Abstract
Both CD4+ T cell help and IL-2 have been postulated to “program” activated CD8+ T cells for memory cell development. However, the linkage between these two signals has not been well elucidated. Here we have studied effector and memory CD8+ T cell differentiation following infection with three pathogens (Listeria monocytogenes, vesicular stomatitis virus, and vaccinia virus) in the absence of both CD4+ T cells and IL-2 signaling. We found that expression of CD25 on antigen-specific CD8+ T cells peaked 3–4 days after initial priming and was dependent on CD4+ T cell help, likely through a CD28:CD80/86 mediated pathway. CD4+ T cell or CD25-deficiency led to normal early effector CD8+ T cell differentiation, but a subsequent lack of accumulation of CD8+ T cells resulting in overall decreased memory cell generation. Interestingly, in both primary and recall responses KLRG1high CD127low short-lived effector cells were drastically diminished in the absence of IL-2 signaling, although memory precursors remained intact. In contrast to previous reports, upon secondary antigen encounter CD25-deficient CD8+ T cells were capable of undergoing robust expansion, but short-lived effector development was again impaired. Thus, these results demonstrated that CD4+ T cell help and IL-2 signaling were linked via CD25 up-regulation, which controls the expansion and differentiation of antigen-specific effector CD8+ T cells, rather than “programming” memory cell traits.
Keywords: infection, memory
Although recent findings have advanced our knowledge of the factors necessary to generate optimal memory CD8+ T cell responses, a full understanding of the signals required remains elusive. CD8+ T cell responses to acute viral or bacterial infections are characterized by three phases. Upon recognition of a specific antigenic epitope, T cells undergo massive proliferation and acquisition of effector functions. Pathogen-specific CD8+ T cells can expand close to 105-fold from a population as small as a few hundred precursors (1). These effector T cells have altered chemokine and homing receptor expression enabling their migration through all peripheral tissues (2, 3). On further antigenic stimulation, the effector CD8+ T cells will kill infected target cells through several mechanisms thereby limiting pathogen growth and dissemination. After this stage, effector CD8+ T cells enter a contraction phase where a majority of the effector cells undergo apoptosis leaving only 5–20% of the cells present at the peak of expansion (4). The last stage is characterized by the maintenance of a heterogeneous population of long-lived, stable CD8+ memory T cells (5). These remaining cells are characterized as stem-cell like because they retain telomerase expression, the ability to self-renew, a high proliferative capacity, and multipotency (5). In acute infections, the persistence of these memory T cells relies on the presence of the common gamma-chain (γc) cytokines IL-7 and IL-15 (6, 7). Upon secondary exposure to the specific antigen, memory CD8+ T cells undergo a more rapid expansion and production of effector cytokines compared to the primary response, which results in rapid control of infection.
Unfortunately, these cardinal rules of memory CD8+ T cell function do not account for the extensive functional and phenotypic complexity of subsets that is known to exist (5). Additionally, exactly when each subset differentiates from the original naïve precursor remains controversial (8). Recent work has demonstrated that a single naïve antigen-specific CD8+ T cell can give rise to all of the effector and memory subsets observed (9). One theory suggests that heterogeneity occurs as early as the first asymmetric division of a naïve cell, where one daughter cell is “fated” to become effector-like through the up-regulation of effector molecules and expression of differentiation markers. Meanwhile, the sister cell retains the ability to differentiate into a memory-like cell (10). It is unclear whether these lineages are set in stone or whether manipulation of cell fate can occur through the presence or lack of secondary stimulation. Recent evidence indicates the latter scenario is likely true because cells that have up-regulated granzyme B (and therefore would be considered effector-like) maintained the ability to form long-lived memory cells (11).
Population heterogeneity continues throughout the expansion phase, as the ability to form memory cells can be correlated with the expression patterns of CD127 and the killer cell lectin-like receptor G1 (KLRG1) (12–14). Briefly, KLRG1high CD127low CD8+ T cells are considered to be more terminally differentiated and die after clearance of the infection and therefore are referred to as short-lived effector cells (SLEC). KLRG1low CD127high CD8+ T cells are referred to as memory precursor effector cells (MPEC) because they possess effector properties, but retain the ability to differentiate into a long-lived memory population. The factors regulating the formation of the SLEC and MPEC populations remain ill-defined. It is well established that three signals are necessary for the activation of naïve CD8+ T cells: (i) TCR stimulation, (ii) costimulation, and (iii) inflammatory cytokines (15), but how variations in these signals may orchestrate effector and memory heterogeneity is not clear. Interestingly, alterations in the amount of antigenic exposure do not seem to skew effector cell differentiation, but the inflammatory milieu is able to alter the SLEC/MPEC ratio (12, 14, 16). Based upon this, we explored whether other known regulators of effector and memory CD8+ T cell differentiation, namely CD4+ T cell help and IL-2 signaling, are critical for the development of SLEC and MPEC populations.
