Abstract
The hippocampus plays a critical role in recognition memory in both monkeys and humans. However, neurophysiological studies have rarely reported recognition memory signals among hippocampal neurons. The majority of these previous studies used variants of the delayed match-to-sample task; however, studies of the effects of hippocampal damage in monkey and humans have shown that another task of recognition memory, the visual paired-comparison, or visual preferential looking task (VPLT), is more sensitive to hippocampal damage than the delayed matching tasks. Accordingly, to examine possible recognition memory signals in the hippocampus, we recorded the activity of 131 hippocampal neurons in two monkeys performing the VPLT. Eighty-eight neurons (67%) responded significantly to stimulus presentation relative to the baseline prestimulus period. A substantial proportion of these visually responsive neurons (36%) showed significant firing-rate modulations that reflected whether stimuli were novel or familiar. Additionally, these firing-rate modulations were correlated with recognition memory performance on the VPLT such that larger modulations by stimulus novelty were associated with better performance. Together, these results provide evidence for a neural signal in the hippocampus that may support recognition memory performance.
Keywords: medial temporal lobe, monkey, single unit
Recognition memory refers to the ability to perceive a previously encountered item as familiar. The neural processing necessary for this ability has long been attributed to structures in the medial temporal lobe (MTL), including the hippocampus and the adjacent entorhinal, perirhinal, and parahippocampal cortices (1–3). However, there remains significant controversy regarding the role of the hippocampus in recognition memory. Several studies have reported impaired recognition memory performance following damage limited to the hippocampus in both humans (4–7) and monkeys (8–10); however, other studies have reported a lack of impairment (11–15). For example, there are inconsistent findings regarding the role of the hippocampus in performance of the delayed nonmatching-to-sample task (8, 10, 13, 14). This task requires subjects to remember a previously encountered visual stimulus or object and to choose a different visual stimulus or object after a delay to receive a reward. While it is widely accepted that the perirhinal cortex is critical for performance of delayed nonmatching-to-sample task (14, 16), these inconsistent findings have called into question the extent to which the hippocampus contributes to performance (13).
Studies in humans have led to the proposal that the hippocampus is essential for recollection, but is not critical for simple recognition memory or judgments of familiarity (17). Studies of developmental as well as adult-onset amnesia have reported cases in which hippocampal damage produced intact recognition memory but impaired episodic memory, or the ability to recollect information pertaining to the specific event during which the stimulus was first encountered (11, 12, 15, 18, but see also 6). The “Remember-Know” procedure has often been used to try to distinguish impairments in simple recognition from deficits in recall (12, 15, 19–21). However, this depends on the assumption that Remember judgments reflect recollection while Know judgments reflect familiarity. It has recently been proposed that these findings can just as easily be explained in terms of memory strength (22), with Remember and Know judgments often reflecting strong and weak memories, respectively (23–27). In support of this idea, activity in the hippocampus as measured by fMRI has been related to memory strength, even for familiarity-based or recognition memories (24).
If the hippocampus is critical for recognition memory performance, hippocampal neurons would be expected to modulate their evoked activity depending on whether a given stimulus is novel or familiar. This kind of modulation has been described among neurons in the entorhinal and perirhinal cortices (28–32); still, physiological studies in nonhuman primates have generally reported only very low percentages of hippocampal neurons (33, 34)—or in many cases no neurons at all (30, 35, 36)—displaying such modulation. This apparent inconsistency between the findings from lesion and physiology studies has added to the controversy surrounding the role of the hippocampus in recognition memory.
Previous neurophysiological studies of recognition memory signals in the monkey MTL have typically involved training monkeys to maintain the representation of a visual stimulus in memory during a delay period to later signal recognition of that stimulus for a reward. Specific variations on this basic task structure used for physiological studies include the delayed match-to-sample task (28, 29, 32), the Konorski conditional delayed matching task (30, 35, 37, 38), and the serial recognition task (33, 34, 38). Another task that has been used to examine recognition memory in monkeys and humans is the visual preferential looking task (VPLT). Unlike the delayed matching tasks, this task does not require any specific training but relies on the subject’s innate preference for novelty. In the VPLT, recognition is assessed by comparing subjects’ preferences for visual stimuli. When given a choice between a novel and a repeated stimulus, control subjects spend about 70% of the time viewing the novel stimulus, which indicates that they have formed a memory of the repeated stimulus. Lesions restricted to the hippocampus in both monkeys and humans produce significant impairment on this task (8, 14, 39, 40). Accordingly, this task may be useful for identifying recognition memory signals in the hippocampus.
