Abstract
The question of whether dedicated progenitor cells exist in adult vertebrate pancreas remains controversial. Centroacinar cells and terminal duct (CA/TD) cells lie at the junction between peripheral acinar cells and the adjacent ductal epithelium, and are frequently included among cell types proposed as candidate pancreatic progenitors. However these cells have not previously been isolated in a manner that allows formal assessment of their progenitor capacities. We have found that a subset of adult CA/TD cells are characterized by high levels of ALDH1 enzymatic activity, related to high-level expression of both Aldh1a1 and Aldh1a7. This allows their isolation by FACS using a fluorogenic ALDH1 substrate. FACS-isolated CA/TD cells are relatively depleted of transcripts associated with differentiated pancreatic cell types. In contrast, they are markedly enriched for transcripts encoding Sca1, Sdf1, c-Met, Nestin, and Sox9, markers previously associated with progenitor populations in embryonic pancreas and other tissues. FACS-sorted CA/TD cells are uniquely able to form self-renewing “pancreatospheres” in suspension culture, even when plated at clonal density. These spheres display a capacity for spontaneous endocrine and exocrine differentiation, as well as glucose-responsive insulin secretion. In addition, when injected into cultured embryonic dorsal pancreatic buds, these adult cells display a unique capacity to contribute to both the embryonic endocrine and exocrine lineages. Finally, these cells demonstrate dramatic expansion in the setting of chronic epithelial injury. These findings suggest that CA/TD cells are indeed capable of progenitor function and may contribute to the maintenance of tissue homeostasis in adult mouse pancreas.
Keywords: ALDH1, CD133, pancreatic, pancreatosphere, stem cell
Although the mammalian pancreas is characterized by steady turnover of differentiated cell types and displays a significant capacity for regeneration following injury, the presence or absence of a dedicated adult pancreatic progenitor population remains controversial. A variety of cell types have been proposed as possible pancreatic progenitors, including preexisting acinar cells (1–3), preexisting β-cells (4, 5), cells associated with ductal epithelium (6, 7), and mesenchymal-like nestin-expressing cells (8). Despite work suggesting that differentiated pancreatic cell types can act as facultative progenitors, additional studies continue to suggest the presence of more-dedicated progenitor cells in adult mouse pancreas (7).
In addition to the cell types listed above, cells known as centroacinar cells have also been considered as possible multilineage pancreatic progenitors. This poorly characterized cell type lies at the junction between acinar cells and the adjacent terminal ductal epithelium, and it is uncertain whether centroacinar and terminal duct cells represent two different cell types or are functionally equivalent. These cells send out projections that contact both endocrine and exocrine cells (9), and have been shown to rapidly proliferate following partial pancreatectomy (10), streptozotocin administration (11), or administration of caerulein (12). Recent work has also identified centroacinar and terminal duct cells as unique domains of activated Notch signaling in adult human, mouse, and zebrafish pancreas (13–16).
Despite considerable interest in these cell populations, the successful isolation of centroacinar/terminal duct cells has not previously been reported, and their progenitor capacities have never formally been assessed. The aim of the present study was to isolate centroacinar and terminal ductal epithelial cells from adult mouse pancreas, and interrogate their capacity to act as multilineage progenitors.
Results
ALDH1 Expression in Embryonic and Adult Pancreas.
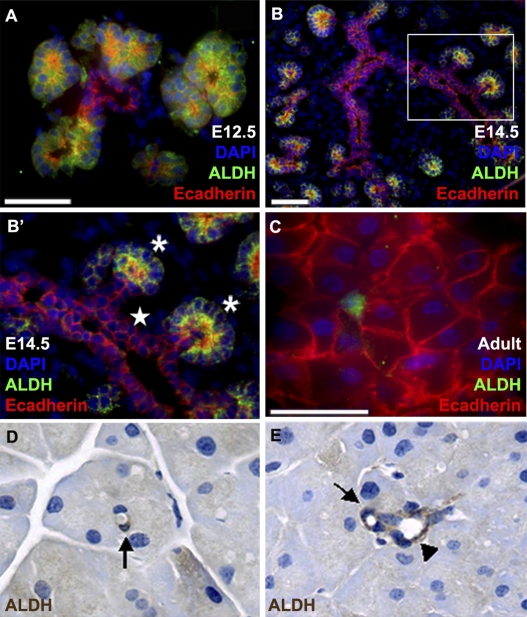
Based on prior studies documenting high levels of ALDH1 enzymatic activity in neural, hematopoietic, and mammary epithelial progenitors (17–19), we characterized temporal and spatial patterns of ALDH1 protein expression in embryonic and adult mouse pancreas (Fig. 1). Using E-cadherin as a marker of pancreatic epithelial cells, we found that ALDH1 protein is first detectable within the developing pancreatic epithelium on E12.5 (Fig. 1A). At this point, expression is restricted to the tips of the branching tubules, recently proposed to represent a multipotential progenitor domain (20). A similar pattern of expression has previously been reported for Aldh1a1 transcripts (21). Expression in the tubular tips (and not in the central trunks) persists through E14.5 (Fig. 1 B and B′), and is subsequently down-regulated in differentiating acinar cells. In adult pancreas, epithelial ALDH1 expression is most frequently observed in centroacinar and terminal ductal epithelial cells (Fig. 1 C–E). Mesenchymal (E-cadherin-negative) ALDH1-expressing cells were also detected surrounding endocrine islets and exocrine acini (Fig. S1).
