Abstract
Neurological dysfunction caused by traumatic brain injury results in profound changes in net synaptic efficacy, leading to impaired cognition. Because excitability is directly controlled by the balance of excitatory and inhibitory activity, underlying mechanisms causing these changes were investigated using lateral fluid percussion brain injury in mice. Although injury-induced shifts in net synaptic efficacy were not accompanied by changes in hippocampal glutamate and GABA levels, significant reductions were seen in the concentration of branched chain amino acids (BCAAs), which are key precursors to de novo glutamate synthesis. Dietary consumption of BCAAs restored hippocampal BCAA concentrations to normal, reversed injury-induced shifts in net synaptic efficacy, and led to reinstatement of cognitive performance after concussive brain injury. All brain-injured mice that consumed BCAAs demonstrated cognitive improvement with a simultaneous restoration in net synaptic efficacy. Posttraumatic changes in the expression of cytosolic branched chain aminotransferase, branched chain ketoacid dehydrogenase, glutamate dehydrogenase, and glutamic acid decarboxylase support a perturbation of BCAA and neurotransmitter metabolism. Ex vivo application of BCAAs to hippocampal slices from injured animals restored posttraumatic regional shifts in net synaptic efficacy as measured by field excitatory postsynaptic potentials. These results suggest that dietary BCAA intervention could promote cognitive improvement by restoring hippocampal function after a traumatic brain injury.
Keywords: branched chain amino acids, cognitive impairment, hippocampus, tramautic brain injury
Every 23 s in the United States an individual experiences a traumatic brain injury (TBI), with a fatality occurring once every 7 min (1). TBI causes serious neurological pathologies culminating in cognitive impairment (2). To date, treatment has typically targeted physical manifestations of the injury, e.g., alleviating increased intracranial pressure, without addressing the underlying mechanisms contributing to injury-associated impairments. Current research has begun to focus on fundamental changes in brain activity with the intention of developing efficacious therapies for mild to moderate TBI patients.
The limbic hippocampus, a brain structure implicated in higher learning and memory, is often damaged in TBI. Lateral fluid percussion injury (LFPI) reproducibly damages this structure, and causes cognitive impairment (3, 4) and regional shifts in network excitability in both area CA1 (decreased net synaptic efficacy) and dentate gyrus (increased net synaptic efficacy) (4–7).
At present, it is thought that astrocytes remove released glutamate from the synaptic cleft and amidate this glutamate to form glutamine for return to the neuron. Because this biological process, known as the glutamate:glutamine cycle, is inefficient, de novo synthesis of glutamate is required to maintain synaptic neurotransmitter pools. Branched chain amino acids (BCAAs) are key amino acids involved in de novo glutamate synthesis (Fig. S1), as ≈50% of brain glutamate contains BCAA-derived nitrogen (8). Previous studies have indicated an essential role for BCAA transamination in the synthesis of glutamate and subsequently GABA. Furthermore, de novo glutamate synthesis contributes ≈40% of the releasable synaptic glutamate. In addition to their conventional synaptic function, glutamate and GABA are integral in cellular metabolism and are major sources of TCA cycle intermediates. Although BCAA administration has demonstrated therapeutic benefits in treating tardive dyskinesia (9), chronic hepatic encephalopathy (10–12), and sepsis (13) the underlying mechanisms remain unknown. Moreover, despite their important role in glutamate and GABA synthesis, no study has mechanistically investigated the efficacy of dietary BCAAs in promoting recovery from TBI. Because alterations in excitation and inhibition directly affect hippocampal function, we hypothesized that dietary BCAAs could restore cognitive performance by ameliorating posttraumatic hippocampal impairment.
Results
For all studies, adult male C57BL/J6 mice received either LFPI or sham injury (4) and were allowed to recover for 7 days. The rationale for selecting 7 days after injury for extensive study is 2-fold: first, this time point is within the clinically relevant “therapeutic time window” (14); and second, results should not be complicated by acute transient alterations induced by brain injury that subside by 48 h. Furthermore, all comparisons were made between ipsilateral slices from sham and LFPI mice, as LFPI also effects the contralateral side (15). Because changes in excitatory and inhibitory transmitter distribution and concentration could explain previously demonstrated changes in net synaptic efficacy after LFPI (16), we quantified the concentration of amino acids in homogenates from the hippocampal region by HPLC. Surprisingly, brain injury did not alter the concentration of glutamate and GABA. This result may reflect an inability to isolate the synaptic pools of glutamate and GABA from the significantly larger metabolic pools that are insular with virtually no cross talk with synaptic pools (17). Interestingly, of the 18 amino acids quantified, the concentrations of only the three branched chain amino acids (BCAAs) were significantly altered after injury (Table 1). Seven days after LFPI, in the ipsilateral hippocampus, the BCAAs (valine, isoleucine, and leucine) were reduced by 50.8%, 21.1%, and 52.3%, respectively. As BCAAs are integral in glutamate and subsequent GABA synthesis, this depletion may be caused by alterations in the underlying mechanism that maintains synaptic pools of these neurotransmitters, thus ultimately impairing hippocampal function.
