Abstract
A subset of neurons in the normal vertebrate nervous system contains double the normal amount of DNA in their nuclei. These neurons are all thought to derive from aberrant mitoses in neuronal precursor cells. Here we show that endogenous NGF induces DNA replication in a subpopulation of differentiating chick retinal ganglion cells that express both the neurotrophin receptor p75 and the E2F1 transcription factor, but that lack the retinoblastoma protein. Many of these neurons avoid G2/M transition and remain alive in the retina as tetraploid cells with large cell somas and extensive dendritic trees, and most of them express β2 nicotinic acetylcholine receptor subunits, a specific marker of retinal ganglion cells innervating lamina F in the stratum-griseum-et-fibrosum-superficiale of the tectal cortex. Tetraploid neurons were also observed in the adult mouse retina. Thus, a developmental program leading to somatic tetraploidy in specific retinal neurons exists in vertebrates. This program might occur in other vertebrate neurons during normal or pathological situations.
Keywords: AChR, cell cycle, dendritic tree, p75NTR, tectum
A debate on the presence of somatic polyploid neurons in vertebrates was raised in the 1960s. Nevertheless, technical limitations prevented a clear conclusion at that time (1). The current belief is that some neurons in the normal vertebrate nervous system are tetraploid (2), but the mechanism leading to this condition remains unclear. Some of these neurons could arise from aberrant mitoses in neural progenitor cells as previously proposed (2, 3). Otherwise, they could also result from a developmental program leading to somatic tetraploidy in specific neuronal populations, as occurs in several organisms whose cells undergo endoreduplication to increase cell size (4).
The vertebrate retina represents an ideal system to analyze this question as this tissue has been suggested to contain tetraploid retinal ganglion cells (RGCs) (5). Furthermore, postmitotic chick retinal neurons are known to reenter the cell cycle in response to the activation of p75NTR by NGF (6). Herein we examine the hypothesis that some RGC neurons may attempt cell cycle reentry driven by NGF in vivo, avoid cell division and apoptosis, and remain in the retina as tetraploid cells with increased cellular size.
Results
The Chick Retina Contains Tetraploid RGCs.
To determine whether the chick retina contains tetraploid RGCs, P1 retinal neurons were dissociated and fixed. The presence of nuclei with a 4C DNA content was then analyzed by flow cytometry in neurons expressing the intracellular marker Islet1, which at this stage is mainly detected in the ganglion cell layer (GCL) (Fig. S1). At P1 the retina is fully functional (7), and 11.82 ± 0.86% (n = 4) of the Islet1-positive neurons were observed to contain double the normal amount of DNA (Fig. 1B), thus demonstrating that a subpopulation of tetraploid RGCs is present in the chick retina. In contrast, retinal neurons lacking Islet1 were consistently diploid (Fig. 1A). The presence of tetraploid RGCs was confirmed in flat-mounts of P1 chick retinas by means of slide-based cytometry (SBC) focused on cells located in the GCL that were retrogradely labeled from the optic nerve with DiI (i.e., RGCs) and then stained with DAPI to quantify the amount of DNA in these neurons (Fig. S2 A, C, and D; Table S1). I-FISH further confirmed this finding (Fig. S2B). I-FISH was performed on neurons derived from partially trypsinized P1 retinas to enrich our preparations in RGCs (38.36 ± 0.92% of the cells were positive for the RGC-specific marker βIII tubulin). We observed that 6.19% of the nuclei (n = 210) contained four copies of a partially inverted tandem repeat previously identified in the pericentric region of chicken chromosome 8 (8).
Fig. 1.
Somatic tetraploid RGCs in the P1 chick and adult mouse retina. P1 chick retina (A and B) and adult mouse retina (C and D). Flow cytometric analysis demonstrates that a subset of Islet1-positive neurons (B and D) are tetraploid (4C) whereas all Islet1-negative (A and C) neurons show a 2C DNA content.
The Mouse Retina Contains Tetraploid Neurons.
As a proof that somatic tetraploidization in a subpopulation of retinal neurons also takes place in other higher vertebrates, dissociated retinal cells from adult mice were immunostained for Islet1, a marker expressed in this species by RGCs and a population of neurons from the inner nuclear layer (9), and analyzed by flow cytometry. This analysis demonstrated that 6.82 ± 2.22% (n = 4) of the murine Islet1-positive cells were tetraploid (Fig. 1D).
p75NTR Is Expressed by Early Differentiating RGCs.
To understand the mechanism responsible for the generation of tetraploid RGCs in the chick retina we focused on the expression pattern of p75NTR at E5, a stage when RGCs are being born and NGF induces cell cycle reentry through p75NTR in vitro (6). As in other neuroepithelia, retinal precursors undergo S-phase at basal positions (i.e., vitreal surface) and mitosis at the apical region, close to the pigment epithelium (10). The first neurons to differentiate in the retina are the RGCs (11), which express the RA4 marker less than 15 min after the end of mitosis (12) and acquire a differentiated morphology as they displace to the basally located GCL (13). In the E5 chick retina, p75NTR is present in migrating and layered RGCs, colocalizing with the early RGC-markers RA4 (12) and βIII tubulin (14), as well as with G4, a chicken glycoprotein mostly expressed by differentiated RGCs in this tissue (15) (Fig. S3). Therefore, p75NTR can be considered as an extremely early RGC marker.
Migrating RGCs Lacking Rb Can Reenter the Cell Cycle.