In the current study, we used the VPLT to examine recognition memory signals in the monkey hippocampus. This task capitalizes on primates’ innate preference for novel over familiar stimuli, requires minimal training, and allows for the measurement of varying degrees of performance. We analyzed the relationship between the activity of isolated hippocampal neurons and performance on the VPLT in monkeys. Here we report that a substantial proportion of hippocampal neurons modulate their firing rates depending on whether pictures are novel or repeated. Furthermore, these modulations in firing rate are associated with trial-to-trial variability in recognition memory performance.
Results
Behavioral Results.
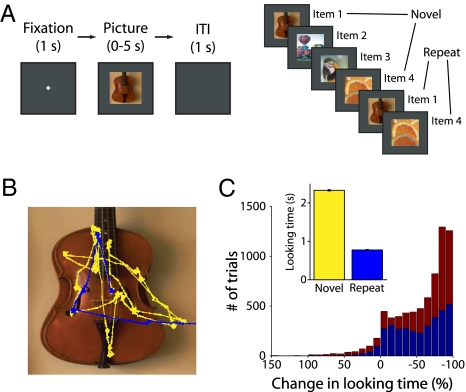
We recorded extracellular spikes from hippocampal neurons in two rhesus monkeys performing the VPLT (Fig. 1A). Each recording session, monkeys were presented with large (11°), complex visual stimuli, one at a time, on a computer screen. Two hundred novel stimuli were each presented twice during a given session, with up to eight intervening stimuli between successive presentations. Each stimulus remained on the screen until the monkey’s gaze moved off the stimulus or for a maximum of 5 s. In this way, the monkey controlled the duration of stimulus presentation, and this duration provided a measure of the monkey’s stimulus preference. We compared the amount of time the monkey spent looking at each stimulus during its first (Novel) and second (Repeat) presentation. Adult monkeys show a strong preference for novelty; therefore, a significant reduction in looking time from the first to the second presentation of a stimulus indicated that the monkey had formed a memory of the stimulus (41). Fig. 1B depicts an example of the monkey’s eye movements during the first (yellow trace) and second (blue trace) presentations of a stimulus. In this example, and across the majority of stimuli, the monkeys spent more time looking at the stimulus when it was novel compared to when it was repeated (Fig. 1C). To control for varying interest in individual stimuli, recognition memory performance was calculated as the absolute change in looking time between presentations as a percentage of the amount of time the monkey spent looking at the first presentation of each stimulus. Across 45 sessions, the monkeys demonstrated robust recognition memory performance. There was a significant (P < 0.001) decrease in looking time for the repeated presentation (average looking times for Novel and Repeat trials were 2.3 s and 0.8 s, respectively). The median reduction in looking time was 70.7% (67.3% in Monkey A and 72.8% in Monkey B). Fig. 1C shows the distribution of the change in looking time across presentations of each stimulus for both monkeys.
Fig. 1.
Behavioral task and performance. (A) VPLT design. Two-hundred novel stimuli were presented in each test session, with up to eight trials intervening between the first and second presentations. Each trial began with a required 1-s fixation period and trials were separated by a 1-s intertrial interval. (B) An example of the monkey’s scan path over the first (yellow) and second (blue) presentations of a stimulus. The monkey spent much less time viewing the stimulus in the second presentation. (C) Combined behavioral data from 45 test sessions in two monkeys. Histogram depicts the change in looking time for all stimuli as a percentage of the amount of time the monkey spent looking at the first presentation of each stimulus (blue: Monkey A; red: Monkey B). A negative change represents stimuli for which looking times were longer during the first presentation. For clarity, trials with a percent-change in looking time of greater than 150% are not shown (these represented a total of 5 trials, or 0.2 trials per session, for Monkey A and 22 trials, or 1.1 trials per session, for Monkey B). (Inset) Mean looking time for first and second presentations of all stimuli. There was a significant (P < 0.001) decrease in looking time for the repeated presentation. Error bars represent SEM.
Pictures were repeated with a variable number of intervening stimuli (see Methods for details), which allowed us to analyze the degree to which performance varied with increasing delays. There was a significant relationship between the change in looking time and number of intervening stimuli (Kruskal-Wallis test, F[8,5848] = 36.48, P < 0.01). As the number of intervening stimuli increased, the median change in looking time became more negative. This effect was driven by trials in which stimuli were repeated without an intervening stimulus, which made up 33% of all trials presented to both monkeys. After removing these trials, there was no significant relationship between behavior and number of intervening stimuli (F[7,3884] = 9.16, P > 0.1). This is consistent with previous findings that control monkeys show very little forgetting in this task across increasing delays (8, 39). When we excluded stimuli that repeated without an intervening stimulus, the population effects for neuronal activity (reported below) remained the same.