Costimulatory signals driven by CD4+ T cells have been shown to be crucial for the optimal differentiation of memory CD8+ T cells in various models (17–19). However, controversy remains as to precisely when CD4+ T cell help is required for memory CD8+ T cell differentiation. Depending on a number of variables in different infection models, CD4+ T cells are required during priming, maintenance, or recall and, in some cases, CD4+ T cell help can be nonessential (17–21). An important variable that alters the requirement for CD4+ T cell help is the pathogen that is under study. For example, the CD8+ T cell response to vesicular stomatitis virus (VSV) infection is largely independent of CD4+ T cell help. In the case of the CD8+ T cell response to Listeria monocytogenes (LM) infection, some reports indicate a CD4+ T cell requirement for primary expansion (21, 22) although others do not (18, 19). However, even when CD4+ T cell help is apparently not required for primary expansion, the “helpless” memory CD8+ T cells respond poorly to challenge (17–19), but there is disagreement in the literature on this point (21, 22). Additionally, in the absence of CD4+ T cell help, immune responses to intranasal infection with vaccinia virus-Western Reserve (VV-WR) or murine γ-herpesvirus generate normal primary responses, but secondary recall is inhibited through the up-regulation of PD-1 (23). Although the critical signal provided by CD4+ T cell help appears to be the ligation of CD40 on antigen presenting cells, IL-2 production by CD4+ T cells may also play a role (24–26). Additionally, previous studies indicate that CD4+ T cell help and IL-2 signaling during priming, although not shown to be synonymous, are not required for the primary CD8+ T cell response to infection but are essential for the formation of a functional memory population (27, 28). Nonetheless, the mechanism by which CD4+ T cells impinge on CD8+ effector and memory T cell subset development remains unclear.
The results presented here demonstrated that optimal differentiation of the SLEC population during primary and secondary CD8+ T cell responses required the expression of CD25 and that CD4+ T cell help, possibly through CD28 costimulation, regulated the expression of CD25 on the pathogen-specific CD8+ T cells. Thus, the helpless condition or the absence of CD25 resulted in a marked reduction of the primary CD8+ T cell response that was largely restricted to the SLEC subset. However, the resulting memory CD8+ T cells readily responded to secondary challenge. Our findings supported a role for CD4+ T cells in promoting IL-2 driven effector CD8+ T cell expansion and survival, but do not support the notion that these factors affect the functionality of memory CD8+ T cells.
Results
CD4+ T Cell Help Is Critical for the Up-Regulation of CD25 by Antigen-Specific CD8+ T Cells.
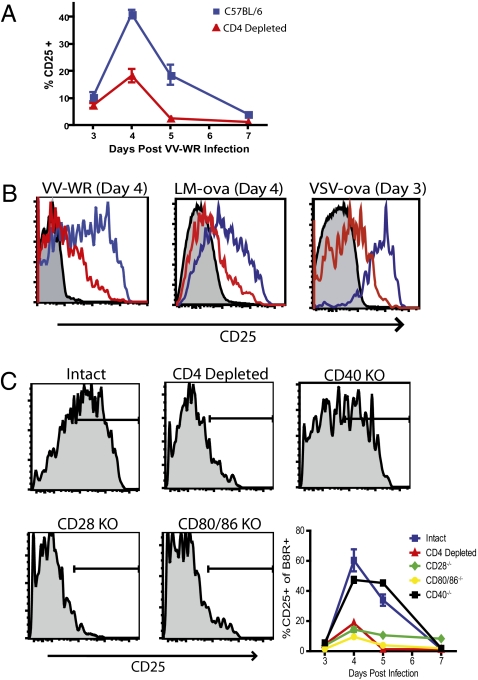
We wished to test whether a link exists between CD4+ T cell help and CD25 expression by CD8+ T cells. To account for potential variability between different systems, we examined responses to LM or VSV expressing ovalbumin (LM-ova; VSV-ova), and VV-WR. C57BL/6 CD8+ T cells up-regulated CD25 transiently with peak expression at approximately 4 days after infection regardless of the pathogen studied (Fig. 1A and B). In contrast, pathogen-specific CD8+ T cells in mice treated with anti-CD4 mAb or in MHC class II−/− mice only weakly up-regulated CD25 (Fig. 1 A and B and Fig. S1). As past studies have shown that CD40 deficient mice have similar responses to helpless mice and agonistic anti-CD40 antibodies can substitute for CD4+ T cell help, we tested whether CD40−/− mice lacked the ability to up-regulate CD25 (25, 29). Surprisingly, VV-specific CD8+ T cells from CD40-deficient animals expressed high levels of CD25, indistinguishable from those in normal mice (Fig. 1C). In contrast, only minimal up-regulation of CD25 was observed in CD8+ T cells from both CD28-deficient and CD80/86-deficient mice (Fig. 1C). The decreased CD25 expression could be a direct result of CD28:CD80/86 signals but might also be a consequence of decreased IL-2 production because CD28:CD80/86 signals are known to regulate IL-2 expression which in turn can regulate CD25 expression (30). To test this hypothesis, helpless mice were treated with 15,000U of rhIL-2 twice a day and subsequently CD25 expression on day 4 after VV infection was determined. Interestingly, rhIL-2 treatment was able to restore CD25 expression by VV-specific CD8+ T cells (Fig. S2). Thus, CD4+ T cell help plays an important role in regulating the expression of CD25 on pathogen-specific CD8+ T cells during infection potentially through CD28-CD80/86 mediated expression of IL-2 from CD4+ T cells.