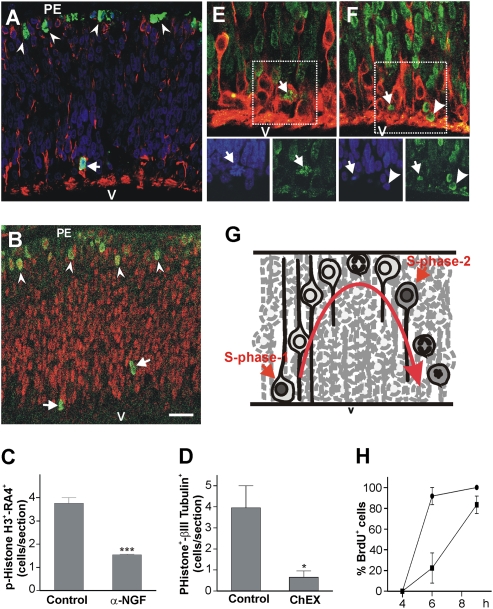
The transcription factor E2F1 is crucial for G1/S-phase progression and Rb is known to prevent E2F1 activity during G1, being the expression of these two proteins tightly controlled during neurogenesis (16). Double labeling with the anti-p75NTR antiserum and an Rb-specific antibody revealed a population of cells containing p75NTR but that lacked Rb expression, representing 25.60 ± 2.06% (n = 3) of all of the p75NTR-positive cells (Fig. S4A). Double labeling for RA4 and Rb confirmed the presence of differentiating RGCs lacking Rb expression (Fig. 2A). By contrast, the transcription factor E2F1 was detected in all of the migrating RGCs as revealed by double immunolabeling with RA4- and E2F1-specific antibodies (Fig. S4B). Double labeling for Rb and E2F1 further demonstrated the existence of retinal cells expressing E2F1 but lacking Rb (Fig. S4C). To study whether migrating RGCs which lack the expression of Rb could reenter the cell cycle in vivo, a 1 h pulse of BrdU was given to E5 chicken embryos and BrdU incorporation in retinal cryosections was studied. Besides the expected incorporation of BrdU into nuclei situated basally, there was also a population of retinal cells in S-phase within the apical half of the neuroepitelium (Fig. 2B). Most of these cells lack Rb (78.29 ± 2.85%; n = 3; Fig. 2B), and express the early RGC marker βIII tubulin (80.45 ± 6.33%; n = 3). These data suggest that some of the cells incorporating BrdU at the apical retina are migrating, postmitotic neurons undergoing DNA replication in vivo. This notion was confirmed by BrdU labeling of both RA4 and βIII tubulin-positive cells with extremely short (15 min) BrdU pulses (Fig. 2 C and D; Table S2), thus supporting the hypothesis that the presence of BrdU in these cells is not a consequence of rapid differentiation (12).
Fig. 2.
During development endogenous NGF induces cell cycle reentry and somatic tetraploidy in RGCs. Cryostat sections (A–D) or flow cytometry (E–J) from E5 chick retinas. A. The arrow points to a migrating RGC expressing RA4 (red) and lacking Rb immunoreactivity (green). The arrowhead indicates an RA4/Rb-double labeled cell. (B) After a 1 h pulse, BrdU (green) was observed in apically-located cells lacking Rb expression (red, see arrows). (C) After a 15 min pulse, most apically-located nuclei positive for BrdU (green) are RA4-positive (red) RGCs (arrows). (D) Arrows indicate the presence of BrdU (15 min treatment; green) in βIII tubulin-positive neurons (red) located at the apical half of the retina. (E) Anti-NGF-antibodies (α-NGF) reduce the proportion of RA4-positive cells incorporating BrdU, when compared to irrelevant antibodies (Control). (F) The anti-p75NTR antiserum ChEX reduces the percentage of βIII tubulin-positive cells incorporating BrdU, when compared to a control antiserum (Control). G. Islet1-negative precursors show a profile of cycling cells in G1, S and G2/M. (H) A subset of Islet1-positive RGCs are tetraploid (4C). (I) Percentage of Islet-1-positive neurons with 4C DNA content as detected by flow cytometry in dissociated retinal cells from embryos treated with hybridoma cells secreting either an irrelevant mAb (Control) or anti-NGF producing hybridoma cells (α-NGF). (J) Percentage of Islet-1-positive neurons with 4C DNA content, as detected by flow cytometry in dissociated E5 retinal cells from eyes injected at E4 with normal rabbit serum (Control) or the anti-p75NTR antiserum ChEX (ChEX). RGCs begin to be produced one day before antibody injection, thus explaining the modest reduction of cells with 4C DNA content. PE: pigment epithelium; v: vitreous body. (Scale bars: A, 15 μm; B–D, 12 μm.) *P < 0.05, **P < 0.01, ***P < 0.005.
NGF Induces Cell Cycle Reentry in Migrating RGCs.
