Abstract
The “One neuron-one neurotransmitter” concept has been challenged frequently during the last three decades, and the coexistence of neurotransmitters in individual neurons is now regarded as a common phenomenon. The functional significance of neurotransmitter coexistence is, however, less well understood. Several studies have shown that a subpopulation of dopamine (DA) neurons in the ventral tegmental area (VTA) expresses the vesicular glutamate transporter 2 (VGLUT2) and has been suggested to use glutamate as a cotransmitter. The VTA dopamine neurons project to limbic structures including the nucleus accumbens, and are involved in mediating the motivational and locomotor activating effects of psychostimulants. To determine the functional role of glutamate cotransmission by these neurons, we deleted VGLUT2 in DA neurons by using a conditional gene-targeting approach in mice. A DAT-Cre/Vglut2Lox mouse line (Vglut2f/f;DAT-Cre mice) was produced and analyzed by in vivo amperometry as well as by several behavioral paradigms. Although basal motor function was normal in the Vglut2f/f;DAT-Cre mice, their risk-taking behavior was altered. Interestingly, in both home-cage and novel environments, the gene targeted mice showed a greatly blunted locomotor response to the psychostimulant amphetamine, which acts via the midbrain DA system. Our results show that VGLUT2 expression in DA neurons is required for normal emotional reactivity as well as for psychostimulant-mediated behavioral activation.
Keywords: amphetamine, midbrain, neurotransmission, reward, striatum
Contrary to a long-held assumption, it has become clear in recent years that most if not all neurons in the central and peripheral nervous system synthesize and release more than a single type of neurotransmitter. Cotransmission of small-molecule neurotransmitters, such as ACh, glutamate, or GABA, together with neuropeptides, is a general phenomenon, both in vertebrates and invertebrates (1). Following the discovery of vesicular glutamate transporters (VGLUT1-3) (2–5), the repertoire of potentially cotransmitting neurons has expanded greatly with the demonstration that monoamine and cholinergic neurons may use glutamate as a cotransmitter. For example, most serotonin (5-HT) neurons and many cholinergic neurons express VGLUT3 (2, 6). The ability of 5-HT, cholinergic, and dopamine (DA) neurons to release glutamate at synapses has been demonstrated in vitro (7–9).
Of the three currently known VGLUTs, VGLUT2 is the member predominantly expressed in the more phylogenetically primitive areas of the brain including the hypothalamus and brainstem. VGLUT2-mediated neurotransmission has been shown to be absolutely critical for respiration, and thus VGLUT2-null mutant mice die from asphyxia (10). VGLUT2 also has been shown to play a role in the hypothalamic neurocircuitry that prevents hypoglycemia (11). Perhaps more surprisingly, higher brain function also is dependent on VGLUT2-mediated neurotransmission, as has been investigated recently by the conditional deletion of VGLUT2 in the cortex and amygdala (12). Although several studies have shown that a subset of DA neurons contains VGLUT2 (13, 14), no study thus far has investigated the functional role of VGLUT2 as a cotransmitter transporter. Thus, very little is known at present about the physiological significance of glutamate cotransmission in monoamine and cholinergic neurons. It has been suggested recently that VGLUT2 expression and glutamate release may be involved in the formation of synaptic contacts by DA neurons (15). The behavioral consequences of glutamate cotransmission in cholinergic and monoamine neurons are undetermined at present; however, if the presence of a VGLUT facilitates the action of the primary transmitter, a reasonable prediction is that in the absence of VGLUT behavioral responses mediated by the primary transmitter should be perturbed. In this report we used a conditional knockout strategy to interrupt the Vglut2 gene selectively in mouse DA neurons. We found that in these mice the behavior-activating effect of amphetamine is severely reduced.
Results
Deletion of VGLUT2 in Midbrain DA Neurons Using a DAT-Cre Transgenic Mouse.
