Abstract
Bisphenol A (BPA), a chemical estrogen widely used in the food-packaging industry and baby bottles, is recovered in human fluids (0.1–10 nM). Recent studies have reported that BPA is hormonally active at low doses, emphasizing the debate of a risk for human health. Estrogen receptors are expressed in the colon, and although the major route of BPA exposure is food, the effects on gut have received no attention. We first examined the endocrine disrupting potency of BPA on colonic paracellular permeability (CPP), experimental colitis, and visceral sensitivity in ovariectomized rats orally exposed to 5 mg/kg/d BPA (i.e., the no observed adverse effect level), 50 μg/kg/d BPA (i.e., tolerable daily intake), or lower doses. BPA dose-dependently decreased basal CPP, with a half-maximal inhibitory dose of 5.2 μg/kg/d, 10-fold below the tolerable daily intake. This correlated with an increase in epithelial tight junction sealing, also observed in Caco-2 cells exposed to 10 nM BPA. When ovariectomized rats were fed with BPA at the no observed adverse effect level, the severity of colitis was reduced, whereas the same dose increased pain sensitivity to colorectal stimuli. We then examined the impact of perinatal exposure to BPA on intestinal permeability and inflammatory response in the offspring. In female rats, but not in male rats, perinatal BPA evoked a decrease of CPP in adulthood, whereas the proinflammatory response of colonic mucosa was strengthened. This study first demonstrates that the xenoestrogen BPA at reference doses influences intestinal barrier function and gut nociception. Moreover, perinatal exposure promotes the development of severe inflammation in adult female offspring only.
Keywords: endocrine disruptor, gut permeability, inflammation, pain, estrogen
The xenoestrogen bisphenol A (BPA), a food contaminant with endocrine disruptor activity, is the monomer widely used to manufacture polycarbonate plastics including baby bottles, infant feeding containers or tableware (plates and mugs), and epoxy resin lining food and beverage cans (1, 2). Significant exposure of humans through diet is evidenced by the presence of BPA in urine, blood, fetal tissues, and amniotic fluid (3, 4). BPA leaches from the polymers into food and water under normal conditions, and exposure to elevated temperatures (boiling, heating) greatly increases its rate of migration (5, 6). There is a global concern for human health as BPA binds to estrogen receptors (ERs) (7), and can interfere with normal sex hormone balance. As a protective measure the US Environmental Protection Agency and European Food Safety Agency have established a tolerable daily intake (TDI) of 50 μg/kg/d, applying an uncertainty factor of 100 to the no observed adverse effect level (NOAEL) of 5 mg/kg/d. However, recent studies revealed that chronic exposure to these reference doses alters some biological endpoints (8, 9), warranting a request for reevaluation of human safe daily intake limits (4, 10). To date, most studies examining the estrogenic impact of BPA have focused on reproductive function (11–13) and, more recently, on brain development (14, 15). In contrast, although the gut is in direct contact with orally absorbed BPA, the endocrine impact on the intestinal barrier function remains an unexplored endpoint.
The intestinal barrier is a highly dynamic interface between external and internal environment of the body, playing an important role in maintaining mucosal immune homeostasis. Mainly dedicated to the absorption of nutrients and water (16, 17), the intestinal epithelium also provides a physical barrier designed to restrict the passage of a broad spectrum of noxious and immunogenic substances from the lumen (18). This is of particular importance in the colon, where the complex microbial composition provides an abundant source of potentially detrimental ligands and antigens that may cause chronic mucosal injury. Maturation of the intestinal barrier function occurs early in life, prenatally and postnatally, and is linked to increased intestinal permeability stimulating the development of the mucosal immune system (19–21). In recent years, several lines of clinical and experimental evidence have reported that estrogens are involved in the development and regulation of the gut barrier. Altogether, a sex difference in intestinal permeability and inflammation (22), the prevalence of irritable bowel syndrome in women (23), the fluctuations of irritable bowel syndrome–associated abdominal pain in women during their menstrual cycle (24), as well as the wide expression of estrogen receptors (ERs) in human fetal and adult colonic mucosa (25, 26) suggest that estrogens play a key role. In animal studies, estrogen action on intestinal permeability, inflammation, and viscerosensitivity are controlled by ERs in gut epithelial cells and sensory neurons, the latter conveying peripheral information to the central nervous system (27–29). Therefore, we assumed that BPA may target these receptors.