To test whether endogenous NGF (17) might be responsible for the cell cycle reentry of migrating RGCs observed in vivo, hybridoma cells secreting an anti-NGF mAb or control hybridoma cells secreting an irrelevant mAb were applied onto the chorioallantoic membrane of E3 chicken embryos, and they were administered a short pulse of BrdU 1 h before sacrificing at E5. The anti-NGF antibody was previously shown to prevent p75NTR-dependent retinal apoptosis in vivo (17). A significant reduction in the incorporation of BrdU was observed in the RA4-positive RGCs of embryos treated with anti-NGF antibodies when compared with control embryos (Fig. 2E). Similarly, injection of the anti-p75NTR ChEX blocking antibody into the eye also significantly reduced the proportion of βIII tubulin-positive cells that incorporated BrdU when compared to the retinas of embryos injected with normal rabbit serum (Fig. 2F). This effect was specific of differentiating RGCs as anti-NGF treatment did not affect the proportion of RA4-negative cells incorporating BrdU (control: 37.93 ± 0.04%, n = 3; anti-NGF: 37.83 ± 0.13%, n = 3; non significant), and ChEX injection did not affect the proportion of βIII tubulin-negative cells incorporating BrdU (control: 38.68 ± 0.50%, n = 3; ChEX: 38.31 ± 0.46%, n = 3; nonsignificant). Because NGF does not induce the expression of neuronal markers in the retinal precursors (6), we conclude that this neurotrophin exerts its proliferative effect only in differentiating RGCs. This view was confirmed in vitro by using dissociated E5 retinal cells cultured under neurogenic conditions for 22 h (see Materials and Methods). As expected, a subpopulation of these cells reduced the levels of Rb in response to differentiation (Fig. S4D), while still maintaining strong E2F1 expression (Fig. S4E). This effect was largely specific of RGCs because 40.96 ± 2.25% (n = 3) of the G4-positive cells were observed to express Rb at low levels, whereas only 4.44 ± 0.08% (n = 3) of the G4-negative cells showed low levels of Rb expression (Fig. S4G). On the contrary, virtually all G4-positive cells contained high levels of E2F1 (Fig. S4F). As expected, BrdU incorporation (1 h pulse) was increased in response to NGF, mostly in differentiating RGCs expressing βIII tubulin (Fig. S4I). Accordingly, electroporation of the luciferase reporter gene under the control of the murine c-Myc core promoter, known to respond to E2F1 (18), demonstrated that NGF can induce E2F1 activity in the retinal cells (Fig. S4H). Although this effect might derive from a putative NGF-dependent decrease of Rb expression, this possibility was ruled out because the percentage of retinal cells expressing low levels of Rb was not affected by NGF (Fig. S4J). In accordance with the hypothesis that NGF acts on RGCs that lack Rb, overexpression of the latter protein reduced the proliferative capacity of the retinal cells and prevented the effect of NGF on BrdU incorporation after 1 h pulse (Fig. S4 K and L).
Most Migrating RGCs that Reenter the Cell Cycle Remain as Tetraploid Neurons.
The fate of the migrating RGCs undergoing cell cycle reentry was assessed in BrdU pulse-chase experiments in E5 chicken embryos followed by immunolabeling of βIII tubulin-positive neurons. When 1 h BrdU pulses in ovo were followed by addition of an excess of dU (Fig. S5 A and B), a subpopulation of βIII tubulin-positive retinal cells undergoing ectopic S-phase could be labeled in vivo, permitting their fate to be followed. BrdU was initially incorporated into about 9% of βIII tubulin-positive retinal cells (Table S2), and this proportion increased to 21.08 ± 1.67% (n = 3) when considering the nonlayered (i.e., migrating) βIII tubulin-positive cells. Two, 4, and 6 h after dU addition, the proportion of βIII tubulin-positive cells that had incorporated BrdU was similar (Table S2), suggesting that this cell subpopulation had still not mixed with precursors in S-phase-1 (Fig. 3G) that had completed G2/mitosis and initiated the process of neuronal differentiation. Many migrating RGCs that replicated their DNA remained as tetraploid cells in the GCL. Accordingly, 6 h after dU addition around 0.4% of the βIII tubulin-positive cells adjacent to the basal surface contained BrdU in their nuclei (Table S2). This percentage rose to around 3.3% 9 h after addition of dU (see arrowhead in Fig. 3F; Table S2), and it augmented even further 15 h after BrdU administration to 5.47% (± 0.28; n = 3). Because there was no evidence of DNA replication (i.e., BrdU incorporation at short time points) in layered RGCs (Table S2), we concluded that the BrdU-positive RGCs observed in the GCL are derived from the βIII tubulin-positive cells that replicated their DNA while migrating. Flow cytometry analysis in dissociated retinal cells isolated from E5 embryos treated with BrdU for 9 h, revealed that 85.77 ± 1.71% (n = 4) of the BrdU-positive cells expressing Islet1 had a 4C DNA content in their nuclei (Fig. S5 C and D). These data indicate that most migrating RGCs that replicated their DNA remained in the GCL with a 4C DNA content. The actual proportion of tetraploid RGCs was measured by flow cytometry in cells expressing Islet1. DNA quantification of dissociated E5 chick retinal cells demonstrated that around 25% of the Islet1-positive cells were tetraploid (Fig. 2H). In contrast, the Islet1-negative cells (i.e., dividing precursors at this developmental stage) showed the typical profile of proliferating cells (Fig. 2G). As expected, the proportion of tetraploid Islet1-positive cells was significantly reduced when endogenous NGF or p75NTR were inactivated with anti-NGF or anti-p75NTR blocking antibodies (Fig. 2 I and J), demonstrating that RGCs depend on the presence of NGF/p75NTR to become tetraploid. The addition of NGF to dissociated E5 retinal precursors, cultured under neurogenic conditions and in the presence of BDNF to prevent G2/M transition (6), supported this conclusion. NGF increased the proportion of cells with a 4C DNA content, as evidenced by SBC (Fig. S4M), an effect that was prevented by the overexpression of Rb (Fig. S4 M–O). Flow cytometric analysis indicated that 20.87 ± 2.14% (n = 3) of the Islet1-positive cells remain tetraploid in the retina at E9, a stage when RGC neurogenesis has been completed (11); indicating that tetraploid neurons survive throughout development.
Fig. 3.