Fig. 1.
ALDH1 expression in embryonic and adult mouse pancreas. (A–C) Immunofluorescent labeling for ALDH1 protein (green) in combination with E-cadherin (red) to mark epithelial structures in E12.5 (A), E14.5 (B and B′), and adult mouse pancreas (C). Image in (B′) represents higher magnification view of area indicated by box in (B). Note restriction of ALDH1 expression to tips of epithelial branches (indicated by asterisks in B′) and not more-central branch trunks (indicated by star). In adult pancreas (C), ALDH1 expression is restricted to a subset of E-cadherin-positive centroacinar cells. (D and E) Immunohistochemical detection of ALDH1 protein (brown) in subsets of centroacinar (arrows) and terminal duct cells (arrowhead). (Scale bars: 50 μM.)
To further characterize ALDH1 expression and ALDH1 enzymatic activity in adult terminal duct/centroacinar cells, we used preparations of peripheral acinar-ductal units freshly isolated from collagenase-digested mouse exocrine pancreas (22). Importantly, these isolated peripheral acinar-ductal units are markedly depleted of large duct and endocrine elements. Compared with total pancreas, peripheral acinar-ductal units exhibited a >400-fold depletion in insulin transcripts, as assessed by RT-PCR (Fig. S2A). When FACS analysis was performed on peripheral acinar-ductal units harvested from transgenic Ins1:DsRed mice expressing red fluorescent protein in β-cells, only 3 of 10,000 cells (0.03%) from this preparation were positive for DsRed.
Additional three-dimensional characterization of ALDH1 protein expression was accomplished using whole-mount fluorescent labeling of isolated peripheral acinar-ductal units. ALDH1 protein was localized in combination with E-cadherin as a marker of epithelial cells, and with either FITC-conjugated Dolichos biflorus agglutinin (DBA) or FITC-conjugated peanut agglutinin (PNA), markers of ductal and acinar cells, respectively. Multichannel imaging confirmed a predominantly centroacinar/terminal ductal location of ALDH1-expressing epithelial cells in adult pancreas (Fig. S3). ALDH1-expressing cells were most often interposed between terminal ductal epithelium and more-peripheral acinar cells. In addition, single epithelial ALDH1-expressing cells were also observed immediately adjacent to terminal ductal epithelium (Fig. S3 A and B). Both DBA-positive and DBA-negative ALDH1-positive cells were identified, whereas ALDH1-positive PNA-positive cells were only rarely identified.
Isolation of ALDH1-Expressing Centroacinar and Terminal Ductal Cells.
In hopes of isolating ALDH1-expressing cells from adult mouse pancreas, we took advantage of a fluorogenic substrate known as “Aldefluor” (StemCell Technologies), which has previously been used in the FACS-based isolation of hematopoietic, neural, and mammary epithelial stem cells (17–19). Before attempting FACS-based isolation of ALDH1-expressing pancreatic epithelial cells, we first applied this reagent to visualize living ALDH1-expressing cells in peripheral acinar-ductal units (Fig. 2). As shown in Fig. 2 E and F, these studies confirmed the centroacinar/terminal ductal location of low-abundance Aldefluor-positive cells in adult mouse pancreas. Aldefluor-positive centroacinar/terminal ductal cells were easily distinguished from adjacent acinar cells by virtue of their small size and lack of zymogen granules. Additional examples of live-cell imaging using the Aldefluor reagent are provided in Fig. S4. These findings implied that anti-ALDH1 immunofluorescence and Aldefluor-based cytofluorescence were labeling a similar centroacinar/terminal ductal population, and further suggested that these cells might be successfully isolated by FACS.