Table 1.
Brain injury causes significant reduction in BCAA concentration in hippocampus
| Amino acid | Sham (nmol/mg protein) | FPI (nmol/mg protein) |
| Valine | 16.33 ± 1.64 | 8.04 ± 1.35* |
| Leucine | 15.03 ± 1.92 | 7.17 ± 1.48* |
| Isoleucine | 10.19 ± 0.51 | 8.04 ± 0.96*† |
| Threonine | 2.58 ± 1.30 | 3.91 ± 0.95 |
| Citrulline | 0.18 ± 0.12 | 0.41 ± 0.91 |
| Tyrosine | 1.87 ± 0.26 | 1.07 ± 0.79 |
| Phenylalanine | 0.08 ± 0.01 | 0.05 ± 0.02 |
Seven days after a fluid percussion injury, the hippocampal formation was removed and analyzed via HPLC to determine concentrations of 18 amino acids. Of the amino acids analyzed, the concentrations of three (valine, leucine, and isoleucine) were significantly altered (P < 0.05) after injury. For both sham and injury, n = 6 hippocampi from six mice, and represent means ± SEM.
*Denotes significance at P < 0.05 confidence level compared with sham values.
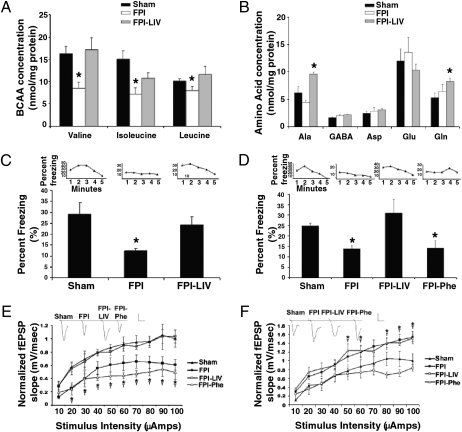
Having observed this significant alteration in the hippocampal amino acid profile, the feasibility of using dietary intervention to restore BCAA concentrations to normal was investigated. Therefore, 2 days after the injury procedure, both LFPI- and sham-injured mice received water that was either untreated (control) or contained a mixture of all three BCAAs (100 mM leucine, isoleucine, and valine, denoted here as LIV). After consuming the treatment for 5 days (starting at 2 days after injury and continuing until 7 days after injury), BCAA levels in the ipsilateral hippocampus were evaluated. Treatment with BCAAs in the drinking water restored BCAA concentration to levels not significantly different from sham animals (Fig. 1A). Furthermore, neither injury nor BCAA treatment after injury significantly altered the remaining tested amino acids, sans glutamine and alanine (Fig. 1B). Dietary intervention did not raise BCAA levels in sham LIV above those seen in the hippocampi from sham animals. Furthermore, consumption of BCAAs did not alter the blood BCAA concentration within injury models, although injured mice alone and undergoing dietary intervention had lower circulating BCAA levels than did sham animals. Sham mice had a total BCAA (i.e., combined leucine, isoleucine, and valine) circulating concentration of 558.75 ± 25.76 nmol/mL, compared with 533.49 ± 37.37 nmol/mL in sham-LIV mice. In LFPI mice, the total BCAA concentration of 509.01 ± 39.44 was not significantly different (P > 0.05) from that seen in LFPI-LIV mice (483.05 ± 19.55). Body weight was not affected by either injury or dietary intervention. Mice were randomly selected from a group of mice having the same initial body weights and assigned to one of four combinations of injury status and dietary intervention. Seven days after injury, and 5 days after consumption of the dietary treatment, no significant difference (P > 0.05) was observed in the body weights of the mice (sham, 23.17 ± 0.43; sham-LIV, 22.65 ± 0.40; LFPI, 21.95 ± 0.48; LFPI-LIV, 22.2 ± 0.60 g).
Fig. 1.
