Abstract
The spleen is the lymphoid organ that induces immune responses toward blood-borne pathogens. Specialized macrophages in the splenic marginal zone are strategically positioned to phagocytose pathogens and cell debris, but are not known to play a role in the activation of T-cell responses. Here we demonstrate that splenic marginal metallophilic macrophages (MMM) are essential for cross-presentation of blood-borne antigens by splenic dendritic cells (DCs). Our data demonstrate that antigens targeted to MMM as well as blood-borne adenoviruses are efficiently captured by MMM and exclusively transferred to splenic CD8+ DCs for cross-presentation and for the activation of cytotoxic T lymphocytes. Depletion of macrophages in the marginal zone prevents cytotoxic T-lymphocyte activation by CD8+ DCs after antibody targeting or adenovirus infection. Moreover, we show that tumor antigen targeting to MMM is very effective as antitumor immunotherapy. Our studies point to an important role for splenic MMM in the initial steps of CD8+ T-cell immunity by capturing and concentrating blood-borne antigens and the transfer to cross-presenting DCs which can be used to design vaccination strategies to induce antitumor cytotoxic T-cell immunity.
Keywords: antigen presentation, infection
The spleen is essential for the induction of immune responses toward blood-borne antigens and has a unique architecture. Arterial blood flow terminates in marginal sinuses situated in the marginal zone (MZ) that surrounds the white pulp containing B-cell follicles and T-cell zones. Marginal sinuses are lined by reticular cells and contain marginal zone B cells and two types of macrophages (Mϕ) (1, 2). Marginal metallophilic macrophages (MMM), characterized by the expression of sialic acid-binding Ig-like lectin-1 (Siglec-1, Sialoadhesin, CD169) (3, 4), are located as a tight network in the inner part of the MZ near the white pulp, whereas marginal zone macrophages (MZM), which specifically express the C-type lectin SIGN-R1, can be found in the outer MZ toward the red pulp (5). Both MZM and a subset of MMM express the type I scavenger receptor MARCO (6). Although MMM and MZM efficiently take up particulate antigens (Ag) present in the blood (7–9), they are hitherto considered not to be important for the generation of T-cell responses (8–10).
In contrast to Mϕ, dendritic cells (DCs) are specialized Ag-presenting cells that have a dominant role in initiating primary T-cell responses. Murine splenic DCs can be divided into two different subsets based on the expression of phenotypic markers: localization and function (11). CD8+ DCs express the C-type lectin DEC205 and are found in the T-cell zone and the outer marginal zone (12). They are specialized in cross-presentation of Ag and in the activation and tolerization of cytotoxic T cells (CTLs) (13–16). Furthermore, they are important for the generation of antitumor specific immune responses and the elimination of tumors in vivo (17). In contrast, CD8− DCs are specialized in the activation of CD4+ T cells. CD8− DCs are mainly localized in the marginal zone and selectively express the C-type lectin DCIR2. Upon activation, all DC subsets migrate to the T-cell zone (18).
In this study, we have investigated the role of splenic Mϕ in the activation of CTL responses. We were able to demonstrate the transfer of Ag to cross-presenting CD8+ DCs and the induction of strong cytotoxic T-cell responses after targeting of Ag to marginal metallophilic macrophages. In addition, MMM were shown to contain adenovirus-encoded Ag and were essential for the generation of CTL responses after intravenous adenovirus vaccination. Our data show a physiological collaboration between MMM and DCs whereby the cross-presenting CD8+ DCs make use of the efficient Ag-concentrating capacity of the MMM. This process can be used for targeting strategies to induce antitumor CTL responses.
Results
Ag Targeting to MMM Results in Efficient CD8+ T-Cell Activation.
To study the role of splenic Mϕ in Ag presentation and T-cell activation, we covalently conjugated the OVA Ag to various monoclonal antibodies (mAb) to specifically target different Mϕ subsets in the spleen. This was achieved by using an mAb to Siglec-1 that is only expressed by MMM, the scavenger receptor MARCO that is strongly present on MZM and on a subset of MMM, F4/80 that is expressed by red pulp Mϕ, and SIGN-R1 that is specifically expressed by MZM. Stainings of spleen cryosections with these mAb confirmed the specific expression patterns on MMM and MZM (Fig. S1A), and upon i.v. injection the antibody-OVA conjugates showed specific binding to specific Mϕ subsets (Fig. S1B).