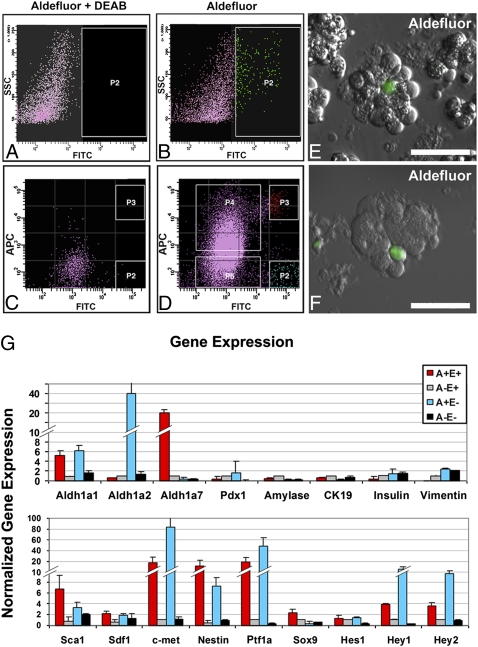
Fig. 2.
FACS isolation of ALDH1-expressing centroacinar/terminal ductal epithelial cells using the Aldefluor reagent. FACS sorting was performed on single cells isolated from peripheral acinar-ductal units depleted of endocrine and large duct elements. (A and B) Gating of Aldefluor-positive cells based on DEAB-sensitive ALDH1 enzymatic activity. y axis indicates side scatter; x axis indicates intensity of Aldefluor signal (A) with and (B) without DEAB. (B and C) Detection of ALDH1 enzymatic activity (C) with and (D) without DEAB, in conjunction with surface detection of E-cadherin protein. y axis represents intensity of labeling with APC-conjugated anti-E-cadherin antibody; x axis indicates intensity of Aldefluor signal. FACS-sorted populations indicated by P2, P3, P4, and P5 in D correspond to Aldefluor-positive, E-cadherin-negative (A+E−), Aldefluor-positive, E-cadherin-positive (A+E+), Aldefluor-negative, E-cadherin-positive (A−E+), and Aldefluor-negative, E-cadherin-negative (A−E−), respectively. (E and F) Imaging of collagenase-digested mouse pancreas using Aldefluor reagent confirms centroacinar/terminal ductal localization of Aldefluor-positive cells, similar to that observed for ALDH1 immunofluorescence (Figs. 1 and 2). Note centroacinar/terminal ductal position and small size of Aldefluor-positive cells relative to larger acinar cells, which are easily identifiable by granular cytoplasm corresponding to apical zymogen granules. (Scale bars: 50 μM.) (G) Quantitative RT-PCR analysis of gene expression in A+E+ cells (red), A+E− cells (white), A+E− cells (blue), and A−E− cells (black). Compared with A−E+ aldefluor-negative epithelial cells, A+E+ aldefluor-positive centroacinar/terminal ductal epithelial cells are enriched for transcripts encoding Aldh1a1, Aldh1a7, Sca1, Sdf1, c-Met, Nestin, Ptf1a, and Sox9. (Scale bars: 50 μM.)
We next pursued FACS-based characterization and sorting of single cells dissociated from peripheral acinar-ductal units. As an initial means to establish specific gating of ALDH1-expressing cells, we employed a pharmacologic inhibitor of ALDH1 enzymatic activity (DEAB). As depicted in Fig. 2 A–D, this strategy allowed for the isolation of a low-abundance cell population characterized by high levels of DEAB-sensitive ALDH1 enzymatic activity, comprising 0.9% ± 0.2% of all sorted cells in adult mouse pancreas. Using an E-cadherin antibody to simultaneously identify epithelial cells, Aldefluor (+) E-cadherin (+) cells were found to represent 0.5% ± 0.13% of all sorted cells in adult mouse pancreas (Fig. 2D).