Accordingly, the present study aimed at comparing the impact of oral exposures to BPA and estradiol benzoate (EB) on the intestinal barrier function. In ovariectomized (OVX) rats exposed for 15 d to the NOAEL or the predicted safe dose TDI, we first examined colonic paracellular permeability under basal conditions (i.e., without inflammation), then inflammatory response through determination of neutrophil infiltration and colonic content of macrophage migration inhibitory factor (MIF), a proinflammatory cytokine targeted by estradiol in colitis (28). Second, we tested the hypothesis that daily oral exposure to BPA may impact pain perception in a model of visceral sensitivity in response to colorectal distension. Third, as low doses of BPA decreased intestinal permeability, which could adversely interfere with the maturing process of the gut, we also evaluated the effects of a perinatal exposure to BPA on intestinal barrier function in the offspring.
Results
Low Doses of BPA Decrease Intestinal Permeability in the OVX Rat.
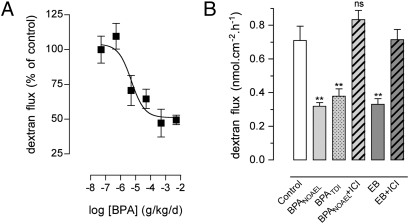
Estradiol affects colonic paracellular permeability (CPP) through ERs in epithelial cells (27). OVX rats were used for complete depletion of endogenous sex hormones, and the influence of a daily oral exposure to BPA was examined on CPP in Ussing chambers. Relative to the vehicle-treated OVX controls, BPA caused a dose-dependent decrease of CPP (Fig. 1A), with a half-maximal inhibitory dose of 5.2 μg/kg/d (95% CI, 0.8–32 μg/kg/d), which is below the human TDI, and maximal inhibition at 0.5 mg/kg/d. To determine whether the decrease in CPP was related to the estrogenicity of BPA, the reference doses were tested in the presence versus the absence of the ER antagonist ICI 182.780, and compared with the effect of EB. As shown in Fig. 1B, the NOAEL and TDI of BPA significantly decreased the paracellular flux to dextran FITC by 55% and 46%, respectively, compared with vehicle-treated OVX controls (P < 0.01). The decrease in CPP was similar to the one observed in EB-treated rats (0.33 ± 0.03 vs. 0.71 nmol·cm−2·h−1 ± 0.09 in controls; P < 0.01), and both BPA and EB effects were prevented in the presence of ICI 182.780 (Fig. 1B).
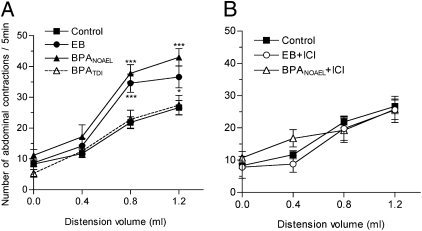
Fig. 1.
Comparative effects of BPA and estradiol on intestinal permeability in OVX rats. (A) Dose effect of BPA (0.5 μg to 5 mg/kg/d for 15 d by mouth) on colonic paracellular permeability (six to eight rats per group; P < 0.05, one-way ANOVA). (B) Effects of 15-d treatment with corn oil (control), BPA NOAEL or TDI, or EB with or without ICI 182.780 (ICI) on paracellular dextran flux (5–13 rats per group). **P < 0.01 vs. controls; ns, not significant on one-way ANOVA. Bars are means ± SEM of triplicate measurements.
Effect of BPA on Tight Junction Proteins and ER Expression in the Colon.
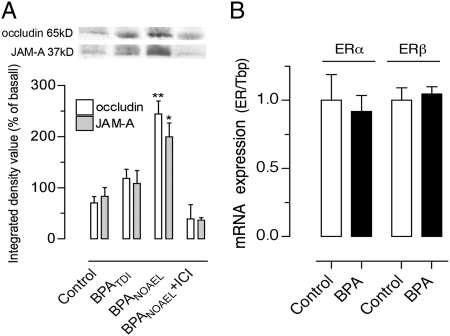
Estradiol enhances the expression of occludin and junctional adhesion molecule (JAM)–A, two transmembrane proteins that sealed intercellular spaces at apical tight junctions (TJs) (27). A dose-related increase in occludin and JAM-A protein levels was observed in the colon of OVX rats exposed to BPA, reaching statistical significance at NOAEL, and this effect was blocked by ICI 182.780 (Fig. 2A). Colonic mucosa analyzed for expression levels of ERα and ERβ mRNA showed predominant expression of ERβ, and BPA at NOAEL did not affect the expression levels of both ERs compared with OVX controls (Fig. 2B).
Fig. 2.
