Abstract
Porphyromonas gingivalis secretes strong proteases called gingipains that are implicated in periodontal pathogenesis. Protein secretion systems common to other Gram-negative bacteria are lacking in P. gingivalis, but several proteins, including PorT, have been linked to gingipain secretion. Comparative genome analysis and genetic experiments revealed 11 additional proteins involved in gingipain secretion. Six of these (PorK, PorL, PorM, PorN, PorW, and Sov) were similar in sequence to Flavobacterium johnsoniae gliding motility proteins, and two others (PorX and PorY) were putative two-component system regulatory proteins. Real-time RT-PCR analysis revealed that porK, porL, porM, porN, porP, porT, and sov were down-regulated in P. gingivalis porX and porY mutants. Disruption of the F. johnsoniae porT ortholog resulted in defects in motility, chitinase secretion, and translocation of a gliding motility protein, SprB adhesin, to the cell surface, providing a link between a unique protein translocation system and a motility apparatus in members of the Bacteroidetes phylum.
Keywords: gingipain, Porphyromonas gingivalis, Flavobacterium, chitinase
Periodontal disease, the major cause of tooth loss in industrial nations (1, 2), is one of the most frequently occurring infectious diseases in humans (3), and a chronic inflammatory disease that results in destruction of periodontal tissue and alveolar bone (4). The Gram-negative anaerobic bacterium Porphyromonas gingivalis is a major periodontal pathogen (5). P. gingivalis secretes extracellular and cell-surface gingipain proteases that are major virulence factors involved in periodontal pathogenesis (6, 7). Gingipains consist of Arg-specific cysteine proteinases (Rgp) encoded by rgpA and rgpB, and the Lys-specific cysteine proteinase (Kgp) encoded by kgp (8–10). Gingipains have signal peptides to allow transit of the cytoplasmic membrane via the Sec machinery, but the mechanism of secretion across the outer membrane is not known. Studies of Gram-negative bacteria belonging to the phylum Proteobacteria have identified at least eight different protein secretion systems (11). Four of these (type I, III, IV, and VI secretion systems) transport proteins across the entire Gram-negative cell envelope and thus do not typically transport proteins with N-terminal signal peptides. Other secretion systems (type II and type V machineries, the two-partner secretion system, and the chaperone/usher system) mediate only the final step (transit across the outer membrane) and rely on the Sec or twin arginine transport systems to escort proteins across the cytoplasmic membrane. P. gingivalis is a member of the Bacteroidetes phylum, and is thus not closely related to the Proteobacteria. Analysis of the P. gingivalis genome suggested that critical components of known bacterial protein secretion systems were lacking in P. gingivalis, suggesting that some other machinery may be involved in gingipain secretion.
Genetic and biochemical analyses indicate that the membrane protein PorT is involved in gingipain secretion (12). Genes related to porT are found in many members of the large and diverse Bacteroidetes phylum, including gliding bacteria such as Flavobacterium johnsoniae and Cytophaga hutchinsonii, and nonmotile anaerobes such as Prevotella intermedia. porT orthologs have not been detected outside of the Bacteroidetes phylum, and they are also lacking from some members of the Bacteroidetes, such as the intestinal anaerobes Bacteroides thetaiotaomicron and Bacteroides fragilis. In this study, 11 additional genes that appear to be involved in gingipain secretion and activation were identified by comparative genome analysis and genetic experiments.
Results
Identification of Genes Involved in Gingipain Secretion by Comparative Genome Analysis and Genetic Experiments.
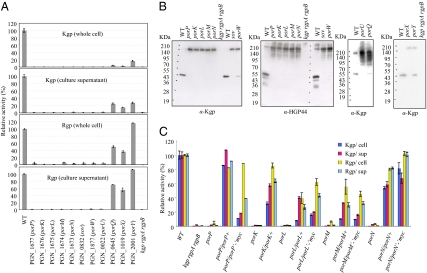
Bacterial protein secretion systems typically require multiple proteins that form a complex in the cell envelope. PorT is required for P. gingivalis gingipain secretion, and may interact with other proteins to form the translocation machinery. porT homologs are found in some other members of the phylum Bacteroidetes, such as C. hutchinsonii ATCC 33406, but not in others, such as B. thetaiotaomicron VPI-5482 (13–15). To identify possible additional components of the gingipain secretion system, we identified 55 genes in addition to porT that were present in P. gingivalis and C. hutchinsonii but absent in B. thetaiotaomicron (Fig. S1 and Table S1). P. gingivalis strains with deletion mutations in 46 of these genes were constructed, and gingipain activities were determined. Mutation of sov (PGN_0832), which was recently reported to be involved in gingipain secretion (16), or of any of nine other genes, which we designated porK (PGN_1676), porL (PGN_1675), porM (PGN_1674), porP (PGN_1677), porQ (PGN_0645), porU (PGN_0022), porW (PGN_1877), porX (PGN_1019), and porY (PGN_2001), resulted in decreased Rgp or Kgp activity in cells and culture supernatants (Fig. 1A). The mutants displayed cell-associated gingipain proproteins with high molecular masses as revealed by immunoblot analysis with anti-Hgp44 and anti-Kgp antibodies (Fig. 1B). These proproteins were inactive, as previously described (12). Introduction of wild-type copies of por genes on plasmids into the appropriate mutants resulted in complementation of the extracellular and cell-surface gingipain defects, confirming the roles of the Por proteins in secretion (Fig. 1C).