Using quantitative RT-PCR to compare the Aldefluor-positive, E-cadherin-positive (A+E+), Aldefluor-negative, E-cadherin-positive (A−E+), Aldefluor-positive, E-cadherin-negative (A+E−), and Aldefluor-negative, E-cadherin-negative (A−E−) populations isolated from adult mouse pancreas, we found the A+E+ population to be significantly enriched for transcripts encoding Aldh1a1 and Aldh1a7, and depleted of transcripts for two other ALDH1 isoforms, Aldh1a2 and Aldh1a3 (Fig. 3G). Aldh8a1 was not detected in any of the samples. Compared with the A−E+ population, A+E+ cells were modestly depleted of transcripts for Pdx1 (P < 0.09), Amylase (P < 0.001), and Cytokeratin-19 (P < 0.01) (markers expressed in differentiated β-cells, acinar cells, and duct cells, respectively). In contrast, A+E+ cells were characterized by high-level expression of Ptf1a, despite that they were depleted of both Amylase transcripts and amylase protein (Fig. 3G and Fig. S5). In addition, these cells were enriched for transcripts encoding Sca-1, SDF1, c-Met, Nestin, Sox9, Hey1, and Hey2, markers previously associated with progenitor populations in pancreas and other tissues. Using immunofluorescent labeling on cytospin preps of FACS-sorted cells, we confirmed marked enrichment for ALDH1 and Sox9 protein, and depletion of amylase in A+E+ cells (Fig. S5). In addition, we also performed FACS analysis to determine the frequency with which Aldefluor (+) cells were also positive for stem cells markers such as CD133 and Sca-1 protein, and observed that over 90% of the Aldefluor (+) cells additionally coexpressed both of these stem cell markers. In contrast, only 0.11% of Aldefluor (+) cells were also positive for the vascular endothelial marker PECAM, whereas 0.08% were positive for the hematopoietic marker CD45. When FACS analysis was performed on peripheral acinar-ductal units harvested from transgenic Ins1-DsRed mice expressing red fluorescent protein in β-cells, all Aldefluor (+) cells were found to be negative for dsRed.
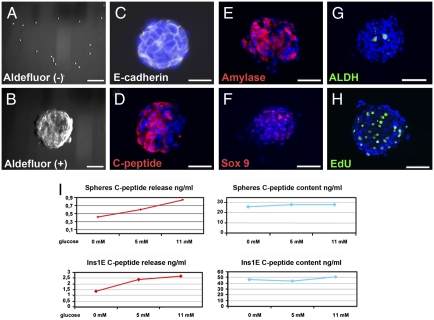
Fig. 3.
Formation, differentiation, and function of pancreatospheres derived from Aldefluor-positive centroacinar/terminal ductal cells. (A and B) A+E+ centroacinar/terminal ductal epithelial cells, but not A−E+ epithelial cells, efficiently form pancreatospheres in suspension culture. (C–G) Expression of E-cadherin (C), insulin C-peptide (D), amylase (E), Sox9 (F), and ALDH1 (G) in day 7 pancreatospheres formed from A+E+ centroacinar/terminal ductal epithelial cells. (H) Cell proliferation in day 7 pancreatospheres as assessed by overnight incorporation of EdU added on day 6 of culture period. (I) ELISA-based assay of stored and secreted insulin C-peptide following overnight incubation of either pancreatospheres or Ins-1 cells in varying concentrations of glucose. Note that pancreatospheres display glucose sensitivity similar to that observed in Ins-1 cells (i.e., ∼2-fold increase in secreted C-peptide in response to 0 vs. 11 mM glucose). (Scale bars: 100 μM.)
Pancreatosphere Assay of Endocrine and Exocrine Progenitor Function.
As an initial screen for progenitor-like activity, we assayed Aldefluor (+) and Aldefluor (–) cells for the ability to form pancreatospheres (Fig. 3), similar to the neurosphere assay commonly used to identify neural progenitors (23). In these assays, A+E+ centroacinar/terminal ductal cells were uniquely able to form spheres in suspension culture. A+E+ cells displayed a sphere-forming efficiency >100 times that of their A−E+ counterparts (Fig. 3 A and B and Table S1). With lower efficiencies, single A+E+ cells were even able to form spheres when plated at clonal density (one cell per well) in 96-well plates (Table S1). Neither of the E-cadherin-negative populations exhibited significant sphere-forming capacity. When cultured over a 5- to 7-day period, pancreatospheres derived from A+E+ cells exhibited strong expression of E-cadherin (Fig. 3C), confirming their epithelial identity, and individual cells within the spheres began to accumulate considerable amounts of either amylase or insulin and insulin C-peptide (Fig. 3 D and E). At 5 days, ≈50% of pancreatospheres displayed expression of amylase, whereas some 30% displayed immunoreactivity to insulin C-peptide. Individual spheres were generally positive for either insulin or amylase, but not both. Small subsets of cells within the spheres maintained ALDH1 expression during the culture period, and also demonstrated nuclear expression of Sox9 protein (Fig. 3 F and G), suggesting the possible maintenance of a self-renewing progenitor pool. This apparent capacity for self-renewal was further supported by the fact that pancreatospheres generated by Aldefluor (+) centroacinar/terminal ductal cells could be subjected to serial enzymatic dissociation, maintaining their sphere-forming capacity over a minimum of three sequential passages at 7-day intervals. In addition, cells within spheres were highly proliferative, as assessed by overnight incorporation of EdU added at either the beginning or the end of the culture period (Fig. 3H).