Hippocampal Neurons Modulate Their Firing Rate with Stimulus Repetition.
We recorded from 131 hippocampal neurons in two monkeys performing the VPLT. For each neuron, the average firing rate across all 200 stimuli was calculated for each of two conditions: Novel and Repeat. The primary response pattern of each neuron (i.e., the directionality and condition specificity) was assessed by analyzing the time period from 100 to 600 ms after stimulus presentation. This duration was chosen to encompass the major part of each visually-responsive neuron’s deviation from baseline firing rate. Eighty-eight neurons (67%) were visually responsive, in that they demonstrated a significant change in firing rate during stimulus presentation compared to baseline during either or both presentations (Table 1). The majority (63%) of these neurons exhibited a decrease in firing rate upon stimulus presentation. There were no significant differences between neurons with enhanced firing rates and those with depressed firing rates in either response latency (131 ± 27 ms and 152 ± 11 ms, respectively; Student’s t-test, P > 0.1) or baseline firing rate (7.63 ± 1.30 spk/s and 7.45 ± 0.97 spk/s, respectively; P > 0.1). Each stimulus was presented exactly twice, once as Novel and once as a Repeat. Because a minimum of 20 to 30 trials are necessary to obtain a reliable measure of firing rate, the experimental design did not allow for an analysis of stimulus specificity. The neuronal effects we describe are averaged across different visual stimuli.
Table 1.
Stimulus response properties of all single units showing significant differences in firing rate between baseline and the 100- to 600-ms period after stimulus onset (P ≤ 0.05)
| Novel only | Repeat only | Both | Total | |
| Visually responsive single units | 21 (25%) | 15 (18%) | 48 (57%) | 84 |
| Increase in firing rate | 5 (24%) | 4 (27%) | 21 (44%) | 30 (36%) |
| Decrease in firing rate | 16 (76%) | 11 (73%) | 27 (56%) | 54 (64%) |
Total hippocampal single units recorded: 131. Percentages in bold are based on the total number of responsive single units; all other percentages calculated from the total number of single units in response category: Novel, Repeat, or Both.
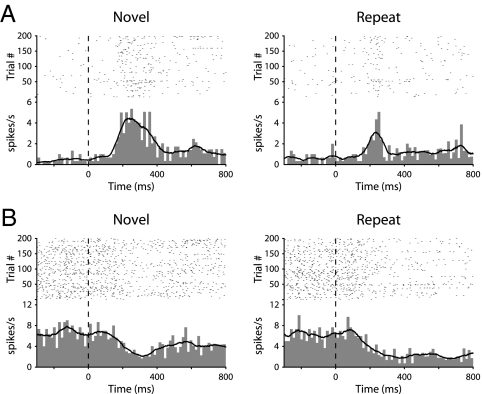
The degree to which the novelty of visual stimuli influenced the activity of hippocampal neurons was measured by analyzing the difference in firing rate across the two conditions (Novel vs. Repeat). The firing rates of 30 visually-responsive units (36%) were significantly modulated by stimulus novelty. These differentially responsive cells fell into four categories, depending on whether their firing rates were enhanced or depressed upon stimulus onset, and whether firing rates were higher for Novel stimuli (novelty responses) or for Repeat stimuli (familiarity responses) (Table S1). Baseline firing rates were not significantly different between novelty response cells (6.3 ± 1.4 spk/s) and familiarity response cells (7.9 ± 2.5 ms; P > 0.1). However, there was a trend for novelty response cells (112 ± 27 ms) to have a shorter response latency than familiarity response cells (200 ± 45 ms; P = 0.09). The responses of two representative differentially responsive neurons are shown in Fig. 2. These data suggest that information about the novelty of visual stimuli is represented in the firing rate of hippocampal neurons in monkeys, consistent with recent findings from human epileptic patients (42, 43).
Fig. 2.
Raster plots, peristimulus time histograms, and smoothed firing rates for two example hippocampal neurons (A and B). The responses of each neuron are averaged for the 200 stimuli, and are plotted separately for Novel (first presentation) and Repeat (second presentation) stimuli.