Consumption of BCAAs restored cognitive performance by correcting injury-induced alterations in net synaptic efficacy. (A) Consumption of 100 mM each of leucine, isoleucine, and valine (LIV) for 5 days restored hippocampal BCAA levels. (B) Consumption of the LIV treatment significantly increased both alanine and glutamine concentrations (P < 0.05). Both retrograde (C) and an anterograde (D) contextual fear conditioning tests demonstrated significant cognitive impairment (P < 0.05) after LFPI. This was completely reversed by the consumption of the LIV treatment. In contrast, phenylalanine consumption (100 mM; LFPI-Phe) had no effect on cognitive performance (P > 0.05). Bars indicate the average percentage of “fear behaviors” per 5 min. Insets show a representative sample of the percentage of “fear behaviors” on a per-minute basis. In area CA1 (E) and dentate gyrus (F), consumption of the LIV treatment restored net synaptic efficacy (P < 0.05), whereas consumption of phenylalanine (100 mM) had no effect (P > 0.05). Insets depict representative waveforms collected at maximal stimulation. Scale bar, 0.5 mV/10 ms. (A and B) n = 6 samples. (C) Sham, LFPI and LFPI-LIV had n = 12, 11, and 9, respectively. (D) Sham, LFPI. LFPI-LIV, and LFPI-Phe had n = 14, 11, 10, and 10, respectively. (E and F) n = 8 samples. For all panels, data are means ± SEM *Means differ significantly from sham values (P < 0.05). †Means of data collected from LFPI-Phe mice differ significantly from sham values (P < 0.05).
Given that dietary intervention restored BCAA concentrations in the ipsilateral hippocampus, we hypothesized that injury-induced cognitive impairment would be diminished or ameliorated. Therefore, an additional set of LIV animals were prepared and subjected to hippocampal-dependent cognitive assessment with both anterograde and retrograde fear conditioning. Conditioned fear response (CFR) uses a single trial, is robust, and can distinguish problems in acquisition, consolidation, and retrieval that are difficult to differentiate with the Morris water maze. Furthermore, previous reports have documented complications using the MWM with mice (18). In addition, CFR has been used to investigate cognitive impairment in mice in a variety of paradigms, including traumatic brain injury (3), cannabinoid administration to transgenic mice (19), and the role of GABA receptors in cognition (20). In the retrograde task, animals were trained before injury. The aversive stimuli in sham animals resulted in a freezing percentage over 5 min, which is significantly (P < 0.05) higher than that observed in the LFPI mice (Fig. 1C, insets). Mice that consumed the LIV treatment for 5 days after injury did not display any reduction in cognitive performance after injury. In fact, the freezing percentage was no different (P > 0.05) from that observed in sham mice (Fig. 1C and insets). Similarly, when animals were tested in the anterograde task, the animals were trained after injury. There was a significant improvement in performance in LFPI animals that consumed the LIV treatment when compared with LFPI mice on water alone (P < 0.05, Fig. 1D and insets). Therefore, dietary intervention appears to completely restore injury-induced hippocampal-dependent cognitive impairment.
To substantiate that cognitive reinstatement was associated with restoration of net synaptic efficacy, brain slices were generated from the animals used for cognitive assessment and regional I/O curves were generated. Slices from LIV animals demonstrated a return to net synaptic efficacy levels not significantly different from those obtained in slices from sham animals in both area CA1 (Fig. 1E and insets) and DG (Fig. 1F and insets). Injury-induced regional alterations in net synaptic efficacy are caused in part by changes in inhibitory transmission, as previously demonstrated by Witgen et al. (4). That is, diminished miniature inhibitory synaptic currents (mIPSCS) in DG together with augmented mIPSC amplitude in area CA1 in slices from LFPI animals. No animal demonstrated improved cognition without a simultaneous restoration of net synaptic efficacy to levels seen in sham animals. To demonstrate that dietary intervention was BCAA specific, another set of injured animals were generated and placed on phenylalanine dietary intervention. Phenylalanine, like the BCAAs, is a large neutral amino acid. These animals showed no cognitive improvement or restoration of network excitability in either hippocampal subregion (Fig. 1 D–F and insets). This supports the contention that dietary amelioration of injury-induced cognitive impairment is directly correlated with restoration of net synaptic efficacy.