Fig. 1.
Kgp and Rgp proteins and activities in wild-type and mutant P. gingivalis. (A) Rgp and Kgp activities in intact cells and in culture supernatants. P. gingivalis cells were grown anaerobically in enriched brain heart infusion medium at 37 °C for 24 h. All cultures had similar cell densities of OD600 of ∼1.0. Kgp and Rgp activities were measured at units per milliliter of cell suspensions or culture supernatants and are indicated as percent of activity relative to that of the wild type. (B) Accumulation of gingipain proproteins with high molecular masses in mutant cells. P. gingivalis cell lysates were subjected to SDS/PAGE and immunoblot analysis with anti-Kgp and anti-Hgp44. (C) Plasmid-mediated complementation. Rgp and Kgp activities in intact cells and in culture supernatants were measured for wild-type, mutant, and complemented cells.
Five of the genes described above, porK, porL, porM, sov, and porW, are similar to F. johnsoniae gliding motility genes gldK, gldL, gldM, sprA, and sprE, respectively (17, 18). In F. johnsoniae, gldN, which is also involved in gliding motility, is located immediately downstream of gldK, gldL, and gldM. The P. gingivalis gldN ortholog (PGN_1673), which we refer to as porN, lies immediately downstream of porK, porL, and porM. A porN defective mutant of P. gingivalis was constructed and found to exhibit almost no extracellular or cell-surface gingipain activities (Fig. 1A). Gingipain activity was restored by complementation with plasmid-encoded wild-type PorN (Fig. 1C). porN mutant cells accumulated unprocessed gingipain proproteins intracellularly (Fig. 1B), further confirming a role for PorN in protein secretion.
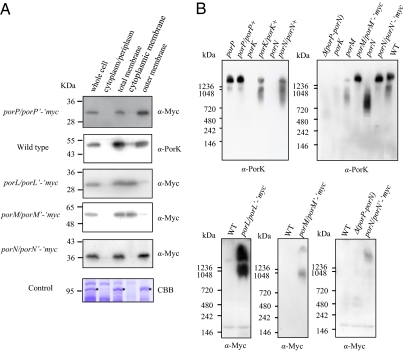
Subcellular Localization of PorP-PorN Proteins in P. gingivalis.
PorK has a hydrophobic amino terminal sequence terminated by a cysteine, which is characteristic of bacterial lipoproteins, whereas PorM, PorN, and PorP have typical signal peptides at their amino termini, and PorL is predicted to have two membrane-spanning helices near its amino terminus. Subcellular fractionation and immunoblot analyses were performed to experimentally determine the locations of these proteins. PorL and PorM were detected in the cytoplasmic membrane fraction, whereas PorK, PorN, and PorP were in the outer membrane fraction (Fig. 2A). Blue native polyacrylamide gel electrophoresis revealed that anti-PorK antibody reacted to a protein band with a molecular mass of more than 1,200 kDa, which disappeared or decreased in intensity in the porM and porN mutants (Fig. 2B). In addition, anti-Myc antibody reacted to a protein band with a molecular mass of more than 1,200 kDa in strains expressing porL’-‘myc, porM’-‘myc, or porN’-‘myc. Thus, these proteins may form part of a protein complex.
Fig. 2.
Subcellular location of PorP, PorK, PorL, PorM, and PorN proteins and formation of a large complex. (A) Subcellular location of the Por proteins. The cell lysates of P. gingivalis strains were subjected to detergent fractionation followed by SDS/PAGE and immunoblot analysis with anti-PorK antibody for the wild-type strain, and anti-Myc antibody for the porP/porP’-‘myc, porL/porL’-‘myc, porM/porM’-‘myc, and porN/porN’-‘myc strains. As a control, a major outer membrane protein, RagA, which was identified by mass spectrometry, is indicated by an asterisk (*). CBB, Coomassie Brilliant Blue staining. (B) Detection of protein complexes by Blue Native PAGE. Cell lysates of P. gingivalis strains were subjected to 3–12% gradient Blue Native PAGE and immunoblot analysis with anti-Myc antibody and anti-PorK antibody.