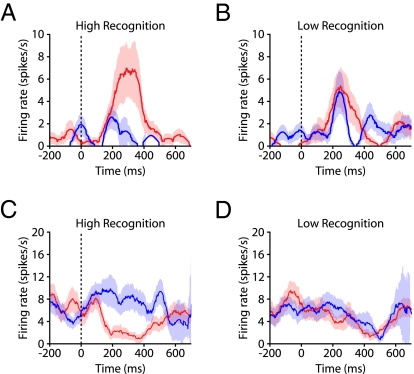
One advantage of the VPLT is that it provides for the ability to analyze the strength of recognition memory by considering the magnitude of the change in looking time for each stimulus across presentations. This offers a distinct advantage over many other recognition memory tasks, where performance for each trial can only be rated as correct or incorrect, and after training, the number of incorrect trials is usually so low that it is difficult to relate modulations in neural activity to performance. We hypothesized that changes in the firing rates of differentially responsive neurons would be correlated with memory strength, assessed through performance on the VPLT. To test this, we defined recognition memory strength as the difference in looking times for the Novel and Repeat presentations, normalized to the looking time during the Novel presentation (as per Fig. 1C). Assuming that this difference in looking time is correlated with the strength of memory encoding, the stimuli with the largest reductions in looking time are those for which the monkey formed the strongest memories. For two example neurons, we calculated the firing rate during both Novel and Repeat presentations for the 30 stimuli for which the monkey showed the best subsequent recognition memory (High Recognition) and the 30 stimuli for which the monkey showed the worst subsequent recognition memory (Low Recognition). Each condition represented ≈19% of all analyzed trials. The firing rate was increased by stimulus onset for one neuron and was decreased for the other neuron (Fig. 3). Both of these neurons showed a significant modulation of firing rate by stimulus novelty for the High Recognition trials (P < 0.05) but not for the Low Recognition trials (P > 0.1).
Fig. 3.
Example differential responses. (A) Firing rates for one enhanced differentially-responsive neuron averaged across Novel (red) and Repeat (blue) presentations, for High Recognition stimuli. (B) Same as in A, but for Low Recognition trials. Red and blue shaded areas represent SEM. Stimulus-evoked firing rates were significantly higher for Novel trials versus Repeat trials in the High Recognition condition (P < 0.05) but not in the Low Recognition condition (P > 0.1). (C and D) Same as in A and B, but for one depressed differentially responsive neuron. Stimulus-evoked firing rates were significantly lower for Novel trials versus Repeat trials in the High Recognition condition (P < 0.05) but not in the Low Recognition condition (P > 0.1).
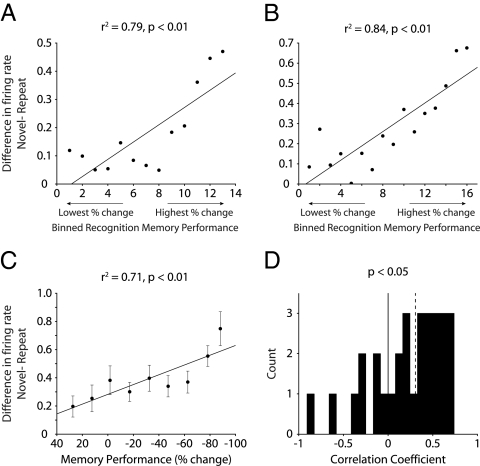
Across the population of differentially responsive neurons, we considered whether firing rate changes were correlated with memory performance throughout each recording session, rather than for just the highest and lowest extremes of memory performance (see SI Text for details). Briefly, we organized the stimuli from each VPLT session by increasing recognition memory performance (least negative to most negative change in looking time). Stimuli were grouped into bins of 30, and within each bin we determined average measures for the difference in firing rates between Novel and Repeat trials and memory performance. The Pearson correlation coefficient was calculated across all bins in the session for each neuron. Fig. 4 A and B depict the relationship between the magnitude of the firing-rate modulation and memory performance for two example neurons. In both cases, firing-rate differences between Novel and Repeat trials were positively correlated with recognition memory performance (P < 0.01). To examine the effects across the population, these data were further sorted into 10 bins based on memory performance (see SI Text for details). Across all differentially responsive neurons, there was a significant correlation between the magnitude of the firing-rate modulation and memory performance (P < 0.01) (Fig. 4C). Fig. 4D shows the distribution of correlation coefficients for the population of differentially responsive cells, which was significantly greater than zero (sign test, P < 0.05). Correlations for enhanced and depressed cells, as well as cells with Novelty and Familiarity responses, are presented in Fig. S1).
Fig. 4.
Correlation between firing rate modulation and memory performance. (A and B) Difference in firing rates for two sample neurons across 30-trial bins organized from trials with lowest to highest percent-change in looking time between encoding and recognition. Black lines represent linear regression of data points. (C) Difference in firing rates across all differentially responsive neurons (n = 30), organized from lowest to highest percent-change in looking time. Memory performance and firing-rate difference were significantly correlated (P < 0.01). Error bars represent SEM. Black line represents linear regression of data points. (D) Histogram of correlation coefficients for all differentially responsive cells. The distribution was significantly positive (sign test, P < 0.05). Dashed line, median.