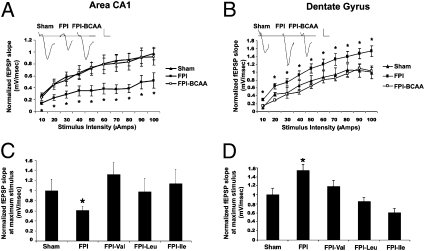
To begin to elucidate the potential mechanism(s) by which dietary BCAAs restore cognitive function and net synaptic efficacy, hippocampal slices from both sham and LFPI mice were subjected to ex vivo application of BCAAs, gas chromotograph–mass spectroscopy (GC-MS) analysis, and Western blot quantification of key enzymes. Seven days after either LFPI or sham procedure, extracellular input/output (I/O) curves were generated in hippocampal slices from the two populations (Fig. 2). Initially a baseline I/O curve in normal artificial cerebrospinal fluid (aCSF) was conducted in the subregion of interest (area CA1 or DG) and then a second I/O curve generated after a 20-min superfusion in modified aCSF containing a combination of valine, isoleucine, and leucine (100 μM each). Plotting the linear slope of the extracellular field excitatory postsynaptic potential (fEPSP) at increasing stimuli substantiated that in slices derived from injured mice, area CA1 shows decreased net synaptic efficacy, whereas net synaptic efficacy in DG is significantly increased when compared with that in slices from sham animals (P < 0.05; Fig. 2 A and B) (4). However, a 20-min application of a mixture containing the three BCAAs completely reversed these shifts in net synaptic efficacy, and restored network excitability to levels obtained in slices derived from sham animals (P > 0.05; Fig. 2 A and B) in both subregions. BCAA treatment had no effect (P > 0.05) on the excitability in any region in slices from sham animals. In addition, for each treatment, the fiber volleys were quantified. Neither injury nor application of BCAAs to slices from sham or sham or injured animals altered fiber volley slope (Table S1).
Fig. 2.
Branched chain amino acids restore net synaptic efficacy. In both area CA1 (A) and the dentate gyrus (B), input/output curves demonstrate that FPI significantly alters net synaptic efficacy. BCAA administration (100 μM each of valine, isoleucine, and leucine) completely restores net synaptic efficacy. Insets depict representative waveforms at maximal stimuli. Scale bar, 0.5 mV/10 ms. In area CA1 (C) and dentate gyrus (D), individual application of leucine, isoleucine, and valine (100 μM) completely restored net synaptic efficacy when compared with either slices from sham or LFPI animals alone. For each panel, n = 8, and data are means ± SEM *Significance when compared with sham values (P < 0.05).
To further investigate this restorative phenomenon, a second set of slices from injured and sham animals were generated and the efficacy of bath application of individual amino acids was examined. Interestingly, in both subregions, individual application of the BCAAs leucine, isoleucine, and valine completely (P < 0.05) restored excitability to normal levels (Fig. 2 C and D).
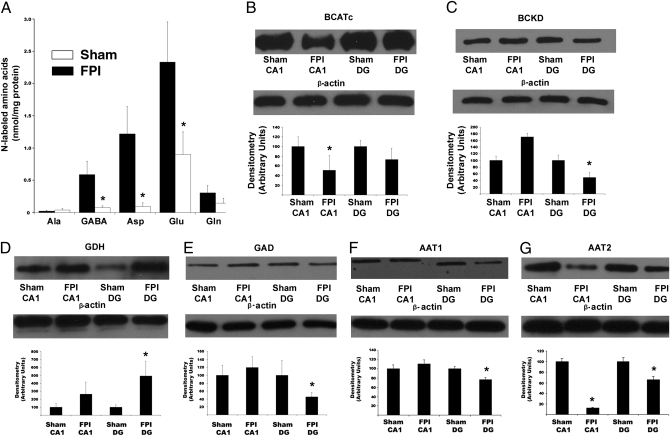
Because of their critical role as nitrogen donors in the brain, a potential mechanism by which BCAAs restore net synaptic efficacy is via transamination reactions involving glutamate and subsequently GABA (Fig. S1). Therefore, hippocampal slices were examined via GC-MS after incubation with 15N-leucine to quantify the metabolic products containing 15N. In slices from injured mice, the amount of 15N-glutamate was reduced by 61.4% when compared with slices from sham animals (Fig. 3A). Similarly, the amount of 15N-GABA from 15N-leucine was ≈86.6% lower in injured slices (Fig. 3A). Interestingly, aspartate production was also altered by brain injury, being reduced by 92.2% in injured mice (Fig. 3 A). Thus the capacity for de novo glutamate and subsequent GABA production from leucine is significantly impaired after concussive brain injury.
Fig. 3.
Brain injury leads to diminished BCAA transamination and alterations in BCAA and glutamate metabolizing enzymes. (A) After incubation with [15N]-leucine (100 μM), LFPI significantly reduced the production of 15N-labeled GABA, aspartate, and glutamate. n = 6, with bars representing means ± SEM. (B) Densitometry analysis of the expression of cytosolic branched chain aminotransferase (BCATc; 49 kDa) reveals a significant reduction (P < 0.05) in area CA1 of injured animals. (C) Branched chain keto acid dehydrogenase (BCKD; 51 kDa) expression significantly decreased (P < 0.05) in the dentate gyrus of injured animals. (D) Glutamate dehydrogenase (GDH; 61 kDa) expression significantly increased (P < 0.05) in the dentate gyrus of injured mice. (E) Glutamic acid decarboxylase (GAD; 65 kDa) was significantly reduced (P < 0.05) in area CA1. (F) Aspartate aminotransferase 1 (AAT1; 46 kDa) was significantly reduced (P < 0.05) in the dentate gyrus. (G) Expression of aspartate aminotransferase 2 (AAT2; 47 kDa) was significantly reduced (P < 0.05) in both the dentate gyrus and area CA1 from injured mice. For each Western blot, n = 4 samples, with densitometry representing means ± SEM *Denotes significance at the P < 0.05 confidence level when compared to sham values.