Effects of BPA on TJ proteins and ER expression in OVX rats. (A) Representative Western blot lanes for occludin and JAM-A and corresponding densitometric analysis of protein levels in the colon of rats fed with BPA at NOAEL, TDI, or vehicle (control), with or without ICI 182.780. Note a dose-related increase of occludin and JAM-A in BPA-treated OVX rats. (B) Relative expression levels of ERα and ERβ mRNA in the colon of OVX rats treated with vehicle (control, set to 1) or BPA NOAEL. Using the same threshold for both ERs, ERβ exponential PCR amplification occurred at 4.2 ± 0.2 cycle thresholds earlier compared with ERα, consistent with predominant ERβ expression. Bars are means ± SEM of duplicate measurements (8–10 rats per group). *P < 0.05, **P < 0.01 vs. vehicle controls (t test).
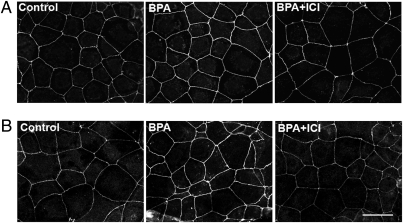
To further investigate whether BPA also affects paracellular sealing in human colonic cells, Caco-2 monolayers were immunostained for occludin and JAM-A. In control cells, labeling occurred as distinct continuous bands lining cell borders (Fig. 3). In BPA-exposed cells (10 nM), occludin and JAM-A staining were markedly increased at TJ level, and ICI 182.780 completely prevented this effect (Fig. 3).
Fig. 3.
Immunofluorescence detection of occludin (A) and JAM-A (B) in Caco-2 cells exposed for 24 h to ethanol vehicle (control) or BPA (10 nM) with or without ICI 182.780 (10 μM). Note that BPA increases occludin and JAM-A staining in epithelial cell membranes, and ICI 182.780 added 24 h before BPA blocked this effect. (Scale bars: 25 μm.)
Effects of BPA on the Inflammatory Response in the OVX Rat.
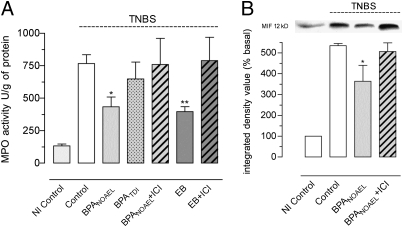
Using the rat model of trinitrobenzene sulfonic acid (TNBS)–induced colitis, the neutrophil infiltration was assessed in the colon of OVX rats receiving EB, BPA, or the vehicle corn oil for 15 d before inflammation. Compared with noninflamed rats, a sharp increase in colonic myeloperoxidase activity (MPO) was observed in all experimental groups 24 h after induction of colitis (Fig. 4A). In inflamed conditions, MPO activity in TDI-treated rats did not differ from controls, whereas, similar to EB treatment, the NOAEL for BPA significantly decreased MPO activity compared with control inflamed rats (−43%; P < 0.05), and coadministration of ICI 182.780 prevented both BPA and EB effects (Fig. 4A). In addition, a 32% decrease in tissue MIF content was observed in BPA NOAEL–treated rats compared with inflamed controls (P < 0.05), and ICI 182.780 blocked this effect (Fig. 4B).
Fig. 4.
Comparative effects of BPA and estradiol on colitis in OVX rats. (A) Colonic MPO 24 h after induction of TNBS colitis in OVX rats exposed for 15 d to corn oil (control), EB, or BPA at NOAEL or TDI with or without ICI 182.780 (ICI). Noninflamed (NI) controls received intracolonic saline solution. (B) Representative Western blot lanes and corresponding densitometric analysis of macrophage MIF in the same protein extracts. Bars are means ± SEM of duplicate measurements (5–13 rats per group). *P < 0.05, **P < 0.01 vs. inflamed controls (one-way ANOVA).
BPA Induces Visceral Hypersensitivity in the OVX Rat.
Colorectal distention (CRD) induces abdominal contractions, and this visceromotor response (VMR) is used to assess visceral pain. Estradiol induces hyperalgesia in response to CRD in rats (29). To test whether BPA affects colorectal sensitivity, we measured the VMR to graded intensities of CRD. Fig. 5A shows that the VMR in the TDI group of rats did not differ from controls. In contrast, at the NOAEL, BPA significantly increased the VMR to noxious stimuli (0.8–1.2 mL) compared with controls (P < 0.001), similarly to EB (Fig. 5A). Coadministration of ICI 182.780 completely prevented the enhanced VMR in the EB- and BPA-treated rats (Fig. 5B).
Fig. 5.