Discussion
Using a behavioral task that is sensitive to restricted lesions of the hippocampus (8, 14, 39, 40), we found that a substantial proportion of hippocampal neurons differentiate between novel and familiar stimuli through changes in firing rate. Furthermore, modulations in firing rate were correlated with variability in recognition memory performance throughout the session. For individual neurons and across the population of differentially responsive neurons, there was a significant positive correlation between the magnitude of the modulation by stimulus novelty and performance, such that changes in firing rate for successive presentations of visual stimuli were greater when these stimuli were better remembered. These findings provide evidence that recognition memory performance may be supported by hippocampal activity at the cellular level.
These data stand in contrast to previous studies of recognition memory signals in the monkey hippocampus. These previous studies used either the Konorski conditional delayed matching task (30, 35, 37) or the serial recognition task (33, 34). In the Konorski conditional delayed matching task, two stimuli (varying in familiarity to the animal) are presented sequentially with a 0.5-s delay, and monkeys are trained to signal whether the two stimuli are the same or different. Despite the relatively large incidence of neurons in cortical areas surrounding the hippocampus whose firing rates decreased with stimulus repetition, no hippocampal neurons showed alterations in firing rates that reflected whether stimuli were novel or had recently been seen (30, 35). One exception was a study by Wilson et al., which reported that 34% of visually-responsive hippocampal units responded differently during the second stimulus presentation depending on whether or not it matched the first stimulus presentation (37). Because these stimuli were already familiar to the monkeys, these signals could reflect neural coding of relative familiarity. However, because the subjects were trained to respond to the right panel for a match and the left panel for a nonmatch, it is also possible that these responses instead reflected spatial coding (22, 44, 45). Other studies used the serial recognition task, in which novel stimuli are presented sequentially, with familiar stimuli intervening at various frequencies. Monkeys are typically trained in a go/no-go paradigm, licking a tube when stimuli are familiar to obtain fruit juice and refraining from licking when stimuli are novel to avoid the taste of saline. Studies using this task have identified very small numbers (<3%) of hippocampal neurons that alter their firing rates for the Novel and Repeat stimulus presentations (33, 34).
One primary difference between the VPLT and these tasks is the degree of stimulus novelty. In the delayed matching task, images depicting a variety of different geometric shapes were used, and these were of varying familiarity to the animal (30, 35, 37, 38). One study in particular described stimuli as often differing only in terms of size while keeping other attributes the same (37). In the serial recognition task, many of the stimuli were considered Novel as long as they were not presented earlier that session. However, the stimuli may have been seen previously, a couple of months (33) or even days (34) prior. In the VPLT, 200 completely novel stimuli were used for each recording session, with a total of 9,000 unique stimuli across all sessions. Because we observed changes in firing rate after only a single stimulus presentation in the VPLT, it is possible that recognition of previously seen stimuli affected the results in previous studies.
Recent studies have suggested that the hippocampus plays a role in working memory (i.e., in tasks requiring active maintenance of stimuli) (46–48). The design of the present study allowed us to examine whether the observed modulations in firing rate were related to the number of intervening stimuli between the Novel and Repeated stimulus presentation (methods and results for this analysis are presented in the SI Text). The data revealed that there was no significant relationship overall between the modulation of the neural response for high and low recognition conditions and the number of stimuli intervening between presentation (Fig. S2). The difference in the firing-rate modulation related to memory strength was not significant when stimuli were presented back to back; however, this difference was significant when there was at least one intervening stimulus. These data support the idea that the neural signal for recognition memory in the hippocampus is not specifically related to working memory.
The VPLT has also been used extensively in rats, where it is called the Visual Paired Comparison task or the Spontaneous Object Recognition task (49, 50; see ref. 51 for review). It has been suggested that the open-field version of this task may not provide a pure assessment of object-recognition memory, but may instead assess memory for objects in a specific context (50). Because the stimuli used in the present study were complex, natural images, it is possible that memory for spatial relational components of the stimuli contributed to the observed modulations in hippocampal neuronal activity. However, it has been shown that hippocampal activity signaling object-context associations often takes many trials to develop (52), while the firing rate changes we see using the VPLT occur after only one presentation. In addition, our results are consistent with previous findings in the human hippocampus (42), where learning-related changes in hippocampal signals were seen after one trial. Importantly, although the task used in that study included a spatial-relational component, the firing-rate modulation in the hippocampus did not depend on performance on that aspect of the task. Taken together, we suggest that these data provide evidence for a recognition memory signal in the hippocampus that is independent of spatial relationships.