To investigate the potential cause(s) of diminished transamination, Western blot analysis of enzymes important in both the astroglial:neuronal nitrogen shuttle and the glutamate:glutamine cycle were examined, as both rely heavily on transamination reactions. Hippocampi from both injured and sham animals were removed and regionally dissected to separate dentate gyrus (DG) from area CA1 (SI Methods). Cytosolic branched chain aminotransferase (BCATc, neuronal isoform) reversibly transaminates BCAAs and α-ketoglutarate to form glutamate and branched chain keto acid (BCKA) and accounts for 70% of brain BCAT activity. This protein demonstrated a significant reduction in area CA1 (P < 0.05, Fig. 3B) but no alteration in expression in the DG. Mitochondrial BCAT (BCATm, astrocytic isoform) was not altered by injury in either region of the brain (P > 0.05). In contrast, branched chain keto acid dehydrogenase (BCKD), which is the rate-limiting, irreversible step in BCAA catabolism, was significantly reduced (P < 0.05) in DG, with no change (P > 0.05) in area CA1 (Fig. 3C). In addition, the expression of glutaminase, glutamate dehydrogenase (GDH) and glutamine synthetase, which are pivotal metabolizing enzymes in glutamate synthesis and transport (Fig. S1) were quantified by Western blot. Neither glutaminase nor glutamine synthetase demonstrated altered expression in either area CA1 or DG. However, GDH, which deaminates glutamate to form α-ketoglutarate for entry into the TCA cycle, demonstrated upregulated expression in the DG subregion from injured animals (Fig. 3D).
Concussive brain injury has been previously demonstrated to alter GABA-mediated inhibition in the hippocampus (4). Therefore, glutamic acid decarboxylase (GAD), which converts glutamate to GABA, was examined. The expression of GAD was significantly down-regulated in DG from injured animals, further corroborating previous data (Fig. 3E) (4). This decreased expression, coupled with the diminished labeling of the GABA precursor glutamate, contributes to the significant reduction in 15N-GABA.
To investigate the mechanism underlying the significant decrease in labeled aspartate production, regionally dissected tissue was examined for the expression of both isoforms of aspartate aminotransferase (AAT1 and AAT2). Both isoforms of this enzyme, which reversibly transfer an amino group from glutamate to oxaloacetate to form aspartate and α-ketoglutarate, were significantly reduced (P < 0.05) in the dentate gyrus (Fig. 3 F and G). However, only AAT2 was significantly reduced (P < 0.05) in area CA1 (Fig. 3G). The reduction in these enzymes, coupled with the decreased conversion of 15N-leucine to 15N-glutamate, likely accounts for the significant decrease in 15N-aspartate synthesis after injury.
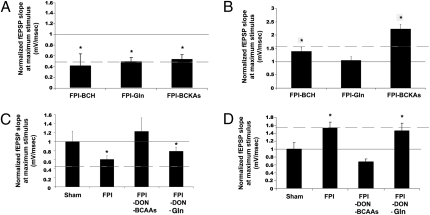
The alterations in transamination reactions caused by brain injury suggest that this mechanism is crucially involved in posttraumatic changes in both net synaptic efficacy and cognition. Furthermore, it provides a potential mechanism for dietary mediated restoration of hippocampal function. However, an alternative pathway independent of transamination would be through catabolism of BCAAs. Branched chain keto acids (BCKAs) are the products of the reversible first step in BCAA metabolism. They can be irreversibly degraded by branched chain keto acid dehydrogense (BCKD) or reaminated by either astrocytic branched chain aminotransferase (BCATm) or the neuronal isoform (BCATc) to their BCAA precursors. Therefore, to determine whether BCAA transamination was mediating restored net synaptic efficacy or acting through a metabolic intermediate, a BCKA mixture (100 μM; α-ketoisocaproate, α-ketomethylvalerate, α-ketomethylbutyrate) was bath applied for 20 min to hippocampal slices. In contrast to BCAA application, BCKA treatment did not restore network excitability in either subregion (P > 0.05; Fig. 4 A and B. These data suggest that oxidative BCAA metabolism does not account for the restorative effects of BCAAs in our TBI model.