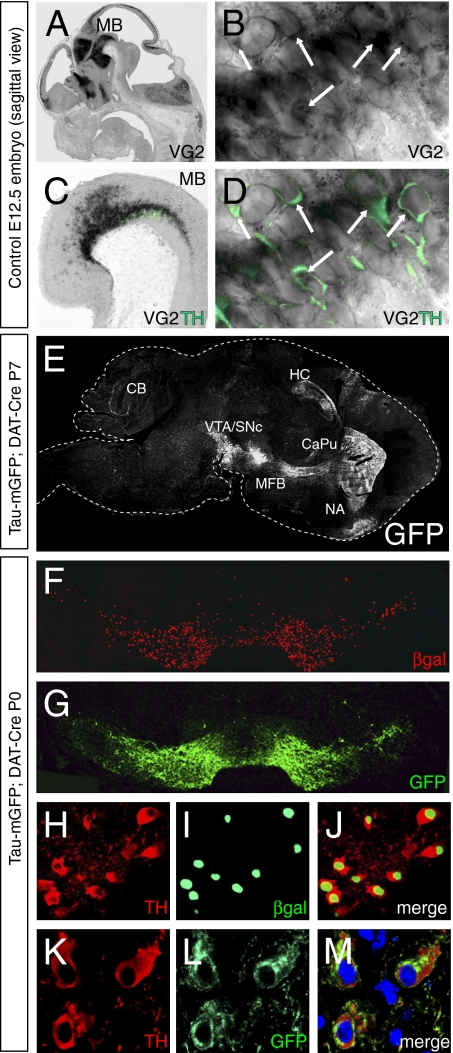
In situ hybridization on cryosections for Vglut2 mRNA expression combined with immunohistochemistry for tyrosine hydroxylase (TH) confirmed previous studies showing Vglut2 expression in subsets of midbrain DA (mDA) neurons. At all embryonic stages analyzed [embryonic day (E)12.5, E14.5, and E16.5] as well as at the first postnatal day (P0), we could readily detect TH-positive neurons in the ventral midbrain that also expressed Vglut2 mRNA (Fig. 1 A–D).
Fig. 1.
Expression of Vglut2 mRNA and DAT-Cre activity in mDA neurons. (A–D) In situ hybridization for Vglut2 mRNA combined with immunohistochemistry for TH on sagittal sections of E12.5 embryo. Vglut2 mRNA is expressed in multiple regions in the embryo, including the ventral midbrain (MB) where DA neurons develop (A). (B) Arrows indicate Vglut2-expressing cytoplasm. Vglut2 mRNA is expressed in TH-immunopositive (green) DA neurons in the MB (Magnification: B ×300) (C). (D) Arrows indicate Vglut2/TH double-positive cells (Magnification: D ×300). (E) Sagittal section of a TaumGFP;DAT-Cre brain at P7 with immunofluorescence for GFP visualizing the mDA neurons in the VTA and SNc and their projection pathway in the median forebrain bundle (MFB) to the target neurons in the striatum (CaPu + NA). GFP projections also are seen in the hippocampus (HC) and the cerebellum (CB). (F and G) Coronal sections of newborn (P0) TaumGFP;DAT-Cre mouse showing immunohistochemistry for β-gal (red) and GFP (green) in the VTA and SNc. (H–L) Coronal sections from TaumGFP;DAT-Cre P0 mouse showing TH (H, red) and β-gal (I, green) immunohistochemistry within the same cells (merged fluorescence in J), and TH (K, red) and GFP (L, green) within another set of cells (merged in M).
A DA transporter (DAT)-Cre transgenic mouse, produced by homologous recombination into the endogenous DAT locus (16), was characterized to ensure the DAT-Cre function. The production of this Cre mouse included restoration of the DAT gene after insertion of the Cre cassette (Fig. S1A), resulting in expression levels of DAT mRNA that are reduced by only 25% (Fig. S1B). This reduction in DAT expression does not affect expression of DA receptors D1 or D2 in the caudate putamen (CaPu) (Fig. S1B). Importantly, the DAT-Cre mouse shows normal levels of DA and its metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid, as well as 5-HT and noradrenaline, in both the CaPu and the nucleus accumbens, as quantified by HPLC (Fig. S1D). The normality of the DA system in the DAT-Cre mice was corroborated further by the normal locomotor response to the psychostimulant amphetamine, which was the same as in wild-type littermate controls (Fig. S1C). Furthermore, we characterized the specificity of the DAT-Cre by using the TaumGFP Cre-reporter mouse (17). By crossing these two mouse lines, TaumGFP; DAT-Cre mice were generated. The TaumGFP reporter allows detection of cell nuclei in which the Cre protein is activated by beta-galactosidase (β-gal) expression, whereas neuronal cell bodies and processes are revealed by expression of a version of GFP fused to the microtubule-associated protein tau (tauGFP), enabling transport of the fusion protein to cytoplasm and fibers. Using GFP immunohistochemistry on sagittal cryosections of E17.5, P0, P7, and adult TaumGFP; DAT-Cre mouse brains, we visualized the mDA neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) and their projections through the median forebrain bundle to the target neurons in the striatum and nucleus accumbens (Fig. 1E). Combined immunohistochemistry for β-gal and GFP on coronal cryosections from the same stage showed the presence of these markers within the same cells in a pattern characteristic for the mDA neurons (Fig. 1 F and G). Combined immunohistochemistry for β-gal/TH as well as for GFP/TH demonstrated that the Cre protein was expressed in DA neurons (Fig. 1 H–M).