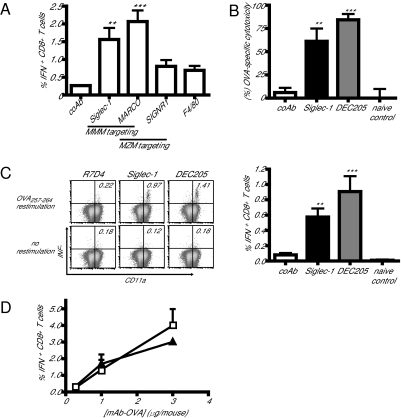
To study whether specific targeting would lead to differences in CD8+ T-cell activation, mAb-OVA complexes were i.v. injected together with activating αCD40 mAb. Seven days after immunization, splenocytes were restimulated in vitro with the H-2Kb OVA257–264 epitope and examined for intracellular IFNγ production as a measurement for OVA-specific CD8+ T-cell induction. Ag targeting to MMM with OVA conjugates directed to both Siglec-1 and MARCO resulted in high numbers of IFNγ-producing CD8+ T cells, whereas immunization with the irrelevant control mAb did not activate CD8+ T cells in vivo (Fig. 1A). Targeting to MZM via SIGN-R1 or red pulp Mϕ via F4/80 resulted in lower CD8+ T-cell responses not significantly different from the irrelevant control mAb (Fig. 1A).
Fig. 1.
Ag targeting to MMM results in efficient CD8+ T-cell priming. (A) Evaluation of CD8+ T-cell priming after targeting to splenic Mϕ subsets. Mice were i.v. immunized with 1 μg indicated mAb-OVA together with 25 μg αCD40 mAb. After 7 days, spleen cells were restimulated in vitro for 5 h with the MHC class I OVA257–264 peptide and stained for CD8, CD11a, and intracellular IFNγ. Graphs show the percentage of CD8+CD11a+ T cells producing IFNγ. (B) Mice were injected with the indicated mAb-OVA complexes and, 7 days later, CFSE-labeled OVA257–264-peptide-coated cells were injected intravenously. After 4 h, the cytotoxicity of OVA257–264-peptide-coated cells was analyzed by FACS. (C) Splenocytes were obtained from mice 7 days after immunization with 1 μg indicated mAb-OVA, plus 25 μg αCD40 mAb. Cells were restimulated in vitro and analyzed for OVA-specific IFNγ production. The bar graph indicates average frequency of IFNγ-positive CD8+CD11a+ T cells in mAb-OVA immunized mice. (D) Mice were immunized with a titration of αSiglec-1-OVA (squares) or αDEC205-OVA (triangles). After 7 days, splenocytes were restimulated in vitro. Control mAb-OVA did not result in T-cell IFNγ production above 0.25% for CD8+ T cells. Error bars indicate SEM, n = 5 mice per group. ***P < 0.01, **P < 0.05 versus control mAb-OVA. P values were calculated by one-way ANOVA with Bonferroni correction (GraphPad Prism 4 software).
Direct comparison of the efficiency of Ag targeting to different Mϕ subsets via conjugation to different Ab has some limitations. Different surface receptors are targeted using Ab which may exhibit differences in affinity and trafficking behavior, which may be further modified due to the chemical conjugation to Ag. These differences may potentially explain the low CD8+ T-cell stimulating capacity of Ab targeting to MZM or red pulp Mϕ. However, with these limitations taken into account, our data indicate that targeting to MMM results in efficient CD8+ T-cell priming.
It has been well-documented that for the generation of CD8+ cytotoxic T cells, the CD8+ dendritic cells expressing DEC205 are crucial and that specific targeting to DEC205 leads to strong CTL activation (16). We therefore wished to compare the efficiency in the generation of CTLs between αSiglec-1-OVA complexes and αDEC205-OVA complexes. As reported previously (16), targeting to CD8+ DCs with αDEC205-OVA resulted in strong CTL activation, as measured by the in vivo killing of OVApeptide-loaded carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes (Fig. 1B) and the frequency of IFNγ-producing CD8+ T cells (Fig. 1C). Targeting to MMM was as effective in CD8+ T-cell induction as direct DC targeting (Fig. 1 B–D). The comparable efficiency over a broad range of conjugate doses (Fig. 1D) is surprising, because the amount of OVA linked to αSiglec-1 mAb was approximately 6-fold less than to αDEC205 mAb (Fig. S1C). Interestingly, we could not detect IFNγ-producing CD4+ T cells after immunization with αSiglec-1-OVA (Fig. S2A).