Fig. 4.
In area CA1 (A) and dentate gyrus (B), slices were exposed to 2-amino-2-norbornane-carboxylic acid (BCH; 100 μM), a nonhydrolyzable BCAA analog, glutamine (100 μM), or a combination of all three branched chain ketoacids (BCKAs; 100 μM). Neither BCKAs nor BCH had a salutary effect in either region, whereas only in the dentate gyrus did glutamine restore net synaptic efficacy. In area CA1 (C) and dentate gyrus (D), the glutaminase inhibitor 6-diazo-5-oxo-l-norleucine (DON; 50 μM) did not block the restorative effect of the combination of BCAAs. In the dentate gyrus (D) but not in area CA1 (C), DON completely blocked the glutamine-mediated restoration of net synaptic efficacy in this region. Solid line represents sham value at maximal stimulation; dotted line represents LFPI value at maximal stimulation. For each panel, n = 8, and data are means ± SEM *Significance when compared with sham values (P < 0.05).
Further, the nonhydrolyzable BCAA analog 2-amino-2-norbornane-carboxylic acid (BCH) had no affect (P > 0.05) on altered net synaptic efficacy in slices from injured animals (Fig. 4 A and B) or in slices from sham animals. Interestingly, the glutamate precursor glutamine was without effect in area CA1 but fully reversed DG hyperexcitability (Fig. 4 A and B).
Normally, BCAA-mediated glutamate synthesis occurs primarily in the astrocyte. Astrocytic glutamate is converted to glutamine for transport back to the neuron (Fig. S1). To examine whether ex vivo BCAA application was using the same directional transport system, experiments were conducted while inhibiting the final step in the recapitulation of synaptic glutamate conversion of glutamine to glutamate by inhibiting glutaminase. Glutaminase is present solely in neurons and converts glutamine into glutamate, and interestingly the expression of this protein was not changed after injury in either subregion. Slices from injured animals were pretreated with the glutaminase inhibitor, 6-diazo-5-oxo-L-norleucine (DON; 50 μM) for 10 min and then continually superfused with DON and either glutamine (100 μM) or the BCAA mixture (100 μM) for 20 min. The addition of DON alone had no affect on the net synaptic efficacy in either area CA1 or DG in slices from LFPI or sham animals. As a positive control to confirm glutaminase inhibition by DON, recordings were first undertaken in the DG where glutamine reversed injury-induced increased net synaptic efficacy (Fig. 4B). Pretreatment and continual application of DON to slices from injured animals completely occluded the restorative affect of glutamine in this region (Fig. 4D). However, DON pretreatment did not occlude or diminish the effect on BCAA-mediated restoration of network excitability in both regions (Fig. 4 C and D). These data provide evidence that BCAAs restore altered net synaptic efficacy by a pathway independent of the glutamate:glutamine cycle.
Discussion
The key finding in this study is that dietary delivery of BCAAs ameliorates hippocampal-dependent cognitive dysfunction together with a restoration of net synaptic efficacy after concussive brain injury. Specifically, in every animal, cognitive improvement occurred only in conjunction with restored net synaptic efficacy. i.v. administration of BCAAs to severely brain damaged patients has previously shown moderately beneficial effects demonstrated by a slight improvement assessed with both the Glasgow Coma Scale and the Disability Rating Scale (cognitive ability for “feeding,” “toileting” and “grooming” reflecting levels of disability). Unfortunately, no specific cognitive testing or underlying mechanism was pursued in these studies (10–12, 21, 22).
Traumatic brain injury causes cognitive impairment and altered ipsilateral net synaptic efficacy. Specifically, an overall decrease in area CA1 net synaptic efficacy and an overall increase in DG net synaptic efficacy has been previously observed (4, 16) and replicated here. We hypothesized that injury-induced alterations in concentrations of the excitatory neurotransmitter glutamate and the inhibitory neurotransmitter GABA contribute to changes in net synaptic efficacy. However, HPLC analysis surprisingly showed no alterations in the overall concentration of these amino acids in hippocampal homogenates. Instead, the concentrations of BCAAs showed a significant reduction in the ipsilateral, but not the contralateral, hippocampus of injured animals. This is probably due to the intrinsic role of BCAAs in maintaining glutamate and thus GABA stores, which likely are under a heightened posttraumatic demand for use by distinct metabolic pathways (e.g., neurotransmission, energy production). Neurons contain multiple “pools” of both glutamate and GABA. The largest pool is the “metabolic pool,” which is approximately 1,000-fold higher than the synaptic pool, and is thought to be insular from the synaptic pool (23). However, because of greatly increased energy demand (24–26), released glutamate could be catabolized in the astrocyte for entry into the TCA cycle. Alternatively, glutamine that is returned to the neuron could be deaminated to form glutamate that is destined for energy production instead of simply replenishing the synaptic pool of glutamate. We speculate that an increased demand for de novo glutamate synthesis toward maintaining the synaptic glutamate pool significantly alters the normal ratio (17:1) of BCAA transamination:BCAA oxidation and leads to a decline in hippocampal BCAA concentration after injury. This would cause irreversible catabolism of BCAAs as a byproduct of increasing demand for glutamate synthesis, thus accounting for their decreased concentration after injury.