We next bred the DAT-Cre mouse line into our conditional Vglut2f/f mouse line, which has been validated and described elsewhere (10, 12). Briefly, this mouse line carries LoxP sites surrounding exons four to six in the genomic Vglut2 sequence that encode three transmembrane domains. The presence of Cre protein in Vglut2-expressing cells in these mice has been shown previously to result in synaptic vesicle malformation and loss of miniature excitatory postsynaptic potentials (10, 18). Thus, the presence of Cre in DAT-expressing mDA neurons is expected to produce dysfunctional VGLUT2 protein, abrogating VGLUT2-mediated glutamatergic neurotransmission. In analyses of coronal sections encompassing the rostral linear nucleus (RLi) and the VTA using combined Vglut2 in situ hybridization and TH immunohistochemistry (Fig. 2A),we were not able to detect any double-positive cells in newborn Vglut2f/f;DAT-Cre sections, whereas such cells were identified in littermate controls (Fig. 2 B and C). We also analyzed Vglut2 expression in Vglut2f/f;DAT-Cre;Tau-mGFP and the Vglut2f/+;DAT-Cre;Tau-mGFP newborn littermate controls. TH- and β-gal–positive neurons in the controls expressed Vglut2, but colocalization was absent in the Vglut2f/f;DAT-Cre;Tau-mGFP DA neurons (Fig. 2 D and E). To confirm the conditional deletion of exons 4–6 in the Vglut2 locus in DA neurons, we performed single-cell RT-PCR experiments in freshly dissociated cells derived from the ventral midbrain (Fig. 2F); 127 GFP-expressing cells were collected from a Vglut2f/f;DAT-Cre;Tau-mGFP newborn mouse. Of these, 74 cells expressed TH alone, and 11 cells coexpressed TH and Vglut2; in total, 85 TH-expressing cells were found. Of the 11 TH/Vglut2 double-positive neurons, 9 showed full deletion of the Vglut2 locus (10.6% of all TH-expressing cells) and thus expressed the gene-targeted allele (Fig. 2G, Left lane). The other two cells expressed the wild-type allele. Among the 127 cells we also found 6 Vglut2-expressing cells (4.7%) that did not express TH (i.e., pure glutamatergic cells). The same experiment was performed on pups at P7. We collected 40 GFP-expressing cells, of which 39 expressed TH and 4 expressed both TH and the Vglut2 gene-targeted allele (10.3% of all TH-expressing cells). Using a littermate Vglut2f/+ control, we collected 70 cells from the same area as in the Vglut2f/f;DAT-Cre;Tau-mGFP mouse; 8 of these cells expressed TH, and 2 of these cells (25%) expressed the Vglut2 wild-type allele (Fig. 2G, Right lane). Note that the controls do not express either DAT-Cre or Tau-mGFP and therefore does not express GFP. In addition, we found four pure glutamatergic cells, all expressing only the wild-type allele. Thus, recombination was specific to the Vglut2f/f;DAT-Cre and Vglut2f/f;DAT-Cre;Tau-mGFP mice and did not occur in the control mice. When Vglut2f/+;DAT-Cre;Tau-mGFP cells were left in standard culture for 1 week, they developed extensive processes that were TH-immunopositive (Fig. 2H).
Fig. 2.
Disruption of Vglut2 mRNA expression in the mDA neurons. (A) Schematic of coronal mouse brain section at bregma −3.08 (modified from ref.39). Aq, aqueduct; IF, interfascicular nucleus; MM, medial mammillary nucleus; PAG, periaqueductal gray; PBP, parabrachial pigmented nucleus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata. (B–E) Vglut2 mRNA expression is seen in control mDA neurons but not in the Vglut2f/f;Dat-Cre mDA neurons, as shown by in situ hybridization. (Left) Control (B and D), Vglut2f/f;Dat-Cre (C), and Vglut2f/f;DAT-Cre;Tau-mGFP (E). Gene-targeted TH-positive DA neurons (red in B–E, Middle, and merged in B–E, Right). In D and E, the nucleus is visualized by β-gal immunohistochemistry (green) to show that DAT-Cre is active in these neurons. (F–H) Multiplex RT-PCR on single cells verifies that gene targeting has occurred in the Vglut2f/f;DAT-Cre;Tau-mGFP DA cells. (F) The pipette is sucking up a freshly dissociated GFP-expressing DAT-Cre cell. (G) The gel picture from electrophoresis of RT-PCR products shows the presence of the gene-targeted Vglut2 allele in the Vglut2f/f;DAT-Cre;Tau-mGFP (KO) cell but not in the control cell (CL). (H) When the GFP-expressing cells are left in culture for 1 week, they develop extensive neurites immunopositive for TH, confirming their DA phenotype. KO, Vglut2f/f;DAT-Cre;Tau-mGFP (knockout); CL, Vglut2f/+;DAT-Cre;Tau-mGFP (control).
