMP model. Genes Cells. 1996;1:293–301. doi: 10.1046/j.1365-2443.1996.24025.x. [DOI] [PubMed] [Google Scholar]
- 21.Hogema BM, et al. Inducer exclusion in Escherichia coli by non-PTS substrates: The role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol Microbiol. 1998;30:487–498. doi: 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]
- 22.Brandman O, Ferrell JE, Jr, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J, Chen C, Lu H, Sun T, Shen P. Two independent positive feedbacks and bistability in the Bcl-2 apoptotic switch. PLoS ONE. 2008;3:e1469. doi: 10.1371/journal.pone.0001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas R, D’Ari R. Biological Feedback. Boca Raton, FL: CRC; 1989. [Google Scholar]
- 25.Feng J, et al. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992;174:6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrell JE, Jr, Xiong W. Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- 27.Goldbeter A, Koshland DE., Jr Ultrasensitivity in biochemical systems controlled by covalent modification. Interplay between zero-order and multistep effects. J Biol Chem. 1984;259:14441–14447. [PubMed] [Google Scholar]
- 28.Koshland DE, Jr, Goldbeter A, Stock JB. Amplification and adaptation in regulatory and sensory systems. Science. 1982;217:220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- 29.Rossi FM, Kringstein AM, Spicher A, Guicherit OM, Blau HM. Transcriptional control: Rheostat converted to on/off switch. Mol Cell. 2000;6:723–728. doi: 10.1016/s1097-2765(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson MR, Blauwkamp TA, Bondarenko V, Studitsky V, Ninfa AJ. Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation. J Bacteriol. 2002;184:5358–5363. doi: 10.1128/JB.184.19.5358-5363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1972. [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 34.Silhavy TJ, Berman ML, Enquist LW. Experiments with Gene Fusions. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.