The complete restoration of net synaptic efficacy after both the ex vivo and in vivo delivery of BCAAs suggests that BCAA supplementation may relieve a local metabolic stress in the hippocampus and improve synthesis, buffering, and maintenance of the synaptic glutamate and GABA pool. The mechanism underlying the observed changes in the BCAA metabolic enzyme levels and the reduced 15N-leucine labeling rates remains to be fully elucidated. However, these novel injury-associated observations are likely to contribute to the impaired capacity for buffering synaptic glutamate and GABA pools. The transamination of α-ketoglutarate to glutamate using BCAA-derived nitrogen occurs despite the diminution of BCATc expression. However, as BCATc is found primarily in the synaptic terminals (27), and it is not the rate-limiting step in BCAA metabolism, it is ideally positioned to use exogenous BCAAs for the synthesis of glutamate.
Ex vivo BCAA supplementation restored net synaptic efficacy even though glutaminase was inhibited. This suggests that the restorative effect is likely to be independent of the glutamate:glutamine cycle, as inhibition of the conversion of glutamine to glutamate did not impede the beneficial effects of BCAAs. Furthermore, the most likely location of impairments are in the neuron, as no changes were observed in the expression of astrocytic enzymes (e.g., BCATm and glutamine synthetase) that would be potentially involved either in reduced concentrations of BCAAs or in BCAA-mediated restorations in net synaptic efficacy. In fact, the primary enzymes involved in BCAA and glutamate metabolism are located exclusively in the neuron, and changes in expression of these proteins would have the greatest impact on neuronal function. Therefore, we believe that BCAAs are being taken up by or transported to area CA1 and DG neurons to directly supply nitrogen necessary for glutamate synthesis in a BCATc-mediated reaction. Furthermore, a selective injury-induced decrease in regional BCATc expression in area CA1 suggests less less BCAA transamination and glutamate synthesis as a basis for this region's decreased net synaptic efficacy. In contrast, decreased expression of BCKD in the DG in injured tissue would be expected to produce a buildup of BCKAs, which limits the synthesis of glutamate. Coupled with the significant reduction in GAD levels, this would also compromise GABA production and thus ultimately increase overall DG excitability.
We propose that concussive brain injury induced regional molecular alterations in BCAA metabolic pathways in turn stress the homeostasis of regional excitatory and inhibitory neurotransmitter pools to produce opposing regional shifts in network excitability in the hippocampus. Specifically, these alterations cause a significant impairment in the hippocampal de novo synthesis capacity for glutamate and GABA using BCAA-derived amino groups. Furthermore, the restoration of leucine, isoleucine, and valine in the ipsilateral injured hippocampus to levels seen in sham animals can be achieved through dietary intervention. This BCAA refurbishing may be sufficient to restore glutamate and GABA pools, to reset net synaptic efficacy, and to eradicate injury-induced cognitive impairment. Indeed, in no animal was cognitive improvement seen without an accompanying restoration in net synaptic efficacy. Although these results show tremendous promise for clinical applications, further study needs to be conducted to determine the relative permanence of the changes mediated by BCAA application. In addition, it will be important to determine the effect of chronic BCAA treatment on improving delayed effects of TBI, which can take months or years to manifest themselves. Presently, no effective interventions are available to reverse or diminish the significant consequences of brain injury, i.e., cognitive impairment. This study demonstrates that dietary intervention with branched chain amino acids ameliorate cognitive impairment together with restoring net synaptic efficacy after a traumatic brain injury.
Methods
Animals.
All experiments were performed on 5–7 week old, 20–25 g, C57BL/J6 mice (Jackson Laboratory). All procedures were approved by the Children’s Hospital of Philadelphia Institution for Animal Care and Use Committee in accordance with international guidelines on the ethical use of animals. The fluid percussion brain injury (FPI) protocol was carried out over 2 days as detailed in SI Methods.