Normal Release in Pure Glutamatergic Neurons of Vglut2f/f;DAT-Cre Mice.
To demonstrate that the loss of Vglut2 expression in DAT-expressing neurons is specific and does not affect VGLUT2-mediated signaling in pure glutamatergic neurons, we performed in vivo amperometry in the brain of anesthetized Vglut2f/f;DAT-Cre and littermate control mice as described in SI Materials and Methods. The Fast Analytical Sensing Technology (FAST), in which a glutamate-selective microelectrode array is used to quantify extracellular glutamate on a subsecond (2 Hz) time scale with a sensitivity of 0.2–0.5 μM, was used to quantify extracellular glutamate in response to a depolarizing challenge stimulus by 70 mM KCl, a concentration that activates glutamatergic fibers (19). We analyzed KCl-evoked glutamate release and reuptake at four levels of the striatum, the dorsal CaPu, the ventral CaPu, the nucleus accumbens core, and the nucleus accumbens shell in Vglut2f/f;DAT-Cre and littermate control mice. The amplitude and the reuptake rate (T80), defined as the time in seconds from maximum rise to 80% decay, were quantified according to Fig. S2. The analyses showed the same level of amplitude in glutamate release (Fig. S2 E–H) and T80 (Fig. S3) in Vglut2f/f;DAT-Cre mice as in littermate controls throughout all six KCl pressure ejections at all striatal levels analyzed (Table S1). Thus, pure glutamatergic signaling, which may be derived from several brain regions including the cerebral cortex is normal in the Vglut2f/f;DAT-Cre mice.
Normal Motor Function but Altered Risk-Taking Behavior in Vglut2f/f;DAT-Cre mice.
To understand the functional role of VGLUT2 expression in mDA neurons, we analyzed the behavior of Vglut2f/f;DAT-Cre mice. Because the mDA neurons that are involved in motor coordination project from the SNc, where Vglut2 is not expressed in the DA neurons, to the dorsolateral striatum via the nigrostriatal pathway (20–22), we hypothesized that motor coordination should be unaffected in the Vglut2f/f;DAT-Cre mice. Therefore, we first analyzed motor behavior using the accelerating rotarod and the beam walk, which measure crude and fine motor coordination, respectively. No difference in performance was observed between Vglut2f/f;DAT-Cre and littermate control mice (Table 1 and Fig. S4A). The verification of normal motor skills of Vglut2f/f;DAT-Cre mice allowed analysis of other types of behavior that require normal motor function. Because VTA neurons project mainly to the ventral striatum, including the nucleus accumbens (23), we next focused on analyses that measure behavior directly relevant to the mesocortical and mesolimbic pathways. Although analysis of cognitive ability, using the baited radial maze that targets working and reference memory function, did not show an alteration in the Vglut2f/f;DAT-Cre compared with control mice (Table 1), analysis of emotional reactivity revealed an alteration. In the elevated plus maze (24), the Vglut2f/f;DAT-Cre mice exhibited the same degree of activity as the littermate controls, in that they entered all areas at the same frequency and spent equally long time in the closed versus the open arms (Table 1 and Fig. S4B). However, the Vglut2f/f;DAT-Cre mice took significantly longer to start moving when placed in the maze, showing an initial escape of movement (Table 1 and Fig. S4C). We then turned to the more sensitive multivariate concentric square field (MCSF) test, which is designed to provoke exploration and behaviors associated with risk assessment, risk-taking, and shelter-seeking in an environment not previously experienced by the mice (12, 25). The purpose of this multivariate design is to gather information that enables a behavioral profiling of the animal. A principal component analysis of the behavior recorded in the MCSF revealed no differences in the overall behavioral profile between the Vglut2f/f;DAT-Cre and control mice (Fig. S4 D–E). However, by analyzing each zone individually (Table S2), we found that the Vglut2f/f;DAT-Cre mice spent less time in the central circle of the open field, indicating an increased avoidance of the open area (Table 1, Fig. S4F, and Table S2). Although the Vglut2f/f;DAT-Cre mice did not show an alteration in shelter-seeking, exploration, or risk-assessment, they did spend significantly more time on the elevated bridge, behavior that is associated with risk-taking (Table 1, Fig. S4G, and Table S2). To analyze the emotional state of the mice further, we performed the forced swim test, but no difference in levels of depression was observed between Vglut2f/f;DAT-Cre and control mice (Table 1).
Table 1.