Migrating RGCs that reenter the cell cycle can either undergo apoptosis or remain as tetraploid neurons. Cryostat sections from the retina of E5 chicken embryos untreated (A and B) or treated with BrdU for 1 h and then with an excess of dU for 6 h (E) or 9 h (F). (A) Basally-located nuclei in mitosis (green) colocalize with RA4-positive RGCs (red, arrow). (B) The phospho-Histone H3 mitotic marker (green) was observed in basally-located nuclei lacking Rb (red, arrows). Precursors undergoing mitosis located apically (arrowheads). (C) Anti-NGF-antibodies (α-NGF) reduce the number of phospho-Histone H3-positive/RA4-positive cells per section when compared to irrelevant antibodies (Control, ***P < 0.005). (D) The anti-p75NTR-antiserum ChEX reduces the number of mitotic figures in βIII tubulin-positive cells per section, when compared to a control antiserum (Control, *P < 0.05). (E) Mitotic figures in βIII tubulin-positive cells (red) that has incorporated BrdU (green, arrow); Bisbenzimide (blue). (Lower) correspond to square in (Upper). (F) A pyknotic nucleus labeled with BrdU (green) in an βIII tubulin-positive cell (red, arrow) and a βIII tubulin-positive/BrdU-positive cell adjacent to the basal (i.e., vitreal) surface (arrowhead) are shown; Bisbenzimide (blue). (Lower) correspond to square in (Upper). (G) Precursors incorporate BrdU at basal (i.e., vitreal) positions, where S-phase-1 takes place, and they divide at the apical (i.e., ventricular) neuroepithelium. Migrating RGCs reenter the cell cycle at apical positions (S-phase-2) and undergo mitosis at basal positions. (H) Percentage of βIII tubulin-positive cells with mitotic figures (circles) or pyknotic nuclei (squares) that had incorporated BrdU at the indicated time points after the addition of dU (n = 3). Sections (C–F and H) were taken through the dorso-ventral axis at the central retina. PE: pigment epithelium; v: vitreous body. (Scale bars: A, 50 μm; B, 40 μm, E, 16 μm, F, 19 μm.)
A Minority of RGCs That Attempt Cell Cycle Reentry Undergo Mitosis Followed by Apoptosis.
In vivo, some of the migrating RGCs were observed to undergo mitosis, as evidenced by double labeling of RA4 and the mitotic marker pH3 (Fig. 3A). Most pH3-labeled RGCs were located basally (Fig. 3 A and B), as opposed to the apically located mitotic nuclei in neuroepithelial cells (arrowheads in Fig. 3 A and B), suggesting that these neurons have completed G2 as they migrate to the GCL. In quantitative terms, the pH3-positive RGCs constitute only a small subset of the RGCs that undergo cell cycle reentry. Thus, although 9.33 ± 0.73% of βIII tubulin-positive cells were observed to incorporate BrdU (15 min pulse; Table S2), only 0.12 ± 0.01% (n = 3) of these cells underwent basally-located mitosis. These results are consistent with the observation that many RGCs that attempt cell cycle reentry remain in a G2-like state (see above). As expected, pH3-labeled RGCs were always positive for the RA4 marker (n = 131 nuclei analyzed; Fig. 3A) and always lacked Rb expression (n = 27 nuclei observed, Fig. 3B). Application of hybridoma cells secreting an anti-NGF mAb or injection of the anti-p75NTR ChEX antibody into the eye significantly reduced the proportion of RA4-positive cells with mitotic figures in their nuclei when compared to control retinas (Fig. 3 C and D). These results further indicate that some RGCs that reenter the cell cycle in response to endogenous NGF can further proceed throughout the cell cycle and undergo mitosis. BrdU pulse-chase experiments in E5 chicken embryos followed by immunolabeling of βIII tubulin-positive neurons confirmed this hypothesis. Thus, 6 h after administration of dU, BrdU was present in around 90% of the βIII tubulin-positive cells that underwent basally-located mitosis (Fig. 3 E and H). In vitro, a fraction of differentiating E5 retinal neurons undergoes mitosis followed by apoptosis when treated with NGF (6). BrdU pulse and chase experiments confirmed these results in vivo. Thus, although 6 h after dU treatment, when most pH3-positive RGCs are labeled with BrdU (see above), BrdU was only evident in around 20% of the βIII tubulin-positive cells showing pyknotic nuclei (Fig. 3H), 3 h later BrdU was already observed in most of the βIII tubulin-positive, pyknotic cells (Fig. 3 F and H). These data indicate that ectopic mitosis was rapidly followed by apoptosis in vivo in the βIII tubulin-positive retinal cells. The reduced amount of RGCs that undergo mitosis (see above) followed by apoptosis agrees with previous published data demonstrating that only 2% of Islet1-positive cells suffer cell death in the embryonic chick retina in vivo (19).
Endogenous BDNF Prevents Ectopic Mitosis.
BDNF is a neurotrophin known to prevent mitosis and apoptosis in differentiating RGCs (6, 20). Accordingly, inhibition of endogenous BDNF in E4 eye explants with TrkB receptor bodies resulted in a dramatic increase of βIII Tubulin-positive cells undergoing mitosis (Fig. 4B). In contrast, manipulation of BDNF levels did not affect BrdU incorporation by these neurons (Fig. 4A). These results indicate that BDNF is crucial for the maintenance in a G2-like state of RGCs that have reentered into the cell cycle in response to NGF.
Fig. 4.