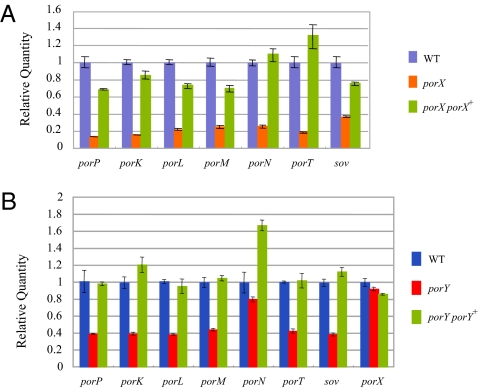
P. gingivalis Genes Regulated by the Putative Response Regulator PorX and the Putative Histidine Kinase PorY.
PorX and PorY are similar to response regulatory proteins and histidine sensor kinases respectively of two-component signal transduction systems, and may have roles in regulation of expression of genes of the transport system. PorX and PorY are “orphan” signal transduction proteins because the expected cognate partners do not appear to be encoded by nearby genes in either case. Given the similar phenotypes of the mutants it is possible that the two proteins function together as a two-component signal transduction system. To determine what genes are regulated by PorX, microarray analysis using a custom tiling DNA array chip with the genome sequence of P. gingivalis ATCC 33277 was performed. The tiling DNA array analysis revealed that 20 genes were down-regulated in the porX deletion mutant to less than 60% of the wild-type parent strain Table S2). porT, sov, porK, porL, porM, porN, and porP, which were among the 20 down-regulated genes, are each involved in gingipain secretion (Fig. S2), and the decreased expression of these genes was confirmed by RT-PCR analysis (Fig. 3A). RT-PCR analysis revealed that these genes were also down-regulated in the porY mutant (Fig. 3B).
Fig. 3.
Response regulator PorX- and histidine kinase PorY-mediated regulation of the expression of porT, sov, porP, porK, porL, porM, and porN. (A) Real-time RT-PCR analysis of gene expression of porP, porK, porL, porM, porN, porT, and sov in the wild-type, porX, and porX porX+ (complemented) strains. (B) Real-time RT-PCR analysis of gene expression of porP, porK, porL, porM, porN, porT, sov, and porX in the wild-type, porY, and porY porY+ (complemented) strains.
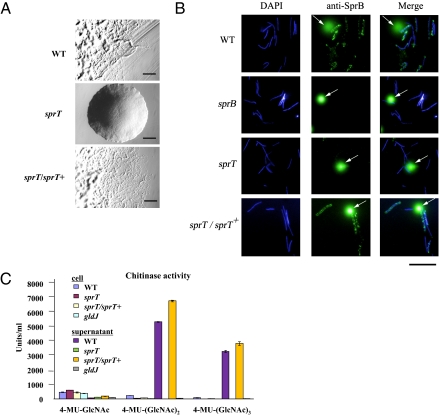
Disruption of the F. johnsoniae porT Ortholog sprT Results in Motility Defects.
P. gingivalis is nonmotile, but many other members of the phylum Bacteroidetes, such as the cellulolytic bacterium C. hutchinsonii, the chitin digesting F. johnsoniae, and the fish pathogen Flavobacterium psychrophilum, exhibit rapid gliding motility over surfaces. Bacteroidete gliding is not closely related to other well-studied forms of bacterial movement, such as flagellar motility, type IV pilus-mediated twitching motility, myxobacterial gliding motility, and mycoplasma gliding motility (19). Fifteen genes (gldA, gldB, gldD, gldF, gldG, gldH, gldI, gldJ, gldK, gldL, gldM, gldN, sprA, sprB, and sprE) that play important roles in bacteroidete gliding have been identified (17–20). F. johnsoniae, F. psychrophilum, and C. hutchinsonii each have a porT ortholog. Because PorT, PorK, PorL, PorM, and PorN appear to function together in P. gingivalis, we hypothesized that the F. johnsoniae PorT ortholog may function with GldK, GldL, GldM, and GldN, and thus have a role in gliding. A F. johnsoniae porT ortholog mutant was constructed and was found to be deficient in gliding motility (Fig. 4A). The mutant formed nonspreading colonies on agar, and individual cells were severely but not completely deficient in gliding in wet mounts. Whereas wild-type cells attached readily to the glass slide and displayed rapid motility, the mutant cells exhibited very limited motility. Most mutant cells failed to attach to the glass, and most of the cells that did attach failed to move. However, extended observation revealed a few cells (typically less than 1 of 1,000) that exhibited occasional slight movements. Because this phenotype is similar to that exhibited by nonspreading sprA mutants (17), we named the gene sprT. Complementation of the sprT mutant with a wild-type copy of the gene on a plasmid restored colony spreading and single-cell motility.