Behavioral characterization of the Vglut2f/f;Dat-Cre mice
| Method | No.Vglut2f/f;DAT-Cre mice | No.littermate control mice | Behavior or characteristic analyzed | Phenotype of Vglut2f/f;DAT-Cre mice |
| Rotarod | 13 | 12 | Motor: crude motor coordination | Normal |
| Beam walking | 22 | 21 | Motor: fine motor coordination | Normal |
| Elevated plus maze | 29 | 29 | Emotion: anxiety | Increased latency of movement |
| Multi concentric square field | 29 | 29 | Emotion: anxiety and risk analysis | Altered risk-taking behavior |
| Forced swim test | 29 | 29 | Emotion: depression | Normal |
| Radial maze | 29 | 29 | Cognition: reference and working memory | Normal |
Vglut2f/f;DAT-Cre Mice Show Blunted Behavioral Response to the DA-Releasing Drug Amphetamine.
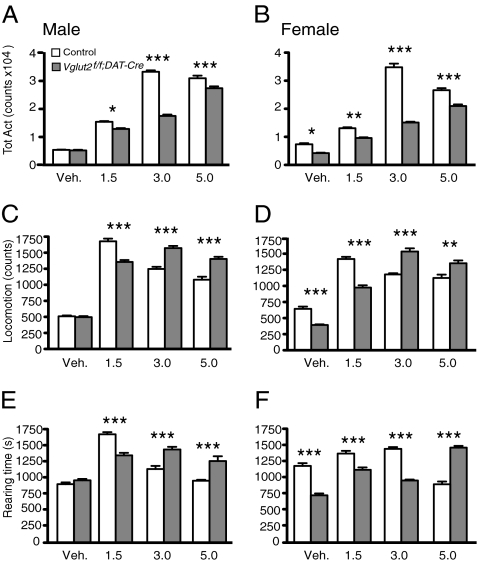
The loss of VGLUT2 expression in DA neurons probably leads to a decrease in excitatory activity of the affected dopaminergic neurons. We therefore were interested in determining the impact of this decrease on functional DA signaling. VTA DA neurons mediate the rewarding effects of psychostimulants such as amphetamine by increasing the level of extracellular DA in limbic areas such as the nucleus accumbens (26). We challenged the mice with three different doses of amphetamine, 1.5, 3.0, and 5.0 mg/kg body weight, administered in a random order. The motor behavior of the mice was recorded for 60 min in a home-cage environment where locomotion, rearing, and total activity were measured. (Statistical data are shown in Tables S3 and S4.) Both male and female control mice responded to the amphetamine injections as expected and reached a maximum response at 3.0 mg/kg, after which their total activity decreased (Fig. 3 A and B, white bars). The behavior of the Vglut2f/f;DAT-Cre mice was strikingly different (Fig. 3 A and B, gray bars). The total activity of the Vglut2f/f;DAT-Cre mice rose continuously with increased amphetamine dose, but the actual level of total activity of the Vglut2f/f;DAT-Cre mice was significantly lower than that of controls at all doses (Fig. 3 A and B; compare white and gray bars).
Fig. 3.
Vglut2f/f;DAT-Cre mice show blunted behavioral response to amphetamine, as measured in a home-cage environment. (A and B) Male and female knockout mice showed a reduced behavioral response to amphetamine as shown by significantly lower total activity at all three doses of amphetamine. (C and D) Male and female knockout mice display a shift in dose-response to amphetamine-induced locomotion. (E and F) Male and female knockout mice show a different dose-response profile regarding rearing behavior in response to amphetamine. A right shift in dose-response in locomotion is apparent in the Vglut2f/f;DAT-Cre mice. Data were analyzed with 1-way ANOVA followed by Tukey´s post hoc test when appropriate. Data are presented as mean ± SEM (n = 9). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Locomotion and rearing also were different in the Vglut2f/f;DAT-Cre mice and in controls. Controls responded to the lowest dose of amphetamine with increased locomotion, but the locomotion decreased gradually with the higher doses of the drug (Fig. 3 C and D, white bars). A similar profile was seen for rearing (Fig. 3 E and F, white bars). Because the total activity was more pronounced at 3.0 mg/kg, the behavioral pattern suggests an increase in stereotypic behavior (scored as total activity minus locomotion and rearing) with the increased dose in the controls. In contrast, the Vglut2f/f;DAT-Cre mice continued to increase their locomotion at 3.0 mg/kg of amphetamine to levels higher than the controls (Fig. 3 C and D, gray bars). At the highest dose, the locomotion of the Vglut2f/f;DAT-Cre mice was lower than with the previous dose but still was significantly higher than in the controls. The pattern of rearing was similar to that of locomotion (Fig. 3 E and F, gray bars). Thus, the total activity of the Vglut2f/f;DAT-Cre mice is lower than that of controls at all doses, but their locomotion and rearing are higher at the higher doses. The Vglut2f/f;DAT-Cre mice thus are less susceptible to stereotypic behavior. The data suggest that the response of the Vglut2f/f;DAT-Cre mice to the DA-releasing drug amphetamine is perturbed.