Supplementary Material
Acknowledgments
The authors are indebted to Ilana Nissim for determination of amino acids levels by HPLC and to Yevgeny Daikhin for measurement of 15N-labeled metabolites at the GC-MS Core. We thank Drs. M.J. Schell, M.B. Robinson, and M. Yudkoff for many helpful and insightful discussions, and Drs. R.G. Kalb and D. Dinges for critiquing earlier drafts of this manuscript. This work was supported by National Institutes of Health/National Institute of Child Health and Human Development grant RO1HD059288 (A.S.C.), and by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants RO1DK053761 (I.N.) and 5T32NS043126-07 (C.M.M). This work is dedicated to Maimon M. Cohen, whose suggestions were instrumental in planting the seeds for this line of investigation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910280107/DCSupplemental.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Oddy M, Coughlan T, Tyerman A, Jenkins D. Social adjustment after closed head injury: A further follow-up seven years after injury. J Neurol Neurosurg Psychiatry. 1985;48:564–568. doi: 10.1136/jnnp.48.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lifshitz J, Witgen BM, Grady MS. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: Evaluation by conditioned fear response. Behav Brain Res. 2007;177:347–357. doi: 10.1016/j.bbr.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witgen BM, et al. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 5.Soltesz I, Smetters DK, Mody I. Tonic inhibition originates from synapses close to the soma. Neuron. 1995;14:1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 6.D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai R, Cohen DM, Henry JF, Burrin DG, Reeds PJ. Leucine-nitrogen metabolism in the brain of conscious rats: Its role as a nitrogen carrier in glutamate synthesis in glial and neuronal metabolic compartments. J Neurochem. 2004;88:612–622. doi: 10.1111/j.1471-4159.2004.02179.x. [DOI] [PubMed] [Google Scholar]
- 9.Richardson MA, Small AM, Read LL, Chao HM, Clelland JD. Branched chain amino acid treatment of tardive dyskinesia in children and adolescents. J Clin Psychiatry. 2004;65:92–96. doi: 10.4088/jcp.v65n0116. [DOI] [PubMed] [Google Scholar]
- 10.Rossi Fanelli F, et al. Use of branched chain amino acids for treating hepatic encephalopathy: Clinical experiences. Gut. 1986;27(Suppl 1):111–115. doi: 10.1136/gut.27.suppl_1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, et al. LIV-EN Study Group. Clinical comparison of branched-chain amino acid (l-Leucine, l-Isoleucine, l-Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV-EN study) Hepatol Res. 2005;31:232–240. doi: 10.1016/j.hepres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Walser M. Therapeutic aspects of branched-chain amino and keto acids. Clin Sci (Lond) 1984;66:1–15. doi: 10.1042/cs0660001. [DOI] [PubMed] [Google Scholar]
- 13.De Bandt JP, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr. 2006;136(1) Suppl:308S–313S. doi: 10.1093/jn/136.1.308S. [DOI] [PubMed] [Google Scholar]
- 14.Grady MS, Lam AM. Management of acute head injury. In: Lam AM, editor. Anesthetic Management of Head Injury. New York: McGraw-Hill; 1995. pp. 87–100. [Google Scholar]
- 15.Tran LD, et al. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- 16.Bonislawski DP, Schwarzbach EP, Cohen AS. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol Dis. 2007;25:163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogen IL, Risa O, Haug KH, Sonnewald U, Fonnum F, Walaas SI. Distinct changes in neuronal and astrocytic amino acid neurotransmitter metabolism in mice with reduced numbers of synaptic vesicles. J Neurochem. 2008;106(4):1515–1524. doi: 10.1111/j.1471-4159.2008.05344.x. [DOI] [PubMed] [Google Scholar]
- 18.Carbonell WS, Maris DO, McCall T, Grady MS. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma. 1998;15:217–229. doi: 10.1089/neu.1998.15.217. [DOI] [PubMed] [Google Scholar]
- 19.Mikics E, et al. The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav Pharmacol. 2006;17:223–230. doi: 10.1097/00008877-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Yee BK, et al. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- 21.Aquilani R, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: A pilot study. Arch Phys Med Rehabil. 2008;89:1642–1647. doi: 10.1016/j.apmr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Aquilani R, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1729–1735. doi: 10.1016/j.apmr.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 23.McKenna MC, Stevenson JH, Huang X, Hopkins IB. Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int. 2000;37:229–241. doi: 10.1016/s0197-0186(00)00042-5. [DOI] [PubMed] [Google Scholar]
- 24.Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: A 13C NMR study. J Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- 25.Vespa PM, et al. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007;35:1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- 26.Dusick JR, et al. Increased pentose phosphate pathway flux after clinical traumatic brain injury: A [1,2-13C2]glucose labeling study in humans. J Cereb Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- 27.Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: Expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.