Based on the distinct progenitor capacities displayed by A+E+ cells, we further examined sorted cell populations for expression of Ngn3, a marker of endocrine progenitor cells (Fig. S2B). Consistent with previous studies (7), we were unable to detect significant expression of Ngn3 in either total adult pancreas or any of the freshly sorted cell populations. However, once the A+E+ cells were placed in culture, they began to generate detectable expression of Ngn3 immediately preceding the onset of insulin expression, further confirming the endocrine progenitor capacity of ALDH1-expressing centroacinar and terminal ductal epithelial cells.
Pancreatospheres Derived from Aldefluor (+) Terminal Ductal/Centroacinar Cells Display Glucose-Responsive Insulin Secretion.
The detection of cells expressing insulin and insulin C-peptide in cultured pancreatospheres prompted assessment of whether these cells were capable of glucose-responsive insulin secretion, a characteristic of functional β-cells. As a positive control, we used Ins-1 cells (clone 832/13) an immortalized β-cell line commonly used for studies of insulin secretion in response to physiological concentrations of glucose. Following overnight incubation of either pancreatospheres or Ins-1 cells in 0, 5, and 11 mM glucose, both culture media supernatants and cell lysates were harvested and assayed for secreted and cellular insulin C-peptide using an ELISA-based assay. Pancreatospheres derived from Aldefluor (+) centroacinar/terminal ductal cells secreted C-peptide in a glucose-dependent manner, with glucose sensitivity similar to that displayed by Ins-1 cells (Fig. 3I).
Aldefluor (+) Adult Terminal Ductal/Centroacinar Cells Can Contribute to Embryonic Endocrine and Exocrine Lineages.
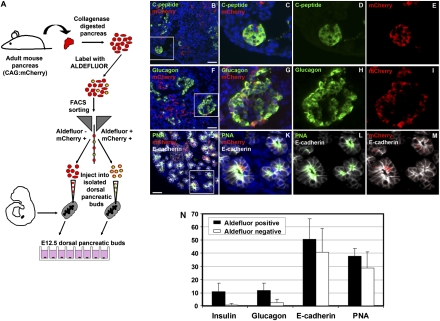
As an even more stringent test for pancreatic progenitor activity, we microinjected isolated Aldefluor (+) and Aldefluor (–) cells into microdissected dorsal pancreatic buds isolated from E12.5 mouse embryos, and assayed for an ability to productively contribute to the developing endocrine and exocrine lineages (Fig. 4). This approach was recently used to document progenitor activity for Ngn3-expressing cells arising following pancreatic duct ligation (7). To trace the lineage of adult-derived donor cells and distinguish them from their embryo-derived counterparts, we isolated Aldefluor (+) and Aldefluor (–) cells from the pancreas of mice carrying a ubiquitously expressed pCAG:mCherry transgene, as schematically depicted in Fig. 4A (pCAG:mCherry mice were kindly provided by Michael Wolfgang, Johns Hopkins University). When compared with Aldefluor (–) cells, Aldefluor (+) cells carried a dramatically enhanced potential to contribute to emerging endocrine lineages within the maturing dorsal buds, as demonstrated by coexpression of mCherry with either C-peptide (Fig. 4 B–E) or glucagon (Fig. 4 F–I). Using superimposed E-cadherin labeling to allow counting of individual mCherry-positive cells, we quantitatively evaluated the ability of adult Aldefluor (+) and Aldefluor (−) cells to enter into embryonic lineages. Seven days following microinjection of Aldefluor (+) cells into E12.5 dorsal buds, expression of glucagon was observed in 11.7% of residual mCherry positive cells, with insulin C-peptide expression observed in an additional 11.6% (Fig. 5N). In contrast, 2.4% of residual mCherry-positive Aldefluor (−) cells expressed glucagon, and only 0.2% expressed insulin C-peptide. Interestingly, the Aldefluor (+) and Aldefluor (–) populations displayed equivalent abilities to enter into nonendocrine epithelial lineages, perhaps reflecting the fact that most of the Aldefluor (–) population was comprised of already differentiated acinar cells. Similar frequencies of E-cadherin, amylase, and PNA positivity were observed in residual mCherry-positive cells derived from either the Aldefluor (+) or Aldefluor (–) populations (Fig. 4 J–N).
Fig. 4.