Gender-Dependent Difference in Dose-Response to Amphetamine in a Novel Environment.
The effect of amphetamine on Vglut2f/f;DAT-Cre mice also was analyzed in an environment novel to the mice. Locomotor behavior was recorded 40 min before and 90 min after injection of either saline or amphetamine as described in SI Materials and Methods. No difference in locomotion between the genotypes was seen before or after saline injection in either males or females. As expected, no significant increase in locomotion was observed in either of the genotypes or genders after injection of 0.75 mg/kg amphetamine (genotype F(1, 24) = 1.029, P = 0.4172 for males; F(1, 96) = 3.679, P = 0.0914 for females) (Fig. S5 A and D). However, when the mice were treated with 1.5 mg/kg body weight of amphetamine, male Vglut2f/f;DAT-Cre mice responded with increased locomotion in the same way as their littermate controls (P = 0.8744) (Fig. S5B), whereas female Vglut2f/f;DAT-Cre mice failed to respond to the psychostimulant (time × genotype interaction F(12, 168) = 5.129, P < 0.0001) (Fig. S5E). At an amphetamine dose of 3.0 mg/kg body weight, the Vglut2f/f;DAT-Cre females had a normal response (P = 0.8675) (Fig. S5F), whereas the males failed to increase their locomotion; instead, their activity remained at the same level as when treated with 1.5 mg/kg amphetamine (genotype × time interaction F(12, 48) = 3.908, P = 0.0003) (Fig. S5C). The results confirm that Vglut2f/f;DAT-Cre mice respond to amphetamine in a blunted fashion. In addition, a gender-dependent difference that was not detected in the home-cage environment was observed in an environment novel to the animals.
Discussion
In this study, we were interested in analyzing the functional role of VGLUT2-mediated neurotransmission by DA neurons. The feature of glutamate cotransmission of DA neurons has been debated during the past two decades, ever since the first demonstration that TH-positive neurons are immunoreactive for phosphate-activated glutaminase (27) and the demonstration that TH-labeled neurons in single-cell cultures generate a fast excitatory current (7). However, the functional role of such dual glutamatergic/dopaminergic signaling by a subset of the classical DA neurons in the VTA is unknown. During the past few years, with the identification of the vesicular glutamate transporters (3–5, 28), it has been possible to analyze the vesicular glutamate packaging properties of the mDA neurons. Several groups, including ours, have shown that a subset of mDA neurons expresses Vglut2 mRNA, a finding that makes the use of glutamate as a neurotransmitter a possibility in these neurons (14, 29, 30). Kawano et al. (29) showed that the area containing the most Vglut2- and TH-expressing neurons was the RLi, with somewhat lower expression in the interfascicular nucleus, the rostral part of the caudal linear nucleus, the parabrachial pigmented nucleus, and the paranigral nucleus of the VTA.
The VTA, which includes the caudal linear nucleus, interfascicular nucleus, parabrachial pigmented nucleus, and paranigral nucleus, is critical for transmitting the rewarding properties of drugs of abuse to the nucleus accumbens shell and core via the ventromedial projections in the median forebrain bundle (23, 31). We recently have shown that the VGLUT2 protein is present in TH-expressing DA nerve terminals within these target regions, thereby verifying the ability of the DA neurons to use glutamate as a cotransmitter (32). The RLi recently was shown to be involved in mediating the rewarding effects of heroin (33). The presence of VGLUT2, specifically in mDA neuronal populations important for mediating rewarding effects of drugs of abuse, suggests a functional role of VGLUT2-mediated neurotransmission in drug-induced mechanisms. A way to analyze this role is by removing Vglut2 expression by gene-targeted mutation specifically in the DA neurons of mice. We produced such a conditional knockout by crossing our Vglut2f/f mice, generated and validated previously in our laboratory (10, 12), with transgenic DAT-Cre mice (16). Our analysis of the basal behavior of the mice did not show any major differences in motor coordination or memory function between the Vglut2f/f;DAT-Cre and littermate control mice, but the Vglut2f/f;DAT-Cre mice showed altered risk-taking behavior. Importantly, we show that VGLUT2-mediated neurotransmission in DA neurons is required for the increase in activity normally observed upon amphetamine provocation. Amphetamine-induced behavior in a home-cage environment differs considerably between the Vglut2f/f;DAT-Cre and littermate control mice. The Vglut2f/f;DAT-Cre mice show a strikingly different dose-response behavior to amphetamine than controls. The total activity is significantly lower in Vglut2f/f;DAT-Cre mice than in the control mice at all doses analyzed. At the higher doses, locomotion and rearing are decreased in the controls, as stereotypic behavior becomes dominating. In contrast, the Vglut2f/f;DAT-Cre mice show less stereotypy but rather increased locomotion and rearing. Because motor function and basal locomotor activity is normal in the conditional mutant mice, these parameters do not contribute to the differential response to amphetamine observed between the genotypes. This finding suggests that the induction of stereotypic behavior seen at higher doses in the control mice does not develop to the same degree in the conditional mutant mice. Importantly, because the effects of acute amphetamine administration are largely mediated by amphetamine-induced DA release in the nucleus accumbens (34, 35), we suggest that the altered response to amphetamine in the Vglut2f/f;DAT-Cre mice results from reduced mesoaccumbens DA transmission. This possibility is supported further by the absence of a measurable indirect effect from other glutamatergic systems projecting to the striatum using the highly sensitive FAST in vivo amperometry method. Our findings thus link loss of VGLUT2-mediated glutamatergic neurotransmission in DA neurons with decreased sensitivity to the psychostimulant drug amphetamine and connect the functionality of the glutamatergic and dopaminergic systems within the same neuron.