Fig. 1.
CD4+ T cell help is required for the expression of CD25 by antigen-specific CD8+ T cells. (A) Time-course showing the percentage of B8R/Kb-specific CD8+ T cells expressing CD25 for mice infected with VV-WR in the presence (blue) or absence (red) of CD4+ T cells for 7 days p.i. (B) Representative histograms show the peak expression of CD25 following infection with VV-WR, LM-ova, or VSV-ova in the presence (blue line) or absence (red line) of CD4+ T cells. Shaded histograms = isotype control. (C) Representative histograms and time-course showing CD25 expression on B8R/Kb-specific CD8+ T cells from intact (blue) or CD4-depleted WT mice (red), CD40−/− mice (black), CD28−/− mice (green), or CD80/86−/− mice (orange) four days p.i.
CD25 Expression Is Necessary for Optimal Expansion of CD8+ T Cells.
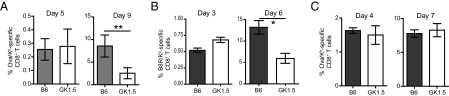
As helpless CD8+ T cells were unable to up-regulate CD25, we examined whether primary responses were compromised in anti-CD4 mAb treated mice. Following infection, antigen-specific CD8+ T cells from all groups of mice underwent an initial robust expansion, but responding CD8+ T cells from LM-ova and VV-WR infected anti-CD4 mAb treated hosts were unable to sustain expansion (Fig. 2 A and B). In contrast, CD4-depleted VSV-ova infected mice mounted a primary response equivalent to that of intact mice (Fig. 2C), confirming previous studies (21). CD8+ T cells in MHC class II−/− mice exhibited a similar phenomenon to CD4-depleted mice in all cases (Fig. S1). Interestingly, optimal expansion of VV-specific CD8+ T cells correlated with conditions that resulted in high levels of CD25 expression (Fig. S3).
Fig. 2.
Early and late expansion of pathogen-specific CD8+ T cells in helpless mice. C57BL/6 mice were either treated with anti-CD4 or left untreated. Mice were subsequently infected with 103 CFU of Lm-ova (A), 2 × 106 PFU of VV-WR (B), or 105 PFU of VSV-ova (C) and tetramer+ splenic CD8+ T cells were monitored. These data are representative of two independent experiments, each containing four to five mice per group. Statistical significance was determined using a Student's t test.