Aldefluor-positive adult pancreatic cells enter both endocrine and exocrine lineages in cultured embryonic pancreas. (A) Schematic of experiment. To trace the lineage of adult cells, Aldefluor (+) and Aldefluor (–) cells were isolated from adult CAG:mCherry transgenic mouse pancreas, microinjected into microdissected dorsal pancreatic buds isolated from E12.5 nontransgenic mouse embryos, and assayed for an ability to productively contribute to the developing endocrine and exocrine lineages. (B–J) Coexpression of mCherry and insulin C-peptide (B–E) and mCherry and glucagon (F–I) confirms capacity of adult Aldefluor (+) cells to contribute to embryonic β- and α-cell lineages, whereas labeling of individual mCherry-positive cells with FITC-conjugated PNA (J–M) confirms ability to contribute to the embryonic acinar lineage. (N) Frequencies with which residual mCherry-positive adult Aldeflouor (+) and Aldefluor (−) cells label for insulin C-peptide, glucagon, E-cadherin, and PNA 7 days after microinjection into microdissected E12.5 dorsal pancreatic buds. All cell counts were determined using E-cadherin labeling to outline the boundary of individual cells. Note that the capacity for endocrine differentiation is predominantly limited to the Aldefluor (+) population, whereas both Aldefluor (+) and Aldefluor (−) cells can productively contribute to the developing exocrine lineages. (Scale bars: 50 μM.)
Fig. 5.
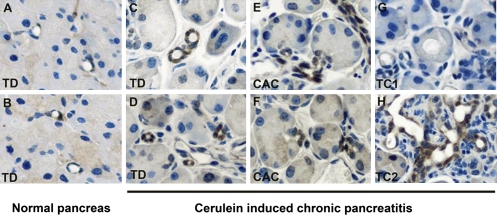
Expansion of ALDH1-expressing centroacinar and terminal ductal epithelial cells in setting of chronic inflammation and regenerative epithelial metaplasia. Following antigen retrieval, ALDH1 protein was detected using immunohistochemistry on pancreatic tissue from normal adult pancreas (A and B) and pancreas harvested from mice with chronic pancreatitis induced by three weekly injections of caerulein (C–H). (A and B) Low-frequency labeling for ALDH1 in terminal ductal (TD) epithelial cells from normal adult pancreas. (C and D) Expansion of ALDH1-expressing terminal ductal epithelium following sequential caerulein administration. (E and F) Similar expansion of ALDH1-expressing centroacinar cells (CAC) following sequential caerulein administration. (G and H) Expression of ALDH1 in caerulein-induced metaplastic type 2 (TC2; H), but not type 1 (TC1; G) tubular complexes.
Expansion of ALDH1-Expressing Centroacinar and Terminal Duct Cells Following Chronic Epithelial Injury.
To evaluate the in vivo behavior of ALDH1-expressing centroacinar and terminal duct cells, we assessed patterns of ALDH1 expression in the setting of chronic inflammation and regenerative metaplasia induced by sequential administration of low-dose caerulein. As previously reported (15), treatment of adult mice with three injections of caerulein (50 μg/kg) per week for 3 consecutive weeks induced a state of chronic pancreatitis followed by near complete regeneration and repair. This process is characterized by inflammatory infiltrates, stromal expansion, and the formation of regenerative metaplastic tubular complexes, which include type-1 tubular complexes previously shown to be acinar cell-derived, and type-2 tubular complexes (TC2), previously shown to be nonacinar derived and presumably arising from proliferating terminal duct cells (15). In contrast to the relatively low abundance of ALDH1-expressing terminal duct and centroacinar cells observed in normal adult pancreas (Fig. 5 A and B), ALDH1-expressing terminal duct (Fig. 5 C and D) and centroacinar (Fig. 5 E and F) cells were markedly expanded in the setting of caerulein-induced chronic pancreatitis. Of note, type-2 tubular complexes were comprised predominantly of ALDH1-expressing cells (Fig. 5H), whereas type-1 tubular complexes showed no evidence of ALDH1 expression (Fig. 5G).
Discussion
In normal adult pancreas, it has been shown that progenitor-like cells capable of in vitro endocrine differentiation can be enriched by flow cytometry using a variety of surface markers (24–27). However, a major challenge has been to unequivocally localize these cells in normal pancreas, as the combinatorial application of multiple cell-surface markers, each expressed along a continuous high-low gradient, has often precluded standard immunohistochemical or immunofluorescent approaches. More recently, Ngn3-positive endocrine progenitor cells were visualized as they arose adjacent to terminal ductal epithelium following pancreatic duct ligation (7), suggesting that, under normal conditions, Ngn3-negative multipotent progenitor cells may also reside in this position.