Comparative effects of BPA and estradiol on VMR to colorectal distension in OVX rats exposed for 15 d to (A) corn oil (control), EB, or BPA NOAEL or TDI, and (B) with ICI 182.780 (ICI) (5–14 rats per group). *P < 0.05, ***P < 0.001 vs. controls (two-way ANOVA).
To further determine whether the enhanced VMR to noxious stimuli resulted from change in muscle tone after EB or BPA exposure, the colonic compliance was determined through the measurement of the pressure–volume relationship using a barostat. Fig. S1 shows a linear relationship between the volume and resulting pressure in all groups of rats. This indicates that neither BPA nor EB affect the muscular tone of colonic wall, and the increase in VMR induced by isovolumic distension did not result from changes in colonic compliance.
Perinatal BPA Alters Mucosal Immune Function in Female Offspring.
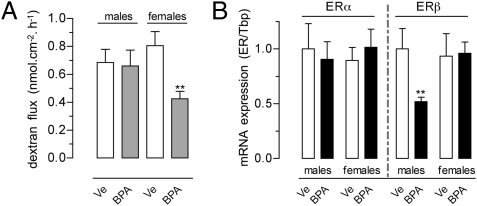
In rats perinatally exposed to the NOAEL of BPA, basal intestinal permeability assessed in adulthood revealed a significant decrease in the colonic dextran flux. This change was sex-specific, observed only in female rats compared with controls assessed at the same estrous stage (−47%; P < 0.01; Fig. 6A). In contrast, perinatal BPA resulted in a marked decrease of ERβ mRNA levels in the colon of male offspring only, with no effects on ERα expression (Fig. 6B).
Fig. 6.
(A) Intestinal permeability and (B) relative expression levels of ERα and ERβ mRNA in the colon of rats perinatally exposed to BPA NOAEL or vehicle (Ve). For each ER, data were calibrated to male controls (set to 1). Bars are means ± SEM of duplicate measurements (4–11 rats per group). **P < 0.01 vs. corresponding controls (t test).
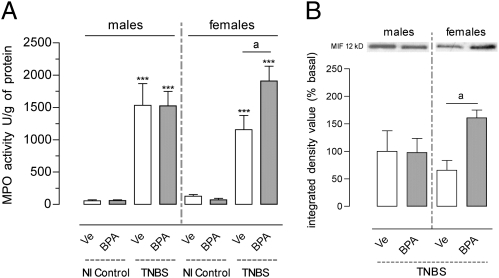
Twenty-four hours after induction of TNBS colitis, MPO activity significantly increased in all inflamed rats, and enhanced MPO activity was observed in females perinatally exposed to BPA compared with vehicle (+40%; P < 0.05), whereas no difference occurred in male rats (Fig. 7A). Similarly, MIF protein levels were increased in inflamed female rats with perinatal BPA compared with controls (P = 0.01), but not in male rats (Fig. 7B).
Fig. 7.
(A) MPO and (B) Western blot analysis of MIF in the colon 24 h after induction of TNBS colitis in rats perinatally exposed to BPA NOAEL or vehicle (Ve). NI, noninflamed controls with intracolonic saline. Bars are means ± SEM of duplicate measurements (five to eight rats per group). ***P < 0.001 vs. corresponding noninflamed controls; aP < 0.05 (t test).
Discussion
Our study shows that the xenoestrogen BPA exerts estrogen-like activities in the intestine of rats orally exposed to reference doses. These effects were observed at the currently accepted NOAEL (5 mg/kg/d) derived from toxicological studies conducted for risk-assessment purposes. Our findings show that, similarly to estradiol, BPA at the NOAEL decreased basal epithelial permeability in the colon and enhanced nociceptive responses from the gut. The effect on intestinal permeability was further observed at the reference safe limit for human exposure (TDI, 50 μg/kg/d), demonstrating that gut epithelial barrier is highly sensitive to low doses of BPA.