Effects of BDNF and TrkB receptor bodies on RGC cell cycle reentry. Eyes from E4 chicken embryos cultured overnight in the presence of vehicle (Control), 2 ng/mL BDNF (BDNF), or 1 μg/mL TrkB receptor body (TrkB/Fc); then treated with 0.5 μ/mL BrdU (1 h) and fixed. Central retina cryosections (12 μm) immunolabeled for βIII tubulin and either BrdU or pH3 were analyzed. (A) Neither BDNF nor TrkB receptor bodies affected the proportion of βIII tubulin-positive neurons incorporating BrdU. (B) Sequestering of endogenous BDNF with TrkB receptor bodies increased the number of βIII tubulin-positive neurons undergoing mitosis. The number of βIII tubulin-positive neurons undergoing mitosis was extremely small when compared to that observed at E5 (Fig. 3 C and D), which is consistent with the reduced levels of apoptosis detected at E4 in comparison with E5 (20). Exogenous BDNF did not change this small number of βIII tubulin-positive neurons undergoing mitosis, likely due to the presence of saturating levels of endogenous BDNF in the explants. ***P < 0.005.
Tetraploidization Correlates with Increased Size and Extensive Dendritic Trees in RGCs.
To define the phenotype of tetraploid RGCs, we focused in the posthatching chick retina. Flow cytometry forward scattering analysis of Islet-1-positive cells isolated from P1 chick retinas indicated that, on average, tetraploid RGCs were larger than diploid RGCs (Fig. S6 A–C). A similar conclusion was obtained from the analysis of the soma size of DiI-labeled RGCs, performed by means of SBC in random retinal areas (Fig. 5A; Fig. S2 C and D). In all analyzed areas, tetraploid RGC somas were significantly larger on average than those of the diploid RGCs (Table S1), indicating that the presence of double amount of DNA is translated into increased soma size. The average size of diploid RGC somas ranged from 60 to 110 μm2 (mean: 75.40 ± 3.44 μm2, n = 15), whereas the soma size averages of the tetraploid RGCs were significantly higher and ranged between 70 and 230 μm2 (mean: 127.73 ± 10.47 μm2, n = 15; ***P < 0.005) (Fig. 5A). These values resemble the bimodal distribution for soma sizes for chick RGCs previously shown by (21). A positive correlation (r = 0.83) between soma sizes of diploid and tetraploid RGCs within the analyzed retinal areas was observed (Fig. 5B), suggesting that tetraploidization results in increased soma size regardless of the initial size of the diploid neurons in which it takes place. The area occupied by the dendritic trees, as evidenced by retrograde DiI labeling, of both diploid and tetraploid RGCs was estimated by SBC in randomly selected retinal areas. This analysis demonstrated that tetraploid RGCs showed larger dendritic trees than those observed in the diploid RGCs (Fig. 5C; see also Fig. S2 C and D). Dendritic fields of diploid RGCs ranged from 136 to 8,904 μm2 (n = 37) whereas dendritic fields of tetraploid RGCs were higher and ranged between 407 and 76,569 μm2 (n = 42). These values are in a similar range as those previously described (21).
Fig. 5.
Characterization of somatic tetraploid RGCs in the P1 chick retina. SBC in DiI retrograde-labeled RGCs (A–C, E, and F) or flow cytometry (D). (A) Estimation of the soma areas of diploid (2C) and tetraploid (4C) RGCs. Dots represent the mean obtained in random retinal regions. Lines represent the range of sizes observed in all of the regions analyzed. (B) Correlation between soma sizes from diploid (2C) versus tetraploid (4C) RGCs in random retinal areas, as estimated from (A). (C) Distribution of dendritic field sizes from diploid (2C) or tetraploid (4C) RGCs as evidenced from SBC. (D) DNA content (DAPI) in dissociated retinal cells double-immunostained with anti-Islet1 and anti-β2AChR antibodies. Most Islet1/β2AChR-double labeled cells were observed to be tetraploid (4C). (E) Simultaneous analysis of DNA content (DAPI) in either total or β2AChR-positive cells located in the GCL demonstrates that cells expressing β2AChR are mostly tetraploid. A few events with DNA content above 4C can be observed (asterisk), likely due to doublets of 2C and 4C cells. (F) A representative plot showing the distribution of soma sizes in the β2AChR-positive cell population located in the GCL.
Tetraploid RGCs Comprises a Specific Population of Retinal Projection Neurons in the Chick.
A subpopulation of less than 10% of RGCs expresses nicotinic AChR β2 subunit (β2AChR) and innervates SGFS-F in the tectal cortex (22). Analysis by flow cytometry in dissociated P1 chick retinal neurons demonstrated that 83.64 ± 2.45% (n = 4) of tetraploid cells are labeled with the anti-β2AChR antibody mAb 270 (22) and that most Islet1/β2AChR-double labeled cells (85.37 ± 1.96%; n = 7) are tetraploid (Fig. 5D); indicating that the majority of tetraploid RGCs belong to the neuronal population that innervate SGFS-F. Analysis by SBC confirmed that most β2AChR-positive cells present in the GCL (Fig. S2E) showed a 4C DNA content (Fig. 5E). In contrast, the entire population of Islet1-positive cells located in the GCL showed a DNA content profile (Fig. S7) equivalent to that previously observed by flow cytometry in cells expressing Islet1 (Fig. 1B); thus indicating that the SBC analysis performed with the anti-β2AChR antibody was specific. The size of the β2AChR-positive somas, as evidenced by SBC (Fig. 5F), was in the same range as that of the tetraploid RGCs (Fig. 5A). Flow scattering analysis confirmed that, on average, the size of Islet1/β2AChR-double labeled RGCs (Fig. S6 E and F) was in the same range as that of tetraploid RGCs (Fig. S6 B and C). This contrasts with the small size of Substance P-positive RGCs, a population of neurons with soma areas of 28–63 μm2 (23) that innervate SGFS-B (22, 23). Therefore, the innervation of specific laminae in the chick optic tectum seems to be associated with the size of the innervating RGCs. To verify whether the expression of β2AChR in tetraploid RGCs is conserved between the chick and mammals we focused in the mouse retina because RGCs in this species are known to express β2AChR (24). By means of flow cytometry we found that only 4.76 ± 0.96% (n = 3) of the tetraploid cells derived from adult mouse retinas expressed β2AChR. Furthermore, only 28.95 ± 0.97% (n = 4) of the β2AChR-positive cells obtained from these retinas showed a 4C DNA content. Therefore, we conclude that β2AChR cannot be considered a marker for tetraploid neurons in the mouse retina.