MMM Specifically Transfer Captured Ags to CD8+ DCs In Vivo.
We showed that OVA targeted to MMM strongly activated OVA-specific CD8+ T-cell responses. Over the years, attempts to attribute a direct role in adaptive immunity to the Mϕ in the marginal zone have failed (8, 10), and in general Mϕ are thought to support T-cell functions only after initial priming by dendritic cells. We therefore hypothesized that CTL generation was a result of Ag transfer to splenic DCs. Because MMM are present in very low numbers in spleen preparations and are extremely difficult to purify, we were not successful in isolating them for in vitro transfer studies. Therefore, we investigated whether DCs could acquire Ag from MMM in vivo. For this, CD11c+ DCs were isolated from mice after immunization with mAb-OVA complexes, and the capacity to present OVA Ag was tested by culturing them with OVA-specific CD8+ T cell receptor transgenic OT-I T cells and OVA-specific CD4+ T cell receptor transgenic OT-II cells. Strong OT-I proliferation was observed after stimulation with CD11c+ DCs isolated from mice injected with αSiglec-1-OVA or αMARCO-OVA complexes that target MMM, whereas CD11c+ DCs isolated from mice injected with αSIGN-R1-OVA or F4/80-OVA complexes that target MZM or red pulp Mϕ, respectively, stimulated weak OT-I proliferation (Fig. 2A). This indicates that Ag targeted to MMM was transferred to DCs for MHC class I presentation. Analogous to our in vivo observations, we did not observe MHC class II restricted OVA presentation to OT-II cells with DCs purified from mice that had been injected with mAb-OVA complexes targeted to MMM or MZM (Fig. S2B).
Fig. 2.
CD8+ DCs efficiently cross-present Ag targeted to MMM for induction of CD8+ T-cell responses. (A) Splenic CD11c+ DCs were purified from animals 16 h after i.v. injection with 2.5 μg indicated mAb-OVA + 25 μg αCD40 mAb. Titrated numbers of DCs were cultured together with 105 purified OT-I T cells (black bars; 3 × 105 DCs/well plus 3-fold dilutions in gray and white bars). After 2 days, cultures were pulsed with 1 μCi [3H]thymidine/well. T-cell proliferation was measured after an additional coculture of 16 h. (B) Similarly, CD11c+ DC presentation of in vivo Ag targeted to MMM (anti-Siglec-1), CD8+ (anti-DEC205), or CD8− (anti-DCIR2) DCs was evaluated. (C) To determine whether mAb-OVA complexes, used for targeting Mϕ subsets in vivo, could directly be taken up by DCs, an in vitro Ag-presentation assay was performed. Single-cell suspensions were obtained from mouse spleens and CD11c+ DCs were purified. CD11c+ DCs (105) were cocultured with 10, 3, or 1 μg/mL mAb-OVA, together with purified OT-I T cells, and [3H]thymidine incorporation was determined as described above (10, 3, and 1 μg/mL mAb-OVA are depicted as black, gray, and white bars, respectively). (D) Mice were injected with 2.5 μg/mouse of the indicated mAb-OVA complexes together with 25 μg αCD40 Ab. CD11c+ DCs were isolated after 16 h. CD11chigh CD8− and CD8+ DCs were FACS-sorted from αSiglec-1-OVA immunized mice and tested for their ability to stimulate naive OT-I cells. [3H]Thymidine incorporation was determined as described above. Different bars indicate titration of DCs in the assay [starting at 1 × 105 DCs/well (black bars) with 3-fold dilutions (gray and white bars)]. Error bars indicate SEM of triplicate wells.