In addition to transcellular exchanges, the paracellular flux is a major physiological component of the epithelial barrier function, and is controlled by the integrity of the apical TJs that seal paracellular spaces between cells (18). In physiological conditions, the intestinal barrier is not impermeable but leaky, allowing fluid transport (17) and, at the colonic level, communication between the mucosal immune system and the commensal flora, playing a major role in antigen sampling and the development of tolerance (30). We have recently shown that the basal permeability in the female colon displays a high sensitivity to circulating estrogens, and is not static, but fluctuates during the reproductive cycle, being decreased in estrous stages under plasma estrogen dominance (27). In rodents as well as in humans, ERβ is the predominant ER in the colon, with very weak expression of ERα (25, 26), and estradiol acts primarily through ERβ to enhance expression of the TJ proteins occludin and JAM-A, thus reducing paracellular spaces (27). The present results show that oral BPA evokes similar ERβ activity, consistent with a higher binding affinity for this receptor (7). First, oral BPA in OVX rats did not affect the ER expression pattern in the colon, and BPA dose-dependently decreased paracellular permeability, an effect prevented by the ER antagonist ICI 182.780. Second, both occludin and JAM-A were found up-regulated in the colon of NOAEL- and TDI-treated rats, as well as in the human Caco-2 cell line exposed to 10 nM BPA [i.e., a concentration commonly found in human fluids (3, 4)], a situation reported only in the presence of an ERβ specific ligand in such cells (27).
Of particular interest is the observation that BPA impacts intestinal permeability at levels below the TDI, i.e., at exposure levels usually considered as safe for humans. There is now growing evidence showing endocrine-disrupting effects of BPA at doses lower than the reference limits (11, 12, 31, 32). The present dose–response study on intestinal permeability shows a half-maximal inhibitory dose for BPA 10 fold below the TDI. Moreover, this dose is threefold lower than the maximal exposition level of 14.7 μg/kg/d reported by the National Toxicology Program in children. It is thus questionable whether a daily gut exposure to low doses of BPA may have implications for human health. Estrogens show antiinflammatory activity in the gut, decreasing the severity of colitis in animal models of human inflammatory bowel diseases (28). Similarly, we showed that BPA at NOAEL in OVX rats provides anti-inflammatory activity at the onset of inflammation, in reducing neutrophil infiltration and tissue level of MIF, a proinflammatory cytokine involved in the pathogenesis of inflammatory bowel disease known as a target for estrogen-mediated protection in the colon (28). Because the TDI BPA had no effect, these findings suggest that a daily BPA exposure does not represent a risk factor in developing inflammation in the adult female gut. Conversely, the water-transporting capacity of the colon is of considerable physiological importance, particularly through its capacity to regulate the passive water secretion through the paracellular pathway (17, 33), and it can be assumed that BPA interferes with this process. Indeed, plasma estrogen dominance during the follicular period of the reproductive cycle inhibits chloride ion secretion in the colon (33), and decreases paracellular spaces (27), 2 mechanisms that limit the transport of water toward the gut lumen, thus leading to body fluid retention. On this basis, the ability of low doses of BPA to permanently decrease the paracellular permeability could enhance salt and water retention in women, as commonly observed in high estrogen states associated with the contraceptive pill or hormone replacement therapy or during pregnancy (34, 35).
Abdominal pain is a recurrent syndrome in intestinal functional disorders and sex hormones influence pain perception in women (23). However, visceral nociception has never been considered as a target for xenoestrogen chemicals such as BPA, although estrogens enhance visceral sensitivity to colorectal stimuli (29). The gastrointestinal tract is highly innervated, and primary sensory afferents convey information between intestine and central nervous system, adapting nociceptive behavior. Estrogen receptors are widely expressed throughout the sensory processing pathway, including dorsal root ganglia and spinal cord (36, 37). Our study showed that BPA induces visceral hypersensitivity to graded intensities of colorectal distension, an effect blocked by ICI 182.780, thus depending on ER binding activity. Because BPA as well as estradiol did not change the compliance of the colon, it is concluded that the hyperalgesic effect is neurally mediated, and not related to changes in the muscular tone of the colon. These data support a putative contribution of chronic low-level exposure to chemical estrogens in the genesis of visceral hypersensitivity states. In the present study, visceral pain was modulated only by a NOAEL dose regimen of BPA, and not by TDI. However, NOAEL is the reference dose in rodents for risk-assessment purposes in classical toxicological studies, considered without any effect in biological functions, and is used as a base to calculate the TDI considered safe for humans. These findings reinforce the idea that reevaluation of BPA’s NOAEL would be necessary to better address public health issues related to this chemical (4, 10).
Toxicological Evaluation of Perinatal Exposure to BPA.