Fig. 4.
Effect of disruption of F. johnsoniae sprT on gliding motility, surface localization of SprB protein, and extracellular chitinase activity. (A) Colony morphology of wild-type, mutant, and complemented strains. Cells were incubated on PY2 agar at 25 °C for 45 h. (Scale bar: 0.5 mm.) (B) Detection of surface localized SprB protein by immunofluorescence microscopy. Cells of wild-type and mutant F. johnsoniae were exposed to DAPI and to anti-SprB antibodies followed by secondary antibodies conjugated to Alexa 488. Arrows indicate InSpeck relative-intensity fluorescence beads. (Scale bar: 10 μm.) (C) Chitinase activities of intact cells and culture supernatants of F. johnsoniae strains with three synthetic substrates for chitinase, 4-MU-GlcNAc, 4-MU-(GlcNAc)2, and 4-MU-(GlcNAc)3.
sprT Mutant Cells Are Defective in Translocation of the Gliding Motility Adhesin, SprB, to the Cell Surface.
There are several possible roles of protein translocation in bacteroidete gliding motility. Mounting evidence suggests that the gliding machinery propels adhesive proteins, such as SprB, along the cell surface (20). The secretion system may be needed for assembly of SprB or other cell-surface components of the motility apparatus on the cell surface. Latex spheres carrying antibodies against SprB attach specifically to wild-type F. johnsoniae cells and are rapidly propelled along their surfaces (20). The antibody-coated spheres failed to bind to cells of sprB mutants. We used antibody-coated spheres to determine whether SprB was present on the surface of cells of sprT mutants. Wild-type and sprT mutant cells produced SprB protein as determined by Western blot analysis (Fig. S3), but spheres carrying antibodies against SprB failed to bind to cells of the sprT mutant (Movies S1–S3). Under the conditions tested, 65 of 100 randomly selected wild-type cells bound and propelled spheres during a 30-s incubation, whereas none of 100 sprT mutant cells bound or propelled spheres. Complementation of the sprT mutant with the wild-type gene on a plasmid restored the ability to bind and propel antibody-coated spheres to wild-type levels (Movie S4). This indicates that SprB is not properly exposed on the cell surface of the sprT mutant, and suggests that SprT may be involved in secretion of this adhesin to the cell surface. Detection of SprB by immunofluorescence confirmed that sprT mutant cells are defective in surface localization of SprB protein (Fig. 4B). Cells with mutations in gldK, gldL, gldM, gldN, and sprA behaved similary to those of the sprT mutant. They produced SprB protein (20) but were deficient in surface localization of SprB as determined by failure to bind spheres coated with anti-SprB antibodies, and by decreased staining by anti-SprB immunofluorescence microscopy.
sprT Mutant Cells Are Deficient in Extracellular Chitinase Activity.
Previous studies showed that many F. johnsoniae mutants defective in gliding also exhibit deficiencies in chitin utilization, but the reason for the lack of chitin utilization has not been elucidated (17). Chitinolytic activity was determined using three synthetic substrates, 4-methylumbelliferyl N-acetyl-β-D-glucosaminide (4-MU-GlcNAc), 4-methylumbelliferyl N, N′-diacetyl-β-D-chitobioside [4-MU-(GlcNAc)2], and 4-methylumbelliferyl β-D-N, N′, N′′-triacetylchitotrioside [4-MU-(GlcNAc)3]. The wild-type strain and a sprT/sprT+ plasmid-mediated complementation strain exhibited strong hydrolytic activities against 4-MU-(GlcNAc)2 and 4-MU-(GlcNAc)3 in their culture supernatants, whereas those of the sprT and gldJ mutants did not (Fig. 4C). The culture supernatants of the wild-type and sprT/sprT+ plasmid-mediated complementation strain contained a putative chitinase encoded by Fjoh_4555 that appeared to be lacking in the culture fluids of sprT mutant cells (Fig. S4). Lysates of Escherichia coli cells expressing recombinant Fjoh_4555 protein also displayed chitinase activity (Fig. S5), supporting the role of Fjoh_4555 in chitin digestion. These findings suggest that SprT and GldJ are involved in chitinase secretion by F. johnsoniae, and that part of the bacteroidete gliding motility machinery is also a unique protein translocation apparatus.