Next, we compared CD11c+ DCs isolated from αSiglec-1-OVA immunized mice to DCs from mice immunized with αDEC205-OVA that targets CD8+ DCs and DCs from mice immunized with αDCIR2-OVA that targets CD8− DCs. Targeting to CD8+ DCs with αDEC205-OVA as well as targeting to MMM with αSiglec-1-OVA resulted in strong OT-I proliferation (Fig. 2B). We did not detect OT-II proliferation after coculture with CD11c+ DCs isolated from αSiglec-1-OVA immunized mice, although targeting to CD8− DCs with αDCIR2-OVA led to strong OT-II proliferation, as previously reported (Fig. S2B) (16). Together, the results show that after targeting to MMM, DCs acquire the Ag and are able to activate Ag-specific CD8+ T cells.
CD11c+ DCs purified from nontreated mice were not able to stimulate OT-I proliferation in the presence of αSiglec-1-OVA in vitro, although strong proliferation was observed in the presence of αDEC205-OVA (Fig. 2C). This indicates that CD11c+ DCs cannot take up, process, and present OVA epitopes from αSiglec-1-OVA in vitro and suggests that the observed in vivo activation of CD8+ T cells via αSiglec-1-OVA was not due to nonspecific targeting of DCs.
Splenic DCs can be separated into CD8+ and CD8− subsets, of which the CD8+ DC subset is specialized in cross-presentation and CTL induction. To determine which DC subset was responsible for the observed CD8+ T-cell priming in vivo, DC subsets were sorted from αSiglec-1-OVA immunized mice and used as stimulators of naive OT-I cells. Only CD8+ DCs but not CD8− DCs from αSiglec-1-OVA immunized mice cross-presented OVA in the context of MHC class I molecules to OT-I T cells (Fig. 2D). These results clearly show that Ag targeting to MMM results in exclusive cross-presentation by CD8+ DCs to CTLs.
Clodronate Depletion of Macrophages Impairs T-Cell Priming via αSiglec-1.
To determine whether MMM were necessary for presentation of αSiglec-1-OVA complexes by DCs, mice were i.v. injected with Cl2MBP (clodronate) liposomes, which are very potent in depleting highly phagocytic cells including red pulp Mϕ, MMM, MZM, and DCs (19). Red pulp Mϕ and DCs repopulate the spleen within 7 days, whereas MMM and MZM take 2 weeks to a month to return to normal numbers. Analysis of spleens 7 days after administration of clodronate liposomes indicated that MMM were effectively depleted, whereas DC populations were present at normal numbers (Fig. 3A). At this time point, αSiglec-1-OVA complexes were injected and CD11c+ DCs were isolated 16 h after immunization. DCs isolated from clodronate liposome-treated mice completely lacked the capacity to cross-present OVA to OT-I cells in vitro (Fig. 3B). In contrast, direct targeting to CD8+ DCs via αDEC205-OVA complexes was not dependent on the presence of splenic Mϕ (Fig. 3B). Accordingly, in vivo OVA-specific cytotoxicity 7 days after immunization with αSiglec-1-OVA was lost in clodronate liposome-treated mice, whereas CTL activity achieved by targeting to CD8+ DCs via αDEC205-OVA was not affected (Fig. 3C). Together, these results indicate that MMM-mediated uptake of αSiglec-1-OVA is essential for the subsequent CD8+ DC cross-presentation to CTL.
Fig. 3.
Clodronate depletion of splenic Mϕ and PTx treatment inhibit Ag presentation by DCs and CTL activation after targeting to MMM. Splenic Mϕ were depleted from the spleen by i.v. injection of 200 μL clodronate liposomes. At day 7, spleens were analyzed for depletion of MMM. MMM were stained for Siglec-1 with SER4 (green) and DCs with αCD11c (red). (A) Original magnifications: ×20. (B) Seven days after clodronate liposome treatment, mice were immunized with indicated mAb-OVA complexes. Sixteen hours after immunization, DCs were purified and used as stimulators of naive OT-I T cells in an ex vivo Ag-presentation assay [starting at 3 × 105 DCs/well (black bars) with 3-fold dilutions (gray and white bars)]. (C) Seven days after Cl2MBP liposome treatment, animals were immunized with 2.5 μg of indicated mAb-OVA and, after 7 days, in vivo killing of OVA-loaded CFSE-labeled cells was analyzed by FACS. (D) Mice were injected i.p. with 500 ng PTx 8 h before immunization with 2.5 μg mAb-OVA together with 25 μg αCD40. Sixteen hours after immunization, DCs were isolated and tested for their capacity to activate naive OT-I T cells in vivo. Different bars indicate titration of DCs in the assay [starting at 1 × 105 DCs/well (black bars) with 3-fold dilutions (gray and white bars)]. Error bars indicate SEM of triplicate wells. Data are of experiments with three mice per group. ***P < 0.01 versus control animals calculated by t test.