Intestinal paracellular permeability is high at birth and mucosal immune mechanisms are immature in neonates. A high-permeability state in early infancy reflects the development of the gut mucosal barrier (38) that precedes a period of epithelial TJ sealing to provide an effective barrier against foreign agents (19, 20). BPA ingested by mothers is present in fetal tissues (39) and human milk (40), and leakage of BPA from polycarbonate baby bottles under real use conditions has been demonstrated (6). As ERβ is the only ER detectable in human fetal colon (41), we speculated that a BPA-mediated alteration of gut permeability in the perinatal period of life may have restricting effects on the developing immune system in the colon. Indeed, the maturing gut is highly permeable to sugars and proteins from human milk to promote infant growth (19, 20), as well as to nucleotides that improve infant immune status (21). Mice prenatally exposed to BPA show immune system impairment, particularly regarding the developing T cell population (42). We showed that rats exposed in utero and during lactation to BPA NOAEL ingested by their dams exhibited intestinal barrier dysfunctions in adulthood, with sex-related differences in gut susceptibility to BPA effects. First, we observed decreased colonic paracellular permeability in the female offspring, as reported herein in OVX rats fed with oral BPA at NOAEL or TDI, but not in male offspring. Furthermore, we showed that neutrophil infiltration in the colon faced with inflammation was dramatically enhanced in female rats only. The latter correlated with an increase in the tissue levels of MIF, a stimulating factor of proinflammatory cytokines in acute colitis (28). It is noteworthy that, in contrast with the antiinflammatory effect of a direct NOAEL exposure in OVX rats, the same dose administered to pregnant and lactating mothers resulted in opposite effects in female offspring in adulthood. It is also remarkable that this deleterious impact was observed during estrus, i.e., at a time of the reproductive cycle when the colon of adult females is more resistant to injury, and produces less proinflammatory cytokines when challenged by experimental inflammation, as a result of a protective influence of plasma estrogen dominance (22, 28). Our results clearly show that the endocrine disruptor effects of BPA vary depending on age of the gut, and that a perinatal exposure has a long-term effect on mucosal immune defenses that predisposes female offspring to enhanced proinflammatory responses in the colon. Interestingly, male offspring perinatally exposed to BPA showed decreased ERβ expression in the colon, an effect not observed in female offspring. This finding supports our hypothesis that a dominant expression of ERβ in the developing gut barrier is critical for the endocrine disruptor activity of BPA, and that a selective down-regulation of ERβ in male offspring contributes to sex differences, making male offspring rats less sensitive to the intestinal effects of the xenoestrogen.
In summary, this study shows that the gastrointestinal tract is a sensitive target for estrogenic food contaminants such as BPA. Despite its weak estrogenic activity, BPA at the reference doses was found to affect the epithelial barrier function and to enhance visceral nociceptive response by binding to ERs. Importantly, our study shows that the perinatal development of the intestinal barrier represents a critical window for endocrine disruptor effects of BPA in the gut, and may represent a risk factor in female offspring for developing severe colonic inflammation in adulthood. In addition, based on high sensitivity to BPA exposure, the colon epithelium may serve as a valuable model for the risk evaluation of low doses of environmental estrogens, and contribute to establish accurate acceptable human exposure levels.
Materials and Methods
Animals and Treatment.
Adult female Wistar rats (Janvier) weighing 180 to 200 g were housed in cages under a 12:12-h light–dark cycle, maintained at 21 ± 1°C. They were fed a standard diet with free access to water. All protocols were approved by the local institutional animal care and use committee in compliance with the European laws on the protection of animals (86/609/EEC).
In a first series of experiments, OVX rats were treated for 15 d by oral gavage as follows: (i) corn oil as vehicle (control), (ii) BPA (Sigma) 5 mg/kg/d (BPA NOAEL), (iii) BPA 50 μg/kg/d (BPA TDI), (iv) EB (Sigma) 0.6 mg/kg/d. Three groups of rats receiving BPA NOAEL and 2 groups treated with EB received a daily s.c. injection of the ER antagonist ICI 182.780 (2 mg/kg/d in olive oil; Tocris) for the last 5 d of treatment. Rats were used for permeability assay in Ussing chambers (5–13 rats per group), or induction of experimental colitis (5–13 rats per group), or assessment of visceral pain (5–14 rats per group). In a dose-dependent experiment, OVX rats were orally treated with BPA (0.5 μg to 5 mg/kg/d) for 15 d (n = 6–8 rats per group) then used for permeability assay.
A second series of experiments was conducted in which BPA at NOAEL or vehicle (corn oil) was orally administered to pregnant females from d 15 of pregnancy until weaning on day 21 postpartum. Male and female offspring rats were then fed with standard diet until adulthood (10 weeks postpartum), and used for permeability assay (8–10 rats per group) or induction of experimental colitis (8–13 rats per group). In female rats, sexual cycle stages were assessed by daily vaginal smears, and only rats in estrus were used.
Ussing Chambers Experiments.