Our results are consistent with findings from hippocampal recordings in human epileptic patients for both visual (42, 43, 53) and verbal memory (54). Significantly, human hippocampal neurons demonstrate modulations in firing rates after a single presentation for visual stimuli (42, 43, 53), similar to the present findings in the monkey hippocampus. One study (53), using a task in which subjects were instructed to make an old or new judgment on sequentially presented pictures, found that 82% of neurons in the human hippocampus were visually responsive. Of these responsive neurons, 18% differentiated between novel and repeated stimuli, with roughly the same number of enhanced and depressed responses. Interestingly, when compared to the responses of MTL cortical neurons in the same study, there was a much higher incidence of depressed responses in the hippocampus (at least 80% of all depressed differentially responsive neurons were recorded in the hippocampus). Along with our findings, this suggests that this response type plays a relatively more important role in memory processing in the hippocampus than in the MTL cortex. Our results are also consistent with Rutishauser et al. (42), who reported 20% of neurons differentiated between novel and familiar stimuli, with about equal numbers of novelty and familiarity neurons.
Previous studies showed that the firing rates of human hippocampal neurons during the encoding of word pairs predicted recall success (54), and hippocampal activation during encoding (measured using fMRI) has been correlated with subsequent item memory strength (24). The robustness of this effect, when averaged across many stimuli, in our analysis as well as others (42), suggests that hippocampal neurons may act in some circumstances as “novelty detectors.” That is, the firing of hippocampal neurons may not necessarily reflect specific information about the stimulus being viewed, but rather a more general novelty or familiarity signal that is common to all stimuli. By contrast, most previous investigations of neural signals in the perirhinal and entorhinal cortices related to recognition memory have demonstrated significant stimulus-specific firing-rate changes related to the repetition of very few stimuli (28, 29, 31, 32, 55). However, there are exceptions; one study (33), for example, reported that many neurons in the perirhinal and entorhinal cortices in the monkey signaled the relative familiarity of stimuli, without controlling for stimulus specificity. In the present study, because each stimulus was only presented twice and we did not explicitly control for stimulus content, we were unable to examine stimulus or category specificity. However, the presence of a significant effect of stimulus repetition on the firing rates of hippocampal neurons when responses were averaged across all stimuli is consistent with the idea that the hippocampus provides an “abstract” recognition memory signal (56). Accordingly, this pattern of activity may support recognition memory by combining stimulus-selective information from the perirhinal and entorhinal cortices with a more general, abstract signal of novelty or familiarity.
In summary, consistent with findings from studies of the effects of lesions of the hippocampus on recognition memory, we found that a substantial number of hippocampal neurons show modulations in firing rate that are significantly correlated with performance on a recognition memory task. These findings support the idea that the hippocampus plays a significant role in recognition memory and provide evidence for a neural signal that may underlie recognition memory performance.
Methods
Electrophysiological Recording, Data Collection, and Preprocessing.
Procedures were carried out in accordance with National Institutes of Health guidelines and were approved by the Emory University Institutional Animal Care and Use Committee. Neuronal recordings were carried out in two adult male rhesus monkeys (Macaca mulatta), which were obtained from the breeding colony at the Yerkes National Primate Research Center. Their mean weight at the start of the experiment was 6.8 ± 1.1 kg, and their mean age was 4 years and 5 months. Before implantation of recording hardware, monkeys were scanned with MRI to localize the hippocampus and to guide placement of the recording chamber. Using this information, a cilux plastic chamber (Crist Instrument Co.) for recording neural activity and a titanium post for holding the head were surgically implanted. We performed postsurgical MRI to fine-tune electrode placement and to determine recording locations.
During testing, each monkey sat in a dimly illuminated room, 60 cm from a 19-inch CRT monitor, running at 120 Hz, noninterlaced refresh rate. Eye movements were recorded using a noninvasive infrared eye-tracking system (ISCAN). Stimuli were presented using experimental control software (CORTEX, www.cortex.salk.edu). At the beginning of each recording session, the monkey performed a calibration task, which involved holding a touch-sensitive bar while fixating a small (0.3°) gray fixation point, presented on a dark background at various locations on the monitor. The monkey had to maintain fixation within a 3° window until the fixation point changed to an equiluminant yellow at a randomly chosen time between 500 ms and 1,100 ms after fixation onset. The monkey was required to release the touch-sensitive bar within 500 ms of the color change for delivery of a drop of applesauce. During this task, the gain and offset of the oculomotor signals were adjusted so that the computed eye position matched targets that were a known distance from the central fixation point.