The mechanisms by which DA and glutamate act as cotransmitters is not completely known, but it has been shown that the glutamatergic signaling properties of the VTA DA neurons allow fast and coincident excitation that results in temporally precise signals, as opposed to the slow and modulatory signal of DA (9). It was shown recently that VGLUT3 and the vesicular acetylcholine transporter colocalize on the same vesicular surface and that gene targeting of Vglut3 leads to loss of cholinergic transmission resulting from the reduced vesicular transport activity of acetylcholine, a feature referred to as “vesicular synergy” (36). In mDA neurons, it is not known whether VGLUT2 and VMAT2 localize to the same synaptic vesicle, although in electron microscopy analysis they appear to be at least in close proximity to each other (32). The close proximity provides a basis for possible mechanisms by which VGLUT2-mediated cotransmission affects amphetamine-provoked DA release. Alternatively, consistent with the reported involvement of several ionotropic receptors in DA neurons in response to cocaine stimulation (37), the early fast release of glutamate could act on glutamate receptors in cis and thereby lower the threshold required for DA release. In the absence of VGLUT2, e.g, in the Vglut2f/f;DAT-Cre mice, the release threshold would increase, and the increased release threshold in turn could account for the blunted behavioral response to amphetamine. A third possibility is that the altered response to amphetamine in the Vglut2f/f;DAT-Cre mice is the result of a developmental defect of the mDA system caused by the targeted deletion of Vglut2. Indeed, in agreement with the recently suggested down-regulation of VGLUT2 in dopaminergic terminals in the adult rat nucleus accumbens (15), glutamate coreleased by DA neurons may play a developmental role, for example in synapse formation (38), and the loss of VGLUT2 in our conditional mutant mice may result in dysfunctional wiring of the DA terminals. Such dysfunction in turn could account for the blunted response to amphetamine in the adult mutant mice.
In summary, we find that loss of VGLUT2 expression in DA neurons leads to a blunted activity-based behavior in response to the DA-releasing psychostimulant amphetamine. The results may be of importance for understanding the functional significance of glutamatergic transmission by classical DA neurons and also may provide insight into the mechanisms of psychostimulant-mediated effects on brain function.
Materials and Methods
Generation of Transgenic Mice.
The mice in the study were kept according to the guidelines of Swedish regulation and European Union legislation. The Uppsala animal ethical committee had approved all studies. The generation of Vglut2f/f mice has been described elsewhere (10). For this study, the Vglut2f/f mice were bred to the DAT-Cre mice (16) producing the Vglut2f/f;DAT-Cre and littermate controls Vglut2f/f and Vglut2f/+ mice. The Vglut2f/f mice also were bred to the TaumGFP mice (17) producing the Vglut2f/f;Tau-mGFP mice, which in turn were bred to DAT-Cre mice to produce the Vglut2f/f;DAT-Cre;Tau-mGFP mice and theVglut2f/+;DAT-Cre;Tau-mGFP littermate controls. In all experiments in this study, littermates were used to ensure that the specificity of observed phenotypes is dependent only on genotype. For all behavioral and electrochemical analyses, mice older that 8 weeks, i.e., adult mice, were used. The experimenter/observer was blind to the genotype of the mice throughout the study.
In Situ Hybridization Histochemistry and Immunohistochemistry.