Immediately after the rats were euthanized, 3 colonic strips from each rat were used to assess paracellular permeability as described by Braniste et al. (27). Strips were mounted in Ussing chambers (Easymount) with a flux area of 0.5 cm2 and bathed in 5 mL of oxygenated Krebs-Henseleit solution (Sigma) at 37°C. Permeability was assessed by measuring mucosal to serosal flux of FITC-labeled 4-kDa dextran (Sigma) during 1 h, and expressed as the flux of dextran crossing 1 cm2 of epithelium per hour (nmol·cm−2·h−1). Data are the means of triplicate measurements.
Induction of Experimental Colitis and Measurement of MPO.
Under anesthesia, colitis was induced by intracolonic administration of TNBS (80 mg/kg in 50% ethanol; Sigma), and control rats received sterile saline solution (28). Rats were euthanized 24 h after treatment and 0.5-cm samples of distal colon were prepared for measurement of MPO activity, a marker of polymorphonuclear neutrophil primary granules (28). Tissues were suspended in potassium phosphate buffer and homogenized on ice. After 3 cycles of freezing and thawing, suspensions were centrifuged at 10,000 × g for 15 min (4°C) and supernatants were discarded. Pellets were resuspended in the detergent hexadecyltrimethylammonium bromide buffer (Sigma) to release MPO from the primary granules. After sonication on ice and centrifugation (10,000 × g, 15 min, 4°C), supernatants were assayed spectrophotometrically for MPO activity. Results were expressed as MPO activity U/g protein.
Protein Extraction and Western Blot.
Colons were homogenized in RIPA buffer (1% Igepal, 0.5% deoxycholic acid, and 0.1% SDS in Tris buffered saline solution 1×; pH 7.4) with protease inhibitors (Roche Diagnostics), and centrifuged at 10,000 × g for 10 min (4°C). Protein concentrations were assessed using the BC Assay Uptima kit (Interchim), and equal amounts of protein were separated by SDS/PAGE and transferred onto nitrocellulose membranes. Blocking and incubation were performed as already described (27, 28) with polyclonal rabbit anti-MIF antibody (Torrey Pines Biolabs), anti-occludin or anti-JAM-A (Zymed Laboratories), and secondary peroxidase-labeled anti-rabbit IgG (Visualizer Detection Kit; Upstate). Bands were visualized using SuperSignal West Femto (Thermo Scientific). Relative values of band density were estimated using ImageJ software (National Institutes of Health).
Real-Time PCR.
Total RNA from stripped colonic mucosa was extracted with TRIzol (Invitrogen) and reverse-transcribed with a high-capacity cDNA reverse transcription kit (Applied Biosystems). SYBR green real-time quantitative PCR assays (primers in Table S1) were performed on an ABI PRISM 7000 SDS (Applied Biosystems). All data were normalized by TATA box binding protein expression levels and were analyzed using LinRegPCR (43).
Colorectal Distension and Colonic Compliance.
Five days before experiments, rats were surgically equipped with NiCr wire electrodes implanted bilaterally into the abdominal external oblique muscle and exteriorized on the back of the neck. Rats were accustomed for 3 d to be in polypropylene tunnels (diameter, 6 cm; length, 25 cm) before CRD. The balloon (2 mm diameter, 2 cm length) consisting of an arterial embolectomy catheter (Fogarty; Edwards) was inserted into the rectum at 1 cm from the anus, then inflated from 0 to 1.2 mL in increments of 0.4 mL, each lasting 5 min. Electromyographic signals of abdominal muscles in response to CRD were amplified (Bio Amp; AD Instruments), filtered (high frequency, 10 kHz; low frequency, 500 Hz), acquired by a PowerLab unit (AD Instruments) at a rate of 1,000 samples per second, then recorded in Chart software. The VMR was expressed as the number of abdominal contractions per 5 min. For compliance measurement, a 4-cm-long latex balloon was connected to computerized barostat (INRA). Colonic pressure and balloon volume were monitored on a potentiometric recorder (L6514; Linseis). For isobaric distensions, balloon was inflated from 0 to 60 mm Hg in increments of 15 mm Hg, each lasting 5 min.
Cell Culture and Immunofluorescence.