In the present study, we have identified a population of cells residing in a centroacinar/terminal ductal position and characterized by unique capacities suggesting progenitor function. Specifically, these cells express high levels of Ptf1a, Sox9, Sca-1, SDF-1, c-Met, and Nestin. Associated with this unique pattern of gene expression, adult Aldefluor-positive centroacinar and terminal ductal epithelial cells carry a unique capacity to form pancreatospheres, as well as to contribute to both endocrine and exocrine embryonic lineages. Together, these findings suggest that at least a subset of cells residing in a centroacinar/terminal ductal location is capable of progenitor function. Though lineage tracing studies in adult pancreas will be required to determine the actual role played by these cells during normal tissue homeostasis, our finding that this population undergoes dramatic expansion in the setting of chronic epithelial injury suggests that these cells are recruited in the context of pancreatic epithelial regeneration. Together with their location at the junction between peripheral secretory cells and more central ductal epithelium, these characteristics suggest similarity between centroacinar/terminal ductal cells and hepatic oval cells, an injury-responsive progenitor cell type similarly capable of multilineage differentiation (28).
In the present study, we exploited high levels of ALDH1 enzymatic activity purely as a marker of centroacinar/terminal ductal progenitors. Our studies therefore do not address whether this enzymatic activity, especially as it relates to synthesis of retinoic acid, plays an important role in the function of these progenitors, or whether they serve as local sources of retinoic acid production in a manner that organizes surrounding cells. Retinoids have previously been shown to exert profound influences on vertebrate pancreas development (29–31), and retinoic acid is a critical component for directed differentiation of human ES cells into insulin-producing β-cells (32). In the hematopoietic system, in vitro inhibition of ALDH1 enzymatic activity has been reported to somewhat paradoxically lead to the expansion of undifferentiated hematopoietic progenitors (33), and studies using gene-targeted mice have suggested that Aldh1a1-specific enzymatic activity is dispensable for hematopoietic and neural progenitor cell activity (34). However, the multiplicity of genes encoding ALDH1 enzymatic activity obviously renders single-gene loss-of-function studies difficult to interpret, and only a subset of ALDH1 family members (Aldh1a1, Aldh1a2, Aldh1a3, and Aldh8a1) appear to carry all-trans retinal dehydrogenase activity (http://www.aldh.org/superfamily.php). In this regard, Aldefluor-positive centroacinar and terminal ductal epithelial cells are characterized by high-level expression of Aldh1a1 and Aldh1a7, and low-level expression of Aldh1a2, Aldh1a3, and Aldh8a1.
Though centroacinar and terminal ductal epithelial cells certainly remain less well-characterized than other pancreatic cell types, our current findings add to an expanding knowledge base regarding these cells. In addition to mounting a proliferative response to various forms of pancreatic injury, centroacinar cells have also been shown to dramatically proliferate following pancreas-specific knockout of Pten, allowing them to act as apparent cells of origin for pancreatic neoplasia (16). Based on immunohistochemical labeling, these cells have also been suggested to undergo in vivo endocrine differentiation following islet injury (11). Relevant to their capacity to act as progenitors, human, mouse, and zebrafish centroacinar cells also appear to be characterized by active Notch signaling (13–16), a feature that they appear to share in common with terminal ductal epithelial cells (15). Surprisingly, Aldefluor-positive centroacinar and terminal ductal epithelial cells did not display up-regulation of Hes1 transcripts, but did exhibit up-regulated expression of Hey1 and Hey2, consistent with an active Notch pathway.
In summary, we have isolated a unique population of centroacinar and terminal ductal epithelial cells from adult mouse pancreas, and shown that these cells carry significant progenitor capacities. Though additional lineage tracing studies will be required to formally establish these cells as dedicated adult pancreatic progenitors, further characterization and manipulation of this population may prove useful in the treatment of human pancreatic disease.
Materials and Methods
Detailed experimental methods are provided in SI Text.
Dissociation of Adult Mouse Pancreas.
All animal studies were approved by the Animal Care and Use Committee at Johns Hopkins University. Peripheral acinar-ductal units, depleted of large ducts and endocrine islets, were prepared as previously described (22).
Aldefluor Assay and Sorting of Aldefluor-Positive and -Negative Cells by FACS.
The Aldefluor Kit (StemCell Technologies) was used according to the manufacturer’s instructions to identify and isolate cells with high vs. low ALDH enzymatic activity. Flow cytometry was performed using a FACSAria (Becton Dickinson) flow cytometer.