Discussion
Findings in this study indicate a link between a protein translocation system and a motility apparatus in members of the Bacteroidetes phylum. Other well-studied bacterial motility machines also function in protein translocation. The flagellar basal body is a rotary motor, but also functions as a type III secretion system involved in flagellar assembly and has served as a paradigm for studies of type III protein secretion in general (11). Similarly, pilus-mediated twitching motility involves movement of proteins across the cell envelope, and is closely related to type II secretion systems.
The protein secretion system identified in this study, which we refer to as the Por secretion system (PorSS), is unique because the proteins involved (PorK, PorL, PorM, PorN, PorP, PorT, PorW, and Sov) do not exhibit sequence similarity to components of known secretion systems. Protein products from rgpA, rgpB, kgp, and hagA appear to be secreted via the P. gingivalis PorSS (12). These proteins have a conserved C-terminal domain (CTD) (21). CTD structures were also found in predicted proteins of other bacteria in the phylum Bacteroidetes, including P. intermedia, Tannerella forsythia, Parabacteroides distasonis, C. hutchinsonii, F. johnsoniae, and F. psychrophilum, each of which also contain porT orthologs (14, 15, 22). Genes encoding such CTDs were not found in B. thetaiotaomicron or B. fragilis, which both also lack porT orthologs, supporting the suggestion that the CTD proteins are secreted using the PorSS. The C-terminal regions of F. johnsoniae SprB (Fjoh_0979) and chitinase (Fjoh_4555) are not closely similar to the P. gingivalis CTD. If the CTD sequences are involved in interaction with the secretion apparatus, they may have diverged considerably within the Bacteroidetes phylum, as have the individual components of the putative secretion systems of distantly related members of this phylum.
The gliding bacteria F. johnsoniae, F. psychrophilum, and C. hutchinsonii possess complete sets of gld, spr, and por genes (Table S3). In contrast, the nonmotile P. gingivalis, P. intermedia, and P. distasonis contain orthologs of some but not all of the gld and spr genes. Some of the missing orthologs, such as gldD, gldF, and gldG, may have essential roles in motility but not in protein export. Many of the gld, spr, and por genes are absent from B. fragilis and B. thetaiotaomicron, which are both nonmotile and appear to lack PorSS.
Bacterial protein secretion has been most intensively studied in members of the phylum Proteobacteria and in Gram-positive bacteria. Few studies of protein secretion have been conducted for the members of other bacterial phyla. Our studies of members of the phylum Bacteroidetes identified what appears to be a unique protein secretion system that may have evolved independently from the previously known systems. The PorSS is involved in pathogenesis of P. gingivalis and in gliding motility and chitin utilization of F. johnsoniae. PorSSs appear to be present in many other bacteroidetes where they may have diverse functions. These include important periodontal pathogens such as P. intermedia and T. forsythia, and common fish pathogens such as F. psychrophilum and Flavobacterium columnare. An improved understanding of the PorSS may aid in control of the diseases associated with these bacteria. The addition of the PorSS to the already extensive list of bacterial protein secretion systems suggests that machinery to transport proteins across the bacterial outer membrane may have evolved independently many times. Analysis of protein secretion in the many other understudied phyla of bacteria may uncover additional secretion machines.
Materials and Methods
Bacterial Strains and Culture Conditions.
Bacterial strains and plasmids used in this study are listed in Table S4. P. gingivalis cells were grown anaerobically (10% CO2, 10% H2, 80% N2) in enriched brain heart infusion medium and on enriched tryptic soy agar (8). For blood agar plates, defibrinated laked sheep blood was added to enriched tryptic soy agar at 5%. For selection and maintenance of antibiotic-resistant P. gingivalis strains, antibiotics were added to the medium at the following concentrations: erythromycin (Em), 10 μg/mL; tetracycline (Tc), 0.7 μg/mL. F. johnsoniae ATCC 17061 (UW101) was the wild-type strain used in this study. F. johnsoniae cells were grown in Casitone yeast extract medium at 30 °C (23). To observe colony spreading as a result of gliding motility, F. johnsoniae cells were grown on PY2 agar at 25 °C (24). To observe gliding of individual cells, F. johnsoniae was grown in motility medium (MM) (25) overnight at 25 °C without shaking. For selection and maintenance of the antibiotic-resistant F. johnsoniae strains, antibiotics were added to the medium at the following concentrations: Em, 100 μg/mL; Tc, 15 μg/mL.
Venn Diagram Analysis.