Ag Transfer from MMM to CD8+ DCs Is Pertussis Toxin-Sensitive.
MMM are located in the MZ, whereas CD8+ DCs reside in the T-cell area of the white pulp and the outer marginal zone (12). Both DC subsets are continuously replaced with precursors from the blood at a rate of nearly 100,000 cells per day (20). We hypothesized that the continuous influx of CD8+ DC precursors from the blood or from the outer marginal zone could be the underlying mechanism by which DCs acquired Ag from the strategically positioned macrophages. Alternatively, MMM could be able to migrate into the T-cell area of the splenic white pulp. Because both processes would involve chemokine receptor-mediated migration, we used pertussis toxin (PTx), which inhibits Gαi protein-coupled receptors including chemokine receptor signaling. Treatment of mice with PTx inhibited Ag transfer from MMM to DCs, whereas direct targeting to CD8+ DCs was unaffected (Fig. 3D). This result supports the scenario that cell migration is required for Ag transfer from MMM to CD8+ DC, although the identity of the migratory cell type, that is, MMM, CD8+ DC, or other, remains to be elucidated.
MMM Are Essential for the Activation of CTLs via Intravenous Adenovirus Immunization.
A widely used vector for the induction of antitumor CTLs is adenovirus (21, 22), and to analyze the role of the spleen in the induction of CTLs by adenoviral vectors, we immunized mice intravenously or subcutaneously with recombinant adenovirus expressing ovalbumin (AdLOG). AdLOG immunization induced OVA-specific CTLs, as indicated by the capacity of immunized mice to kill OVApeptide-coated CFSE-labeled splenocytes within 4 h after injection (Fig. 4A), and by expansion of OVA-specific CD8+ T cells (Fig. S3 A and B). Intravenous injection of AdLOG was significantly more efficient in activating CTL responses as compared to s.c. injection (Fig. 4A). Interestingly, removal of the spleen completely abrogated the induction of OVA-specific CTLs after i.v. administration of AdLOG (Fig. 4 A and B and Fig. S3 A and B) and prohibited the induction of antitumor responses (Fig. S3 C and D). CTL induction in splenectomized mice could partially be restored by autotransplantation of whole spleen parts, but not by injection of splenocytes in single-cell suspension (Fig. 4B). Using GFP-expressing adenovirus, we could clearly show that, upon i.v. injection, virus-encoded proteins were detected in Siglec-1 expressing MMM (Fig. 4C). These results indicate that after i.v. adenovirus administration the spleen is the major site for CTL activation, that intact splenic tissue organization is needed for in vivo T-cell activation, and that MMM contain virus-encoded proteins.
Fig. 4.
Both splenic Mϕ and DCs are required for functional CTL induction after adenoviral infection. (A) Three days after splenectomy, mice were infected i.v. or s.c. with 5 × 109 viral particle (VP) AdLOG. Five days after infection, in vivo cytotoxicity was measured using CFSE-labeled OVA257–264-peptide-coated cells. (B) Mice were splenectomized and transplanted with isolated splenocytes, pieces of spleen, or left untreated. Seven days after surgery, mice were infected i.v. with 5 × 109 VP AdLOG. In vivo cytotoxicity was determined 5 days after infection. Error bars indicate SEM, n = 3 mice in control group, n = 4 in SplX, and n = 6 in retransplanted group. ***P < 0.01 versus control, **P < 0.05 versus SplX, calculated by t test (GraphPad Prism 4). (C) Twelve hours after infection with Adgfp, spleens were analyzed for GFP expression by immunofluorescence. MMM are detected in red (SER4) and anti-GFP staining is in blue. Original magnifications: 20. (D) For Mϕ depletion, wild-type mice were injected with 200 μL clodronate liposomes and for DC depletion, CD11cDTR mice were injected with 800 ng diphtheria toxin. After 24 h and 7 days, mice were infected i.v. with 5 × 109 VP AdLOG. In vivo cytotoxicity was determined 5 days after infection.