Discussion
Overall, these results demonstrate that endogenous NGF acting through p75NTR forces cell cycle reentry of Rb-negative differentiating retinal neurons, thereby generating tetraploid RGCs. These neurons have large somas and extensive dendritic trees, and most of them express a marker known to be specific of RGCs innervating SGFS-F in the optic tectum. This represents an example of growth-factor-induced somatic polyploidy in vertebrates.
We have shown that most RGCs that reactivate the cell cycle can survive in vivo as a result of a developmental program aimed to induce neuronal somatic tetraploidy, which results in morphological diversity among retinal projection neurons. Tetraploid RGCs in the chick show features of primate parasol cells; a population of RGCs equivalent to α-Y cells in the cat (25) with large somas and wide receptive fields, which are involved in motion processing. Parasol cells make up about 10% of the RGCs, they establish contacts with cholinergic amacrine cells, and they project to specific layers of the lateral geniculate nucleus, the major retinorecipient tissue in mammals (26, 27). Whether or not tetraploid RGCs in the chick are the counterpart of parasol cells requires further analysis. Although in mammals the β2AChR subunit is not specifically expressed by tetraploid RGCs, it may have been replaced by other nicotinic AChR subunits in these organisms. In this regard, medium- to large-sized RGCs have been shown to express β4AChR subunits in the ground squirrel (28).
We show that a minority of postmitotic RGCs that reenter cell cycle in vivo, driven by NGF, undergo mitosis followed by apoptosis, which is consistent with prior reports (6, 19). Notably, the data reported here supports the notion that a major fate for postmitotic RGCs that reenter cell cycle in vivo is replication without cell division, with survival instead of apoptosis.
Our results are consistent with previous observations in vitro that neurite-bearing PC12 cells (29) and sympathetic neurons (30) continue to synthesize DNA in the presence of NGF, resulting in the appearance of cells with high DNA content in culture. To date, the mechanisms used by NGF to trigger cell cycle reentry remain unknown. Our results show that cycle reentry is restricted to the Rb-negative subpopulation of RGCs. It is possible that p75NTR-dependent inhibition of CMAGE in these migrating RGCs could release E2F1 activity and force them to reenter the cell cycle (31). BDNF is a neurotrophin known to prevent mitosis and apoptosis in differentiating RGCs (6, 20), which acts in our system before the classical period of target-dependent neuronal survival. Our results indicate that blockade of BDNF favors mitosis in migrating RGCs, whereas DNA synthesis in these neurons is not affected by this neurotrophin. Therefore, cooperation between NGF and BDNF seems to be required for the induction of tetraploid RGCs and their maintenance in a G2-like state.
Reactivation of the cell cycle triggers neuronal death during both development and disease (32). Our work extends this view because cell cycle reentry can also be associated to an alternative fate—neuron survival as large tetraploid cells, as occurs in the differentiating neurons we describe. Our results demonstrate that induction of neuronal tetraploidy in response to NGF and p75NTR occurs in the normal developing nervous system, correlating with changes in neuronal morphology. Whether induction of large tetraploid neurons in the adult brain in response to endogenous NGF/proNGF and p75NTR (33, 34) may lead to altered neuronal function and changes in neuronal circuits that could trigger neurodegeneration remains unexplored. In this regard, neurons from Alzheimer’s disease patients have been shown to become tetraploid as the disease progresses, in association with a decrease of neuronal numbers (2).
Materials and Methods
Chicken Embryos and Mice.
Fertilized chicken eggs (from Granja Santa Isabel, Córdoba, Spain) were incubated at 38.5 °C as previously described (6). Adult C57BL6/J mice were also used in this study. All experiments were performed in accordance with the European Union guidelines and they were previously approved by the CSIC animal ethics committee.
Primary Antibodies and Immunostaining.
For immunocytochemistry, cells were fixed for 15 min at room temperature with 4% PFA. For immunohistochemistry, embryos were fixed for 8 h at 4 °C with 4% PFA, cryopreserved in PBS containing 30% sucrose, and embedded in the OCT compound Tissue-Tek. Cryosections (12 μm) or cultures were stained with using standard procedures (see SI Materials and Methods for details, as well as for a list of the antibodies used in this study).
Plasmids.
The pcDNA3 vector was purchased from Invitrogen. The pGEM-pG6416 plasmid was obtained by inserting the chick pG6416 inverted repeat region (8) into the pGEM-T Easy vector from Promega. The source of the other plasmids used in this study is described in the SI Materials and Methods.
In Vivo BrdU Treatment.
BrdU (200 μg) was applied to the chorioallantoic membrane of E5 chicken embryos. Some embryos were killed after 1 h, whereas other embryos received dU (20 mg) and killed at different time points (for details, see SI Materials and Methods).
Treatment of Embryos with Hybridoma Cells.
Hybridoma (2 × 106) were applied to the chorioallantoic membrane of E3 chicken embryos and 2 days later they were killed (see SI Materials and Methods for details).
Eye Injections of Anti-p75NTR.