The pairwise reciprocal best hits were calculated among P. gingivalis ATCC 33277, B. thetaiotaomicron VPI-5482, and C. hutchinsonii ATCC 33406. Homologous genes were identified by BLASTP searches using E-value threshold ≤ 10−10. Orthologous genes were defined as reciprocal best hits.
Construction of Bacterial Strains.
Construction and complemetantion of P. gingivalis deletion mutants and of a F. johnsoniae sprT mutant were performed as previously described (8, 24) and are detailed in SI Text. Construction of an E. coli strain expressing Fjoh_4555 is described in SI Text. Primers used in this study are listed in Table S5.
Enzymatic Assays.
Kgp and Rgp assays were performed as previously described (12) and are detailed in SI Text.
For chitinase activity assay, F. johnsoniae cells were incubated in MM (25) at 25 °C overnight with shaking at 170 rpm. Bacterial cells and culture supernatants were separated by centrifugation at 10,000 × g for 10 min at 4 °C. Cells were suspended in the original volume of a buffer consisting of 137 mM NaCl, 2.7 mM KCl, 10 mM Na2PO4, 2mM KH2PO4, pH 7.4 (PBS). Chitinase activity was determined with the synthetic substrates 4-methylumbelliferyl N-acetyl-β-D-glucosaminide (4-MU-GlcNAc; Sigma); 4-methylumbelliferyl N, N’-diacetyl-β-D-chitobioside [4-MU-(GlcNAc)2; Sigma]; 4-methylumbelliferyl β-D-N, N′, N′′-triacetylchitotrioside [4-MU-(GlcNAc)3; Sigma] using a chitinase assay kit (Chitinase Assay Kit, Fluorimetric; Sigma) according to manufacturer's instructions. Enzyme assays were performed in triplicate. One unit of the enzyme activity was defined as the amount of enzyme able to release 1 μmol of 4-methylumbelliferone per minute. Chitinase activity is indicated as units per milliliter of cell suspensions or culture supernatants. For measurement of recombinant chitinase activity in E. coli BL21(DE3) harboring pKF003, cells were grown in LB broth at 37 °C until OD600 of 0.6. Cells were placed on ice for 10 min, supplemented with 0.1 mM isopropyl-β-D-thiogalactopyranoside and incubated at 16 °C for 15 h. Cells were harvested by centrifugation, resuspended in PBS, disrupted by sonication, and chitinase activity was determined as described. The recombinant chitinase activity is indicated as units per microgram of protein.
Quantification of Gene Expression by Real-Time RT-PCR.
Total RNA was isolated from P. gingivalis cells grown to midexponential phase (OD600 of ∼1.0) by using an RNeasy Mini Kit (Qiagen Science). DNA was removed with RNase-Free DNase. cDNA was generated in a reaction mixture containing a random primer (Promega), dNTP mixture, RNase inhibitor, DTT, SuperScript III Reverse Transcriptase (Invitrogen), and DEPC-treated water. Real-time quantitative PCR (qPCR) was performed using Brilliant SYBR Green II QPCR Master Mix (Stratagene) with Mx3005PTM Real-Time PCR System (Stratagene) according to the manufacturer's instruction. Primers for the real-time qPCR are listed in Table S5 and were designed using Primer3 program. Real-time qPCR conditions were as follows: 1 cycle at 95 °C for 10 min, and 35 cycles of 95 °C for 30 s, and 60 °C for 1 min. At each cycle, accumulation of PCR products was detected by the reporter dye from the dsDNA-binding SYBR Green. To confirm that a single PCR product was amplified, after the PCR, a dissociation curve (melting curve) was constructed in the range of 55 °C to 95 °C. All data were analyzed using Mx3005P software. The expression level of each targeted gene was normalized to that of the 16S rRNA gene, which was used as a reference. All PCR reactions were carried out in triplicate. The efficiency of primers binding was determined by linear regression by plotting the cycle threshold (CT) value versus the log of the cDNA dilution. Relative quantification of transcript was determined using the comparative CT method (2−ΔΔCT) calibrated to 16S rRNA. qPCR experiments were performed multiple times independently with comparable results.
Subcellular Fractionation.
Subcellular fractionation of P. gingivalis cells was performed as previously described (12) and is detailed in SI Text.
Protein Preparation and Western Blot Analysis.
Western blots were performed to detect SprB in extracts of wild-type and mutant cells of F. johnsoniae. Overnight cultures were grown in MM at 25 °C without shaking. Cells were pelleted by centrifugation at 3,800 × g for 10 min and suspended in a buffer consisting of 10 mM Tris, 8 mM MgSO4, pH 7.5 (TM). Cells were lysed by boiling for 5 min in SDS/PAGE loading buffer. Protein (25 μg), as determined by BCA assay (Pierce), was loaded per lane, and proteins were separated on 3–8% Criterion XT Tris-acetate acrylamide gels (Bio-Rad) and transferred to nitrocellulose membranes. SprB was detected with SuperSignal West Pico (Pierce) using a FOTO/Analyst LuminaryFx Workstation (Fotodyne).