Analogous to our experiments with Ag-mAb targeting, we wished to establish the role of MMM and DCs in adenoviral specific CTL responses using cell depletion. We first used CD11c diphtheria toxin receptor (CD11cDTR) transgenic mice. Diphtheria toxin treatment of these mice leads to elimination of CD11c+ DCs and both Mϕ subsets present in the marginal zone (23) and completely prohibited the generation of CTLs after AdLOG immunization (Fig. 4D), whereas nontreated CD11cDTR and diphtheria toxin-treated wild-type mice elicited strong CTL responses (Fig. S4). When Mϕ were eliminated using the clodronate liposomes 7 days before adenoviral transfer again no CTL activation was observed (Fig. 4D). This result shows that the presence of DCs is not sufficient for the induction of CTL responses and that Mϕ are crucial for CTL generation after adenovirus infection. Together, these results indicate an important role for splenic Mϕ and specifically MMM in the uptake of adenovirus-encoded Ag and the subsequent generation of CTL responses specific for these Ag.
Targeting to MMM Elicits Antitumor CTL Responses.
Having established the importance of splenic macrophages for CTL induction and a putative specific role for the MMM based on virus localization, we wished to study whether specific targeting to MMM could be used to induce tumor vaccination. Using B16 melanoma cells expressing OVA, we could show that prophylactic vaccination with αSiglec-1-OVA as well as with αDEC205-OVA resulted in a strong inhibition of outgrowth of B16 tumors expressing OVA (Fig. 5). These results indicate that targeting to MMM may provide an efficient vaccination strategy for the induction of antitumor immune responses.
Fig. 5.
In vivo targeting to MMM results in functional antitumor CTL responses. Mice were i.v. immunized either with 5 μg αSiglec-1-OVA, 5 μg αDEC205-OVA (both together with 25 μg αCD40), or 5 × 107 pfu AdOVA. After 7 days, mice were injected with B16 melanoma cells expressing OVA and luciferase in the portal vein. Tumor growth was measured on day 5 after tumor implantation by in vivo imaging (IVIS200; Xenogen). Error bars indicate SEM, n = 3–4 mice per group. ***P < 0.01 versus control. P values were calculated by one-way ANOVA with Bonferroni correction (GraphPad Prism 4 software).
Discussion
In this study, we addressed the function of splenic marginal zone Mϕ subsets in the induction of CTL responses. We have demonstrated that MMM in the splenic MZ transfer Ag exclusively to CD8+ splenic DCs, leading to efficient cross-presentation and activation of CTLs. This collaboration between MMM and dendritic cells was demonstrated for both protein Ag and adenovirus infection. Finally, by specific targeting of tumor Ag to MMM, we were able induce antitumor CTL and inhibit tumor outgrowth. In conclusion, we have identified in the spleen a unique interaction between the innate immune system and the adaptive immune system, leading to efficient capture and presentation of blood-borne Ags.
Transfer of Ag from one cell to the other has previously been demonstrated between Ag-carrying DCs migrating from peripheral tissues and lymph-node-resident CD8+ DCs in lymph nodes draining the skin (24) or the lungs (25). Lymphatic drainage to the spleen has not been demonstrated and, although it cannot be excluded that Ag-carrying DCs from peripheral tissues may enter the spleen via the blood, the observations we present here clearly demonstrate Ag capture by macrophages and subsequent Ag presentation by dendritic cells. Although the precise mechanism involved in the actual cellular transfer remains to be elucidated, the anatomy of the spleen is ideally suited to accommodate the process. On the one hand, the MMM are strategically positioned to capture Ags from the slowly percolating bloodstream of the marginal zone, and on the other hand CD8+ DCs are continuously replaced by precursors from the blood (20). This replacement involves tethering in the marginal zone and migration into the T-cell zone, thereby passing the ring of MMM at the border of the white pulp.
In addition, there is a substantial population of CD8+ DCs present in the outer marginal zone (12). Little is known about their dynamics, but it can be envisaged that they can also migrate into the T-cell zone upon local activation and will pick up Ags from the MMM in the process. Alternatively, the MMM themselves may migrate into the T-cell zone and pass Ags to residing CD8+ DCs. However, in spite of careful examination, we could not detect any translocation of MMM into the T-cell zone after antigenic stimulation, making this route of Ag transfer very unlikely.