The morning of the day a vaginal plug was inserted after mating was designated E0.5. Embryos were collected at E12.5, E14.5, and E16.5. In the morning of E19, pups were born and staged as P0. Tissue was prepared as previously described (10, 12). The protocols for in situ hybridization and immunohistochemistry were described previously (10). Mouse TH (Chemicon), rabbit β-gal (ICN/Cappel), and chicken GFP (Abcam) were used. Images were captured on a Zeiss LSM 510 Meta confocal microscope and analyzed using Volocity software (Improvision).
Multiplex Single-Cell RT-PCR.
The procedure for multiplex single-cell RT-PCR has been described previously (14). Primers were designed based upon sequences deposited in the GenBank database (www.ncbi.nlm.nih.gov/nucleotide). The primers were designed to bind to nonhomologous areas and not to interact with each of the other primers in the multiplex PCR.
Motor Activity.
Home-cage environment. The experiment was performed as previously described (12). In short, nine mice were chosen randomly from each treatment group, and both saline and amphetamine were administered by i.p. injection (10 mL/kg) before activity monitoring, with 2–3 days in between the administration of saline or different doses (1.5, 3.0, and 5.0 mg/kg) of amphetamine.
Supplementary Material
Acknowledgments
The authors thank Professor Nils-Göran Larsson at the Max Planck Institute for Biology of Ageing, Cologne, Germany for sharing the DAT-Cre mouse, Assistant Professor Erika Roman, Uppsala University, Sweden, for help with statistical analysis of the MCSF data, Professor Greg Gerhardt and members of his laboratory at the University of Kentucky, Lexington, KY, for discussions concerning in vivo amperometry, and Professor Sylvia Arber at the University of Basel, Basel, Switzerland, for providing the TaumGFP mouse. This work was supported by Grants 2004-5567, 2007-3630/4479, K2005-33X-15327, K2009-61X-03185-39-3, 529-2008-7420 and 2007-5742 from the Swedish Medical Research Council, by grants from the Swedish Brain Foundation and from the Knut and Alice Wallenberg, Åke Wiberg, Magnus Bergwall, Åhlén, and Socialstyrelsen foundations, by Swedish Foundation for International Cooperation in Research and Higher Education Institutional Grants for Younger Researchers, Swedish Brain Power, and by Uppsala University. K.K. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation.
Footnotes
L.O. is co-owner of a company owning the commercial rights to a genetic mouse model of Parkinson’s disease, the generation of which requires use of the DAT-Cre mouse. None of the other authors declare any conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910986107/DCSupplemental.
References
- 1.Hökfelt T. Neuropeptides in perspective: The last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 2.Gras C, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog E, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellocchio EE, Reimer RJ, Fremeau RTJ, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 5.Fremeau RTJ, Jr, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 6.Fremeau RTJ, Jr, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulzer D, et al. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh CY, Danik M, Manseau F, Trudeau LE, Williams S. Chronic exposure to nerve growth factor increases acetylcholine and glutamate release from cholinergic neurons of the rat medial septum and diagonal band of Broca via mechanisms mediated by p75NTR. J Neurosci. 2008;28:1404–1409. doi: 10.1523/JNEUROSCI.4851-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuhma N, et al. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallén-Mackenzie A, et al. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallén-Mackenzie A, et al. Restricted cortical and amygdaloid removal of vesicular glutamate transporter 2 in preadolescent mice impacts dopaminergic activity and neuronal circuitry of higher brain function. J Neurosci. 2009;29:2238–2251. doi: 10.1523/JNEUROSCI.5851-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal Bo G, et al. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Mendez JA, et al. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bérubé-Carrière N, et al. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- 16.Ekstrand MI, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moechars D, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- 20.Oades RD, Halliday GM. Ventral tegmental (A10) system: Neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 21.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: Potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 22.Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1-6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 25.Meyerson BJ, Augustsson H, Berg M, Roman E. The Concentric Square Field: A multivariate test arena for analysis of explorative strategies. Behav Brain Res. 2006;168:100–113. doi: 10.1016/j.bbr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- 28.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 29.Kawano M, et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M. In: Distributional Maps of Tyrosine-Hydroxylase-Immunoreactive Neurons in the Rat Brain. Classical Transmitters in the CNS, Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, editors. Amsterdam: Elsevier; 1984. pp. 277–379. [Google Scholar]
- 32.Descarries L, et al. Glutamate in dopamine neurons: Synaptic versus diffuse transmission. Brain Res Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E. Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacology. 2006;31:1475–1488. doi: 10.1038/sj.npp.1300946. [DOI] [PubMed] [Google Scholar]
- 34.Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- 35.Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gras C, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 37.Engblom D, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz Y, Luccarelli J, Kim M, Wang M, Sulzer D. Glutamate controls growth rate and branching of dopaminergic axons. J Neurosci. 2009;29:11973–11981. doi: 10.1523/JNEUROSCI.2927-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd Ed. New York: Academic; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.