For preparation of protein samples of culture supernatants of F. johnsoniae, cells incubated in MM at 25 °C overnight with shaking at 170 rpm were centrifuged at 10,000 × g for 10 min at 4 °C. Solid ammonium sulfate was added to the culture supernatant to 50% (wt/vol) saturation. After centrifugation for 15 min, the pellets were dissolved in 10 mM Hepes (pH 7.4) and dialyzed overnight against 10 mM Hepes (pH 7.4) in a Slide-A-Lyzer Dialysis Cassette (3,500 MWCO; Pierce) and subjected to SDS/PAGE.
Blue Native PAGE.
P. gingivalis cells from a 0.5-mL culture were harvested by centrifugation at 10,000 × g for 10 min at 4 °C, and suspended with 200 μL of sample buffer containing 1% n-dodecyl-β-D-maltoside (DDM). After sonication, the samples were centrifuged at 20,000 × g for 30 min at 4 °C to clarify. The samples were supplemented with Coomassie Blue G-250 at a final concentration of 0.25% and electrophoresed on a nondenaturing polyacrylamide gel (NativePAGE Novex 3–12% Bis-Tris Gels; Invitrogen).
Mass Spectrometry.
A gel plug containing proteins was subjected to the following procedures: washing with 50% (vol/vol) acetonitrile, washing with 100% acetonitrile, reduction with 10 mM DTT, alkylation with 55 mM iodoacetamide, washing/dehydration with 50% (vol/vol) acetonitrile, and digestion for 10 h with 10 μg/mL trypsin. The resulting peptides were extracted from the gel plug with 0.1% (vol/vol) trifluoroacetic acid/50% (vol/vol) acetonitrile and concentrated using C-18 ZipTips (Millipore). Digests were spotted on a MALDI target using α–cyano-4-hydroxycinnamic acid as a matrix. Spectra were acquired on a 4800 MALDI TOF/TOF Analyzer (Applied Biosystems). MS/MS spectra were acquired automatically.
Preparation of Antisera.
Rabbit antiserum against a peptide derived from the amino acid sequence F387GLYDMAGNVAEWT400 of PGN_1676 (PorK) in which a cysteine residue was synthesized at the N terminus of the peptide and conjugated to keyhole limpet hemocyanin was purchased from Sigma Genosys. Preparation of anti-Kgp and anti-Hgp44 antisera has been described previously (12, 26). c-Myc antibody was obtained from Sigma.
Tiling Microarray Analysis.
Custom tiling microarrays spanning the whole genome of P. gingivalis ATCC 33277 with 25-mer probes each of which was eight-bases shifted on the genome sequence were purchased from Affymetrix. Antisense biotinylated cDNA was prepared from 10 μg of total RNA according to the Affymetrix GeneChip prokaryotic one-cycle target preparation protocol (Affymetrix). In short, reverse transcriptase (Superscript II; Invitrogen,) and random hexamer primers were used to produce DNA complementary to the RNA. The cDNA products were then fragmented by DNase I and labeled at the 3′ termini with terminal transferase and biotinylated Gene Chip DNA labeling reagent (Affymetrix). The fragmented and labeled cDNA was hybridized to the GeneChip at 45°C for 16 h. Staining, washing, and scanning procedures were carried out according to the GeneChip Expression Analysis Technical manual (Affymetrix). Hybridization was performed three times with the labeled cDNAs independently prepared. Signal intensities were quantified with the GeneChip Operating Software (Affymetrix), and further data analyses were performed with a microarray genomic analysis program (Insilico Molecular Cloning Array edition, In Silico Biology) and Microsoft Excel software (Microsoft). The normalization constant from the 16S rRNA gene was used to calculate the calibrated ratio for every CDS within the P. gingivalis ATCC 33277 genome.
Binding and Movement of Protein G–Coated Polystyrene Spheres on F. johnsoniae Cells.
Movement of surface localized SprB was detected as previously described (20) and is detailed in SI Text.
Detection of Surface Localized SprB on F. johnsoniae Cells by Immunofluorescence Microscopy.