The transfer of Ag by MMM to cross-presenting CD8+ DCs results in CD8+ T-cell immunity. This process exists parallel to direct uptake and phagocytosis by CD8+ DCs themselves, as is illustrated by the effects of direct targeting to DEC205 on CD8+ DCs. Why would two mechanisms be present for uptake and cross-presentation of Ags to CD8+ T cells? Obviously, MMM and CD8+ DCs differ in the expression of uptake receptors and localization. MMM express pathogen recognition receptors, such as MARCO and Siglec-1 (1), that are absent on CD8+ DCs. Perhaps even more important, MMM are strategically positioned to take up Ag from the blood. They are located just beneath the endothelial cells lining the marginal sinus and are very well known to capture Ag present in the blood. In contrast, CD8+ DCs that are situated in the T-cell zone or marginal zone function poorly in capturing particulate and soluble substrates (12). This is illustrated by our experiment in which we infected mice that had been previously treated with clodronate liposomes and which lacked Mϕ, including MMM, but still contained DCs. Even though these mice contained functional DCs, they were not able to activate CTLs in response to adenoviral infection. This finding underlines the importance of Mϕ in the marginal zone for the CTL response against pathogens.
One of the main functions of Mϕ is to eliminate harmful pathogens. In DCs, lysosomal acidification and proteolytic degradation are delayed in favor of cross-presentation, which impairs the microbial killing capacity of DCs (26). In contrast, Mϕ such as MMM are well-equipped and even necessary for elimination of blood-borne pathogens, as shown for Listeria monocytogenes and Lymphocytic choriomeningitis virus (8, 9). Instead, CD8+ DCs have been shown to be permissive for L. monocytogenes entry and spreading in the spleen (27). The efficient uptake of pathogens by MMM followed by elimination as well as Ag transfer to CD8+ DCs would be very advantageous and even necessary for immediate survival as well as for the induction of adaptive immune responses.
Here we show that specific targeting of tumor Ag to splenic MMM leads to cross-presentation by CD8+ DCs and the induction of antitumor CTL responses. Adenovirus-mediated gene therapy to induce CTL responses against tumors and chronic viral infections has received considerable interest and has already led to clinical trials (28, 29). Our data suggest that the enormous potential of adenoviral vectors to induce stable, noncontracting CTL responses is based on their Ag uptake by MMM. We speculate that MMM continuously transfer adenoviral Ag to CD8+ DCs in the MZ. Targeting Ag to MMM via antibodies against Siglec-1 might mimic the reservoir effect of adenovirus, but without the danger of an adenoviral infection. Because also Siglec-1+ Mϕ have been detected near DEC205+ DCs in human spleen (30), further studies on targeting MMM would be of utmost interest for the development of optimal antitumor vaccination strategies.
Materials and Methods
Further details are available in SI Materials and Methods.
Mice.
C57BL/6 mice were obtained from Charles River or Janvier. CD11cDTR mice were a gift from Günter J. Hämmerling (Heidelberg, Germany). OT-I and OT-II mice were bred at the animal facility of the VU University Medical Center and have transgenic Vα2Vβ5 T-cell receptors that recognize OVA257–264/H2-Kb and OVA323–339/I-Ab, respectively. All mice were kept under specific pathogen-free conditions and were used in accordance with local animal experimentation guidelines. Splenectomy and autotransplantation are described in detail in SI Materials and Methods.
Adenovirus Infection, Tumor Injection, and In Vivo Imaging.
Mice were infected with different recombinant adenoviruses expressing OVA, GFP, melanoma tumor Ag TRP2, and luciferase or injected with B16 melanoma tumor cells expressing luciferase, ovalbumin, and GFP. Mice were evaluated using in vivo imaging (IVIS) and for in vivo cytotoxicity.
Depletion of Macrophages and DCs and PTx Treatment.
Mice were injected i.v. with Cl2MBP-containing liposomes (19) to eliminate macrophages, and DCs were depleted in CD11cDTR mice by injection of diphtheria toxin. Mice were injected intraperitoneally with pertussis toxin.