Following the calibration task, the monkey was tested on the Visual Preferential Looking Task. The monkey initiated each trial by fixating a white cross (the fixation target, 1°) at the center of the computer screen. After maintaining fixation on this target for 1 s, the target disappeared and a square picture stimulus subtending 11° was presented. Stimuli were obtained from Flickr. A total of 9,000 stimuli were used in this study. The stimulus disappeared when the monkey’s direction of gaze moved off the stimulus, or after a maximum looking time of 5 s. The VPLT was given in 51 daily blocks of 6, 8, or 10 trials each, chosen pseudorandomly, for a total of 400 trials each day. The median delay between successive presentations was 8.1 s. Reward was not delivered during blocks of the VPLT; however, five trials of the calibration task were presented between each block to give the monkey a chance to earn some reward and to verify calibration. The number of trials in each VPLT block was varied to prevent the monkey from knowing when to expect the rewarded calibration trials.
The recording apparatus consisted of a multichannel microdrive (FHC Inc.) holding a manifold consisting of a 23-gauge guide tube containing four independently moveable tungsten microelectrodes (FHC Inc.), with each electrode inside an individual polyamide tube. Electrode impedance was in the range of 1 to 2 MΩ, and electrode tips were separated horizontally by 190 μm. For each recording, the guide tube was slowly lowered through the intact dura mater and advanced to ∼3.5-mm dorsal to the hippocampus with the use of coordinates derived from the MRI scans. The electrodes were then slowly advanced out of the guide tube to the hippocampus. No attempt was made to select neurons based on firing pattern. Instead, we collected data from the first neurons we encountered in the hippocampus. At the end of each recording session, the microelectrodes and guide tube were retracted. All recordings took place in the anterior part of the left hippocampus. Recording sites were located in the CA3 field, dentate gyrus, and subiculum.
Data amplification, filtering, and acquisition were performed with a Multichannel Acquisition Processor system from Plexon Inc. The neural signal was split to separately extract the spike and the LFP components. For spike recordings, the signals were filtered from 250 Hz to 8 kHz, further amplified, and digitized at 40 kHz. A threshold was set interactively, to separate spikes from noise, and spike waveforms were stored in a time window from 150 μs before to 700 μs after threshold crossing. Each recording typically yielded two-to-six units; single units were sorted offline using Offline Sorter (Plexon, Inc.).
Data Analysis.
All analyses were performed using custom programming in Matlab (The Mathworks, Inc.) and using FieldTrip (http://www.ru.nl/fcdonders/fieldtrip), an open-source toolbox for the analysis of neurophysiological data.
We recorded from 131 hippocampal units in two monkeys (67 in Monkey A and 64 in Monkey B, respectively). For each neuron, the average firing rate was calculated for the period including prestimulus fixation, as well as stimulus presentation, for each trial. A baseline period of 800 ms preceding stimulus onset was used to calculate the average background firing rate for each neuron. The response latency for each neuron was determined by first calculating the spike-density function of the neuron’s firing activity for each trial using a Gaussian kernel with a standard deviation of 100 ms, dividing this smoothed activity into 10-ms bins starting with stimulus onset, then finally using a Student’s t-test to compare the activity in each bin, across trials, to the baseline firing rate. Upon identifying the first instance in which three consecutive bins showed a significant difference (P < 0.05) from the baseline firing rate, the onset time of the first bin was designated as the response latency for the neuron.
Significant responsiveness to visual stimuli was determined by first calculating the average firing rate for the period from 100 to 600 ms after stimulus onset for each trial, then using a Student’s t-test to compare this activity for all trials in either the Novel or Repeat conditions to the average firing rate during a baseline period of 800 ms preceding stimulus onset. For trials where the monkey’s looking time was less than 600 ms, the firing rate after the monkey’s scan path left the picture boundary was not included when calculating the average firing rate. Neurons passing the criteria of significance to P < 0.05 for the trials in each condition were designated as visually responsive for that condition. To designate neurons as differentially responsive, the same 500-ms time period was used to calculate average firing rate for each trial; a Student’s t-test was used to determine whether the firing rates across trials of the Novel condition were significantly different from firing rates across trials of the Repeat condition for each neuron. A Gaussian kernel with a standard deviation of 100 ms was used to smooth neuronal firing rates for visualization purposes in Figs. 2 and 3.
Supplementary Material
Acknowledgments
We thank M. Tompkins and E. Stanley for technical assistance. This research was supported by the Yerkes National Primate Research Center through base Grant RR00165 from the National Institutes of Health, Emory Alzheimer’s Disease Research Center Grant AG025688 (to E.A.B.), the National Institute of General Medical Science (M.J.J.), and National Institute of Mental Health Grants MH080007 (to E.A.B.) and MH082559 (to M.J.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908378107/DCSupplemental.