Wild-type and mutant cells were examined by immunofluorescence microscopy to identify cell-surface localized SprB. Cells were grown overnight in MM medium at 25 °C. Ten microliters of cells were diluted in 140 μL of MM and fixed with 1% formaldehyde for 15 min. Cells were collected on 0.4-μm Isopore membrane filters (Millipore) by filtration. Cells were washed three times with 200 μL of PBS and blocked with 0.1% BSA in PBS for 30 min. After removal of the blocking solution by filtration, cells were exposed to 200 μL of a 1:200 dilution of purified anti-SprB (20) in PBS with 0.1% BSA for 90 min. Cells were washed five times with 200 μL of PBS and exposed to 200 μL of a 1:5,000 dilution of secondary antibody conjugated to Alexa 488 (Invitrogen) in PBS + 0.1% BSA. Cells were incubated 60 min in the dark, the liquid was removed, and cells were washed five times with PBS. During the final PBS wash, 5 μL of InSpeck 0.3% relative intensity fluorescence beads (Invitrogen) were added as controls. The final wash was removed by filtration, the filters were mounted on glass slides with 6 μL of VectaShield with DAPI (Vector Laboratories Inc.), coverslips were applied, and samples were observed using a Nikon Eclipse 50i microscope. Images were captured with a Photometrics CoolSNAPES camera with exposure times of 1,500 ms (DAPI) and 500 ms (Alexa 488).
Supplementary Material
Acknowledgments
We thank T. Hayashi for helpful discussions and comments on the manuscript. This research was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan Grants 18018032 and 20249073, by the Global COE Program at Nagasaki University (K.N.), and by National Science Foundation Grant MCB-0641366 (to M.J.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912010107/DCSupplemental.
References
- 1.Papapanou PN. Epidemiology of periodontal diseases: An update. J Int Acad Periodontol. 1999;1:110–116. [PubMed] [Google Scholar]
- 2.Irfan UM, Dawson DV, Bissada NF. Epidemiology of periodontal disease: A review and clinical perspectives. J Int Acad Periodontol. 2001;3:14–21. [PubMed] [Google Scholar]
- 3.Armitage GC. Periodontal diseases: Diagnosis. Ann Periodontol. 1996;1:37–215. doi: 10.1902/annals.1996.1.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 5.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 6.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtis MA, Aduse-Opoku J, Rangarajan M. Cysteine proteases of Porphyromonas gingivalis. Crit Rev Oral Biol Med. 2001;12:192–216. doi: 10.1177/10454411010120030101. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, et al. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 10.Curtis MA, et al. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodontal Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 11.Economou A, et al. Secretion by numbers: Protein traffic in prokaryotes. Mol Microbiol. 2006;62:308–319. doi: 10.1111/j.1365-2958.2006.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato K, et al. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J Biol Chem. 2005;280:8668–8677. doi: 10.1074/jbc.M413544200. [DOI] [PubMed] [Google Scholar]
- 13.Naito M, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008;15:215–225. doi: 10.1093/dnares/dsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie G, et al. Genome sequence of the cellulolytic gliding bacterium Cytophaga hutchinsonii. Appl Environ Microbiol. 2007;73:3536–3546. doi: 10.1128/AEM.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duchaud E, et al. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat Biotechnol. 2007;25:763–769. doi: 10.1038/nbt1313. [DOI] [PubMed] [Google Scholar]
- 16.Saiki K, Konishi K. Identification of a Porphyromonas gingivalis novel protein sov required for the secretion of gingipains. Microbiol Immunol. 2007;51:483–491. doi: 10.1111/j.1348-0421.2007.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 17.Nelson SS, Glocka PP, Agarwal S, Grimm DP, McBride MJ. Flavobacterium johnsoniae SprA is a cell surface protein involved in gliding motility. J Bacteriol. 2007;189:7145–7150. doi: 10.1128/JB.00892-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun TF, Khubbar MK, Saffarini DA, McBride MJ. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol. 2005;187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nat Rev Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 20.Nelson SS, Bollampalli S, McBride MJ. SprB is a cell surface component of the Flavobacterium johnsoniae gliding motility machinery. J Bacteriol. 2008;190:2851–2857. doi: 10.1128/JB.01904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seers CA, et al. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen KA, Travis J, Potempa J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? J Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride MJ, Kempf MJ. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J Bacteriol. 1996;178:583–590. doi: 10.1128/jb.178.3.583-590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal S, Hunnicutt DW, McBride MJ. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc Natl Acad Sci USA. 1997;94:12139–12144. doi: 10.1073/pnas.94.22.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, McBride MJ, Subramaniam S. Cell surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J Bacteriol. 2007;189:7503–7506. doi: 10.1128/JB.00957-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamaguchi A, et al. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia. Microbiology. 2003;149:1257–1264. doi: 10.1099/mic.0.25997-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.