Human colonic cell line Caco-2 were routinely grown in phenol-red free DMEM (MidiMed), as previously described (27). Cells plated on glass coverslips were exposed in triplicate to BPA (10 nM) for 24 h in charcoal stripped fetal bovine calf serum, with or without ICI 182.780 (10 μM) added 24 h before BPA. All chemicals were predissolved in 100% ethanol, then diluted with the medium to final concentration, whereas control cells were exposed to vehicle ethanol (final dilution <0.1%). Caco-2 monolayers were fixed and permeabilized in methanol–acetic acid (95%/5%) for 20 min at −20°C. Blocking and incubation were performed as described (27) with polyclonal rabbit anti-occludin (1:100 in PBS solution) or anti-JAM-A (1:25 in PBS solution; Zymed Laboratories), and secondary Alexa fluor 488–conjugated donkey anti-rabbit (1:2,000 in PBS solution; Molecular Probes/Invitrogen). Cell monolayers were examined under a Nikon 90i fluorescence microscope.
Statistical Analysis.
Results are expressed as means ± SEM. Statistical significance was assessed by Student t test or ANOVA followed by Bonferroni post hoc test for multiple comparisons when appropriate (Prism 4 software; GraphPad). A P value <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Daniel Zalko for valuable comments on the manuscript. This work was supported by Grant ANR-06-PNRA-008-04 from Agence Nationale de la Recherche and by Ministère de l’Enseignement Supérieur et de la Recherche and Institut National de la Recherche Agronomique (AP AlimH 2008).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907697107/DCSupplemental.
This article is a PNAS Direct Submission.
References
- 1.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 2.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat AM, et al. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maragou NC, Makri A, Lampi EN, Thomaidis NS, Koupparis MA. Migration of bisphenol A from polycarbonate baby bottles under real use conditions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:373–383. doi: 10.1080/02652030701509998. [DOI] [PubMed] [Google Scholar]
- 7.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 8.Dairkee SH, et al. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res. 2008;68:2076–2080. doi: 10.1158/0008-5472.CAN-07-6526. [DOI] [PubMed] [Google Scholar]
- 9.Wetherill YB, et al. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther. 2006;5:3181–3190. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- 10.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6) Suppl:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 11.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 12.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timms BG, et al. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin BS, et al. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 16.Geibel JP. Secretion and absorption by colonic crypts. Annu Rev Physiol. 2005;67:471–490. doi: 10.1146/annurev.physiol.67.031103.153530. [DOI] [PubMed] [Google Scholar]
- 17.Masyuk AI, Marinelli RA, LaRusso NF. Water transport by epithelia of the digestive tract. Gastroenterology. 2002;122:545–562. doi: 10.1053/gast.2002.31035. [DOI] [PubMed] [Google Scholar]
- 18.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–386. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Colomé G, et al. Intestinal permeability in different feedings in infancy. Acta Paediatr. 2007;96:69–72. doi: 10.1111/j.1651-2227.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaller JP, et al. Effect of dietary ribonucleotides on infant immune status. Part 1: Humoral responses. Pediatr Res. 2004;56:883–890. doi: 10.1203/01.PDR.0000145576.42115.5C. [DOI] [PubMed] [Google Scholar]
- 22.Homma H, et al. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- 23.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- 24.Heitkemper MM, et al. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol. 2003;98:420–430. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 26.Konstantinopoulos PA, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 27.Braniste V, et al. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houdeau E, et al. Sex steroid regulation of macrophage migration inhibitory factor in normal and inflamed colon in the female rat. Gastroenterology. 2007;132:982–993. doi: 10.1053/j.gastro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 31.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 32.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Mahony F, Harvey BJ. Sex and estrous cycle-dependent rapid protein kinase signaling actions of estrogen in distal colonic cells. Steroids. 2008;73:889–894. doi: 10.1016/j.steroids.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Fruzzetti F, et al. Effect of an oral contraceptive containing 30 microg ethinylestradiol plus 3 mg drospirenone on body composition of young women affected by premenstrual syndrome with symptoms of water retention. Contraception. 2007;76:190–194. doi: 10.1016/j.contraception.2007.05.080. [DOI] [PubMed] [Google Scholar]
- 35.Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids. 1996;61:166–171. doi: 10.1016/0039-128x(96)00007-4. [DOI] [PubMed] [Google Scholar]
- 36.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Papka RE, et al. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 38.Neu J. Perinatal and neonatal manipulation of the intestinal microbiome: a note of caution. Nutr Rev. 2007;65:282–285. doi: 10.1301/nr.2007.jun.282-285. [DOI] [PubMed] [Google Scholar]
- 39.Zalko D, et al. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect. 2003;111:309–319. doi: 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 41.Takeyama J, et al. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. J Clin Endocrinol Metab. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- 42.Yan H, Takamoto M, Sugane K. Exposure to bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect. 2008;116:514–519. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruijter JM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.