The anti-p75NTR antiserum or normal rabbit serum were injected (1 μL diluted 1/10 in PBS) into the vitreal space of E4-4.5 chick eyes. Embryos were incubated until E5 and then their retinas were subjected to immunostaining or flow cytometry (for details, see SI Materials and Methods).
Electroporation.
Small fragments (approximately 10 mm2) of E5-6 chick retinas were electroporated with different plasmid combinations using four 50 milliseconds pulses of 25 V at a 500 milliseconds frequency (see SI Materials and Methods for details).
Cell Cultures.
Dissociated E5 chick retinal cells were plated on 10-mm round glass coverslips coated with 500 μg/mL poly (D-L) ornithine and 10 μg/mL natural mouse laminin, and cultured at 37 °C in DMEM/Nutrient Mixture F12 HAM containing N2 supplement (DMEM/F12/N2). For details, see SI Materials and Methods.
Explant Cultures.
E4 eye explants were cultured at 37 °C in DMEM/F-12/N2 containing either 2 ng/mL BDNF, 1 μg/mL TrkB receptor bodies, or vehicle (see SI Materials and Methods for details). Finally, a 1 h pulse of 0.5 μg/mL BrdU was given to label cells in S-phase and then the explants were fixed with 4% PFA for 1 h.
Flow Cytometry.
E5 or P1 chick retinas or adult mouse retinas, placed in 1 mL Ca2+-Mg2+-free PBS containing 3 mg/mL BSA, were treated with 0.5 mg/mL trypsin for 5 min (E5 chick retina) or for 8 min (P1 chick retina); or with 0.25 mg/mL trypsin for 5 min (adult mouse retina) at 37 °C. Reactions were stopped by adding 50 μL of a solution containing 10 mg/mL trypsin inhibitor prepared in PBS. 10 μL of DNase I prepared at 1 μg/μl in PBS, and 5 μL of 500 mM EDTA pH 8.0 were then added; and the cells were subsequently dissociated by gentle trituration and fixed ON at 4 °C in 70% Ethanol/PBS. Flow cytometry analysis of these cells was performed as indicated in SI Materials and Methods.
DiI Retrograde Labeling of RGCs and Nuclear Staining.
Small crystals of DiI were implanted into the optic nerve stumps of P1 chick eyes (see SI Materials and Methods for details). The eyeballs lacking the lens and vitreous body were then incubated at 37 °C in 1% PFA in PBS for 4–6 weeks (soma size analysis) and for 6 months (dendritic arbor analysis). The retinas were dissected, incubated for 30 min with 100 ng/mL DAPI in PBS/0.1% Tween 20, washed with PBS/0.1% Tween 20 and PBS (30 min each), and flat mounted with PBS/glycerol (1:1) on glass slides.
SBC.
The relative DNA content of the DiI-labeled, β2AChR-positive, or Islet1-positive RGCs in flat-mounts of fixed P1 chick retinas, or dissociated E5 retinal cells transfected with RFP and cultured under neurogenic conditions was determined by the integral DAPI fluorescence values obtained by means of SBC. SBC analysis was performed with an automated Olympus IX81 Scan^R fluorescence microscope-based imaging platform, from Olympus, equipped with an ORCA-AG C8484-05G01 digital camera, from Hamamatsu Photonics, using 20× magnification objectives (for details, see SI Materials and Methods).
I-FISH.
Suspensions of dissociated P1 chick retinas, enriched in RGCs, were obtained by limited trypsinization (0.5 mg/mL trypsin, for 5 min). These cells were plated onto coverslips previously coated with PLO, maintained in DMEM/F12/N2 for 1 h to allow their attachment to the substrate, and then fixed for 10 min with either 4% PFA (for βIII tubulin immunostaining), or ethanol/glacial acetic acid (3:1) (for I-FISH). I-FISH was performed as previously described (8). The probe, derived from the pG6416 IRR sequence (see above), was labeled with biotin using the Biotin-Nick Translation Kit from Roche following the protocol recommended by the supplier. See SI Materials and Methods for details.
Immunohistochemical Data Analysis and Statistics.
Dorsoventral sections containing both the lens and optic nerve exit were examined using a Leica TCS SP5 confocal set-up, and around 100 labeled cells were analyzed per embryo. Quantitative data are shown as mean ± SEM obtained from at least three different embryos or experimental conditions. The statistical differences of the means were analyzed using the Student’s t test (one-tailed).
Supplementary Material
Acknowledgments
We thank R. Diez del Corral, P. de la Villa, and N. López-Sánchez for scientific comments; P. Lastres, G. Gómez-Mariano, and E. Abanto for technical assistance; M.V. Chao, L.F. Reichardt, S.C. McLoon, A. Rodríguez-Tébar, and E.J. de la Rosa for antibodies; and K. Yashikawa, M. Campanero, and S. Wilson for plasmids. The mAbs G3G4 (S. Kaufman), 40.2D6 (T.M. Jessell), AMV-3C2 (D. Boettiger), and mAb 270 (J. Lindstrom) were obtained from the Developmental Studies Hybridoma Bank (University of Iowa). This work was supported by the Ministerio de Ciencia e Innovación, La Caixa Foundation, FUNDALUCE (J.M.F.), and Programa de Biociencias Medicina Individualizada Translacional en Inflamación y Cáncer-Comunidad de Madrid (A.d.l.H).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906121107/DCSupplemental.