References
- 1.Mahut H, Zola-Morgan S, Moss M. Hippocampal resections impair associative learning and recognition memory in the monkey. J Neurosci. 1982;2:1214–1220. doi: 10.1523/JNEUROSCI.02-09-01214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 3.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99(1):22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37(1):171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 7.Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- 8.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez P, Zola-Morgan S, Squire LR. Damage limited to the hippocampal region produces long-lasting memory impairment in monkeys. J Neurosci. 1995;15:3796–3807. doi: 10.1523/JNEUROSCI.15-05-03796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 12.Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- 13.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia: implications for the acquisition of semantic memory? J Cogn Neurosci. 2001;13:357–369. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- 16.Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 18.Adlam AL, Malloy M, Mishkin M, Vargha-Khadem F. Dissociation between recognition and recall in developmental amnesia. Neuropsychologia. 2009;47:2207–2210. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastin C, et al. Dissociation between recall and recognition memory performance in an amnesic patient with hippocampal damage following carbon monoxide poisoning. Neurocase. 2004;10:330–344. doi: 10.1080/13554790490507650. [DOI] [PubMed] [Google Scholar]
- 20.Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15:203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- 21.Yonelinas AP, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 22.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn JC. Remember-know: a matter of confidence. Psychol Rev. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- 24.Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson W. The role of decision processes in remembering and knowing. Mem Cognit. 1996;24:523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- 26.Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- 27.Wais PE, Mickes L, Wixted JT. Remember/know judgments probe degrees of recollection. J Cogn Neurosci. 2008;20:400–405. doi: 10.1162/jocn.2008.20041. [DOI] [PubMed] [Google Scholar]
- 28.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 29.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riches IP, Wilson FAW, Brown MW. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobotka S, Ringo JL. Investigation of long-term recognition and association memory in unit responses from inferotemporal cortex. Exp Brain Res. 1993;96(1):28–38. doi: 10.1007/BF00230436. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 33.Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 34.Rolls ET, Cahusac PMB, Feigenbaum JD, Miyashita Y. Responses of single neurons in the hippocampus of the macaque related to recognition memory. Exp Brain Res. 1993;93:299–306. doi: 10.1007/BF00228398. [DOI] [PubMed] [Google Scholar]
- 35.Brown MW, Wilson FAW, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409(1):158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- 36.Rolls ET, et al. Hippocampal neurons in the monkey with activity related to the place in which a stimulus is shown. J Neurosci. 1989;9:1835–1845. doi: 10.1523/JNEUROSCI.09-06-01835.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson FAW, Brown MW, Riches IP. Neuronal activity in the inferomedial temporal cortex compared with that in the hippocampal formation: Implications for amnesia of medial temporal lobe origin. In: Woody CD, Alkon DL, McGaugh JL, editors. Cellular Mechanisms of Conditioning and Behavioral Plasticity. New York: Plenum; 1988. pp. 313–328. [Google Scholar]
- 38.Wilson FA, Riches IP, Brown MW. Hippocampus and medial temporal cortex: neuronal activity related to behavioural responses during the performance of memory tasks by primates. Behav Brain Res. 1990;40(1):7–28. doi: 10.1016/0166-4328(90)90038-g. [DOI] [PubMed] [Google Scholar]
- 39.Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- 41.Wilson FAW, Goldman-Rakic PS. Viewing preferences of rhesus monkeys related to memory for complex pictures, colours and faces. Behav Brain Res. 1994;60(1):79–89. doi: 10.1016/0166-4328(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 42.Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 44.Brown MW. Hippocampal and perirhinal functions in recognition memory. Nat Rev Neurosci. 2008;9:405. doi: 10.1038/nrn2154-c1. author reply 405. [DOI] [PubMed] [Google Scholar]
- 45.Squire LR, Wixted JT, Clark RE. Review authors’ response. Nat Rev Neurosci. 2008;9:405. [Google Scholar]
- 46.Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 47.Rissman J, Gazzaley A, D’Esposito M. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex. 2008;18:1618–1629. doi: 10.1093/cercor/bhm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- 49.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- 51.Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127(1–2):159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 52.Wirth S, et al. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 53.Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. J Cogn Neurosci. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- 54.Cameron KA, Yashar S, Wilson CL, Fried I. Human hippocampal neurons predict how well word pairs will be remembered. Neuron. 2001;30:289–298. doi: 10.1016/s0896-6273(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 55.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911(1):175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.