Immunizations and Detection of T-Cell Responses.
Mice were injected with 1 μg mAb-OVA plus 25 μg activating αCD40 mAb (1C10) intravenously. After 7 days, splenocytes were restimulated in vitro to detect OVA-specific CD8+ T-cell and CD4+ T-cell responses.
Isolation of T Cells and DCs and Ex Vivo and In Vitro Ag-Presentation Assays.
CD8+ T cells and CD4+ T cells were purified from OT-I and OT-II transgenic mice, respectively, and DCs were isolated from OVA-mAb immunized mice or from nonimmunized mice. OT-I and OT-II proliferation was measured by the incorporation of [3H]thymidine.
Coupling of Ovalbumin to mAbs and OVA ELISA.
Ovalbumin was covalently coupled to mAb MOMA-1 (specific for Siglec-1), ED31 (specific for MARCO), F4/80, NLDC145 (specific for DEC205), 33D1 (specific for DCIR2), R7D4 (negative control recognizing an idiotypic determinant on a mouse B-cell lymphoma), and 22D1 (specific for SIGN-R1). Efficiency of OVA coupling to mAb was determined by ELISA.
Confocal Microscopy.
Cryosections from spleens from mice injected with mAb-OVA (20 μg) or control B6 mice were stained with mAb specific for DCs and Mϕ subsets and the presence of injected mAb-OVA.
Supplementary Material
Acknowledgments
We thank N. Van Rooijen for providing clodronate liposomes and T. B. Geijtenbeek for providing SA-coated TransFluorSpheres and for comments on the manuscript. We thank R. E. Mebius, R. Roozendaal, S. van der Pavert, and M. Knippenberg for comments on the manuscript, and G. J. Hämmerling for providing CD11cDTR mice and B16gfpluc tumor cells. This work was supported by grant NWO917.46.311 from the Dutch Scientific Research program (J.M.M.d.H.) and by grant SFB704 funded by the German Research Foundation (DFG) to A.L.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909541107/DCSupplemental.
References
- 1.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 2.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraal G, Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986;58:665–669. [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker PR, Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989;169:1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geijtenbeek TB, et al. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 6.Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. The macrophage receptor MARCO. Microbes Infect. 2000;2:313–316. doi: 10.1016/s1286-4579(00)00296-3. [DOI] [PubMed] [Google Scholar]
- 7.Claassen IJ, Osterhaus AD, Claassen E. Antigen detection in vivo after immunization with different presentation forms of rabies virus antigen: Involvement of marginal metallophilic macrophages in the uptake of immune-stimulating complexes. Eur J Immunol. 1995;25:1446–1452. doi: 10.1002/eji.1830250547. [DOI] [PubMed] [Google Scholar]
- 8.Aichele P, et al. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol. 2003;171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 9.Seiler P, et al. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–2633. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
- 10.Ciavarra RP, Buhrer K, Van Rooijen N, Tedeschi B. T cell priming against vesicular stomatitis virus analyzed in situ: Red pulp macrophages, but neither marginal metallophilic nor marginal zone macrophages, are required for priming CD4+ and CD8+ T cells. J Immunol. 1997;158:1749–1755. [PubMed] [Google Scholar]
- 11.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 12.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Haan JMM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnorrer P, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 17.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Smedt T, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 21.Toes RE, et al. Protective anti-tumor immunity induced by vaccination with recombinant adenoviruses encoding multiple tumor-associated cytotoxic T lymphocyte epitopes in a string-of-beads fashion. Proc Natl Acad Sci USA. 1997;94:14660–14665. doi: 10.1073/pnas.94.26.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TC, Dayball K, Wan YH, Bramson J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J Virol. 2003;77:13407–13411. doi: 10.1128/JVI.77.24.13407-13411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Probst HC, et al. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Belz GT, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 27.Neuenhahn M, et al. CD8α+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Molinier-Frenkel V, et al. Longitudinal follow-up of cellular and. humoral immunity induced by recombinant adenovirus-mediated gene therapy in cancer patients. Hum Gene Ther. 2000;11:1911–1920. doi: 10.1089/10430340050129521. [DOI] [PubMed] [Google Scholar]
- 29.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 30.Pack M, et al. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008;123:438–446. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.