Pancreatosphere Formation Assay.
Pancreatosphere formation assays on sorted Aldefluor-positive and -negative cells were performed by plating cells in 24-well ultra-low attachment plates (Corning) at a density of 6 cells/μL. Cells were grown for 5–7 days. For quantitative assays of pancreatosphere formation, cells were sorted directly into 96-well ultra-low attachment plates (Corning) at a density of 1, 10, or 100 cells per well (0.01 cell/μL; 0.1 cell/μL; 1 cell/μL). Serial passages were performed by dissociating spheres using the NeuroCult Chemical Dissociation Kit (StemCell Technologies), selecting viable cells based on trypan blue exclusion, and replating at a density of 6 cells/μL.
Insulin (C-peptide) Secretion Assays.
Assays of pancreatosphere insulin secretion were performed after 7 days in culture. Pancreatospheres were washed and then incubated in D-glucose-free RPMI supplemented with 0.25% BSA at three different glucose concentrations (0 mM, 5 mM, and 11 mM). After 12 h, we removed media, lysed pancreatospheres in 1 M glacial acetic acid, and determined insulin C-peptide levels by ELISA (ALPCO).
Injection of Aldefluor-Positive/mCherry Cells in E12.5 Embryonic Pancreas.
E12.5 dorsal pancreatic buds were isolated and injected with 1,000 Aldefluor-positive or -negative cells freshly harvested from adult pCAG:mCherry transgenic mouse pancreas. pCAG:mCherry mice were kindly provided by Michael Wolfgang, Johns Hopkins University. The injected dorsal bud explants were cultured in vitro for 7 days as previously described (35).
Supplementary Material
Acknowledgments
We thank Dr. Michael Wolfgang for generously providing pCAG:mCherry mice; Drs. Michael Shamblott, Mehboob Hussain, Michael Parsons, and members of the Leach Lab for many helpful discussions; Danielle Blake for outstanding technical and administrative support; and Dr. Mark Soloski, Lee Blosser, and Ada Tam of the Johns Hopkins Flow Cytometry Core for expert FACS analysis. This work was supported by the Chicago Diabetes Project (M.R., J.J., and S.D.L.), the Juvenile Diabetes Research Foundation (J.J.), an American College of Surgeons Clowes Award (to S.P.T.), and National Institutes of Health Grants DK61215 (to S.D.L.), DK070636 (to J.J.), and DK071329 (to S.P.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912589107/DCSupplemental.
References
- 1.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA. 2009;106:7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 4.Fendrich V, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Zulewski H, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 9.Leeson TS, Leeson R. Close association of centroacinar/ductular and insular cells in the rat pancreas. Histol Histopathol. 1986;1:33–42. [PubMed] [Google Scholar]
- 10.Hayashi KY, et al. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66:163–174. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- 11.Nagasao J, Yoshioka K, Amasaki H, Mutoh K. Centroacinar and intercalated duct cells as potential precursors of pancreatic endocrine cells in rats treated with streptozotocin. Ann Anat. 2003;185:211–216. doi: 10.1016/s0940-9602(03)80025-0. [DOI] [PubMed] [Google Scholar]
- 12.Gasslander T, Ihse I, Smeds S. The importance of the centroacinar region in cerulein-induced mouse pancreatic growth. Scand J Gastroenterol. 1992;27:564–570. doi: 10.3109/00365529209000120. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 14.Parsons MJ, et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strobel O, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanger BZ, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Storms RW, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corti S, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Oström M, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means AL, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GP, II, et al. Production of neurospheres from CNS tissue. Methods Mol Biol. 2008;438:135–150. doi: 10.1007/978-1-59745-133-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53:2143–2152. doi: 10.2337/diabetes.53.8.2143. [DOI] [PubMed] [Google Scholar]
- 25.Ku HT. Minireview: Pancreatic progenitor cells—recent studies. Endocrinology. 2008;149:4312–4316. doi: 10.1210/en.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci USA. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima Y, et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Rountree CB, et al. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 29.Martín M, et al. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 31.Stafford D, et al. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- 32.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 33.Chute JP, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi BP, Yilmaz OH, Duester G, Morrison SJ. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood. 2009;113:1670–1680. doi: 10.1182/blood-2008-05-156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esni F, Miyamoto Y, Leach SD, Ghosh B. Primary explant cultures of adult and embryonic pancreas. Methods Mol Med. 2005;103:259–271. doi: 10.1385/1-59259-780-7:259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.