References
- 1.Swartz FJ, Bhatnagar KP. Are CNS neurons polyploid? A critical analysis based upon cytophotometric study of the DNA content of cerebellar and olfactory bulbar neurons of the bat. Brain Res. 1981;208:267–281. doi: 10.1016/0006-8993(81)90557-6. [DOI] [PubMed] [Google Scholar]
- 2.Mosch B, et al. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang AH, et al. Chromosome segregation defects contribute to aneuploidy in normal neural progenitor cells. J Neurosci. 2003;23:10454–10462. doi: 10.1523/JNEUROSCI.23-32-10454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: More for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 5.Museridze DP, Svanidze IK, Macharashvili DN. Content of DNA and dry weight of the nuclei of neurons of the external geniculate body and retina of the eye in guinea pigs. Sov J Dev Biol. 1975;5:269–272. [PubMed] [Google Scholar]
- 6.Frade JM. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci. 2000;113:1139–1148. doi: 10.1242/jcs.113.7.1139. [DOI] [PubMed] [Google Scholar]
- 7.Mey J, Thanos S. Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev. 2000;32:343–379. doi: 10.1016/s0165-0173(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Li J, Leung FC. Partially inverted tandem repeat isolated from pericentric region of chicken chromosome 8. Chromosome Res. 2002;10:73–82. doi: 10.1023/a:1014226412339. [DOI] [PubMed] [Google Scholar]
- 9.de Melo J, et al. Dlx2 homeobox gene transcriptional regulation of Trkb neurotrophin receptor expression during mouse retinal development. Nucleic Acids Res. 2008;36:872–884. doi: 10.1093/nar/gkm1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frade JM. Interkinetic nuclear movement in the vertebrate neuroepithelium: Encounters with an old acquaintance. Prog Brain Res. 2002;136:67–71. doi: 10.1016/s0079-6123(02)36007-2. [DOI] [PubMed] [Google Scholar]
- 11.Prada C, Puga J, Pérez-Méndez L, López R, Ramírez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 12.Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 13.Prada C, Puelles L, Génis-Gálvez JM. A golgi study on the early sequence of differentiation of ganglion cells in the chick embryo retina. Anat Embryol (Berl) 1981;161:305–317. doi: 10.1007/BF00301828. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M, Rutishauser U, Silver J. Formation of the retinal ganglion cell and optic fiber layers. J Neurobiol. 1991;22:85–96. doi: 10.1002/neu.480220109. [DOI] [PubMed] [Google Scholar]
- 15.Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusek JC, Greene RM, Pisano MM. Expression of the E2F and retinoblastoma families of proteins during neural differentiation. Brain Res Bull. 2001;54:187–198. doi: 10.1016/s0361-9230(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 17.Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 18.Hiebert SW, Lipp M, Nevins JR. E1A-dependent trans-activation of the human MYC promoter is mediated by the E2F factor. Proc Natl Acad Sci USA. 1989;86:3594–3598. doi: 10.1073/pnas.86.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Díaz B, Serna J, De Pablo F, de la Rosa EJ. In vivo regulation of cell death by embryonic (pro)insulin and the insulin receptor during early retinal neurogenesis. Development. 2000;127:1641–1649. doi: 10.1242/dev.127.8.1641. [DOI] [PubMed] [Google Scholar]
- 20.Frade JM, et al. Control of early cell death by BDNF in the chick retina. Development. 1997;124:3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- 21.Naito J, Chen Y. Morphologic analysis and classification of ganglion cells of the chick retina by intracellular injection of Lucifer Yellow and retrograde labeling with DiI. J Comp Neurol. 2004;469:360–376. doi: 10.1002/cne.11010. [DOI] [PubMed] [Google Scholar]
- 22.Yamagata M, Sanes JR. Target-independent diversification and target-specific projection of chemically defined retinal ganglion cell subsets. Development. 1995;121:3763–3776. doi: 10.1242/dev.121.11.3763. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich D, Keyser KT, Karten HJ. Distribution of substance P-like immunoreactive retinal ganglion cells and their pattern of termination in the optic tectum of chick (Gallus gallus) J Comp Neurol. 1987;266:220–233. doi: 10.1002/cne.902660208. [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crook JD, et al. Y-cell receptive field and collicular projection of parasol ganglion cells in macaque monkey retina. J Neurosci. 2008;28:11277–11291. doi: 10.1523/JNEUROSCI.2982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britto LR, Rogers SW, Hamassaki-Britto DE, Duvoisin RM. Nicotinic acetylcholine receptors in the ground squirrel retina: Localization of the beta 4 subunit by immunohistochemistry and in situ hybridization. Vis Neurosci. 1994;11:569–577. doi: 10.1017/s0952523800002479. [DOI] [PubMed] [Google Scholar]
- 29.Ignatius MJ, Chandler CR, Shooter EM. Nerve growth factor-treated, neurite-bearing PC12 cells continue to synthesize DNA. J Neurosci. 1985;5:343–351. doi: 10.1523/JNEUROSCI.05-02-00343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: Chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci. 1987;7:3739–3748. doi: 10.1523/JNEUROSCI.07-11-03739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Sánchez N, González-Fernández Z, Niinobe M, Yoshikawa K, Frade JM. Single mage gene in the chicken genome encodes CMage, a protein with functional similarities to mammalian type II Mage proteins. Physiol Genomics. 2007;30:156–171. doi: 10.1152/physiolgenomics.00249.2006. [DOI] [PubMed] [Google Scholar]
- 32.Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Hu XY, et al. Increased p75(NTR) expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Exp Neurol. 2002;178:104–111. doi: 10.1006/exnr.2002.8018. [DOI] [PubMed] [Google Scholar]
- 34.Podlesniy P, et al. Pro-NGF from Alzheimer’s disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosis. Am J Pathol. 2006;169:119–131. doi: 10.2353/ajpath.2006.050787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.