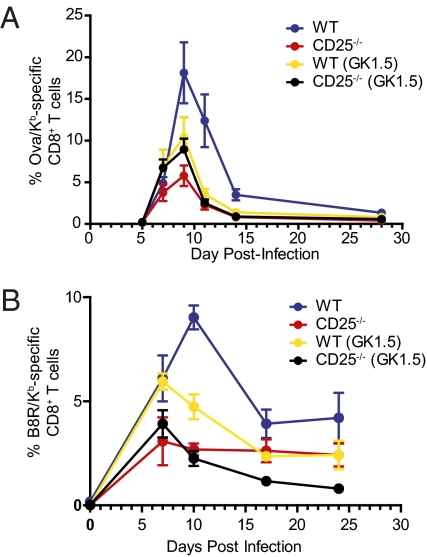
To determine whether expression of CD25 was responsible for the disparity between responses to different infections, we generated C57BL/6:CD25−/− mixed bone marrow chimeras as previously described (27, 28). The generation of mixed bone marrow chimeras is necessary as CD25−/− mice develop autoimmunity and lymphoproliferative disease at an early age due to the absence of regulatory T cells (31). Chimeric mice were infected with LM-ova or VV-WR and a kinetic analysis of peripheral-blood pathogen-specific CD8+ T cells was conducted. Although both populations of CD8+ T cells expanded equivalently through days 4–6, the CD25−/− cells failed to undergo sustained expansion and were rapidly outnumbered by the WT cells in all cases (Fig. 3 A and B), which is in contrast to previously published reports (27, 28). A similar disparity between the expansion of C57BL/6 cells and CD25−/− cells at the peak of the LM-ova CD8+ T cell response was also observed in lymphoid and peripheral tissues of infected mice, indicating that this effect was not isolated to the peripheral blood (Fig. S4). Following VSV infection, the decreased response of CD25−/− cells was substantially less severe than that observed for LM-ova and VV-WR infection (Fig. S5). Nevertheless, SLEC development was impaired, but because fewer SLEC developed after VSV infection compared to LM infection, the overall response was less affected.
To examine whether the observations in the helpless mice and CD25-deficient cells were linked, we treated mixed chimeras with CD4 depleting antibody. When CD4+ T cells were depleted from chimeric mice infected with LM-ova, no statistical difference in the percentage of Ova/Kb-specific cells was observed between CD4-deficient WT and CD25−/− cell populations (Fig. 3A). Similar results were observed for anti-CD4 treated VV-WR infected chimeras during the expansion phase; however during the early stages of the memory phase, a small but statistically significant difference between the percentage of B8R-specific WT and CD25−/− CD8+ T cells in CD4-depleted mice was observed (Fig. 3B). Overall, these results suggested that decreased IL-2 signaling in helpless mice, as a result of lower CD25 levels, was responsible for the reduced accumulation of pathogen-specific CD8+ T cells.
Fig. 3.
CD25-mediated signals are necessary for the optimal expansion of effector CD8+ T cells. Time course showing the percentage of Ova/Kb-specific CD8+ T cells (A) and B8R/Kb-specific CD8+ T cells (B) from the blood in mixed bone marrow chimera mice infected with 103 CFU of LM-ova or 2 × 106 PFU of VV-WR, respectively. The CD8+ T cell population was first divided into WT cells (blue) or CD25-deficient cells (red) and then the percentage of antigen-specific CD8+ T cells was monitored longitudinally in the blood.
Short-Lived Effector CD8+ T Cell Differentiation Is Regulated by IL-2.
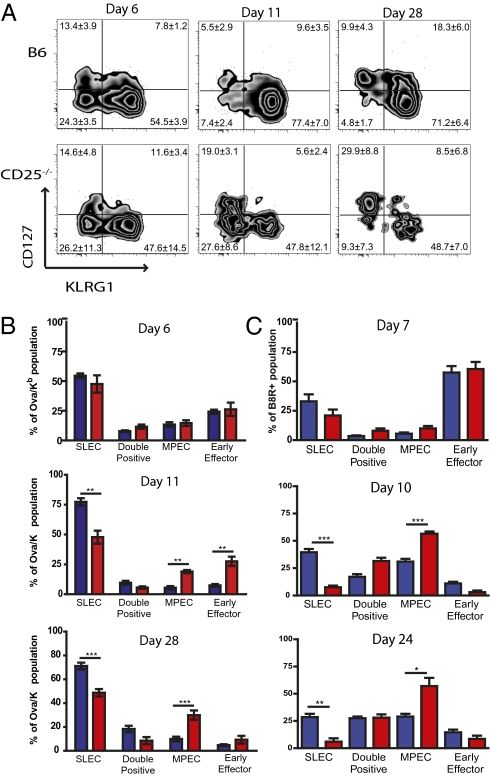
We next examined the role of IL-2 signaling in development of the SLEC and MPEC populations. Expression of CD25 on SLEC and MPEC Ova/Kb-specific CD8+ T cells was analyzed five days after LM-ova infection. Furthermore, a third effector CD8+ T cell population was observed at this time point, which was KLRG1low CD127low [herein termed “early effector cells” (EEC)]. CD25 expression was greatest on the EEC subpopulation, with intermediate expression levels in the SLEC population, and the MPEC population expressing the lowest CD25 levels (Fig. S6). Therefore, we tested whether CD25-deficiency affected the expansion of a particular effector cell subpopulation after LM-ova or VV-WR infection. Interestingly, before the peak of the responses (days 6/7) effector cell differentiation was similar between WT and CD25-deficient CD8+ T cells. However, by the peak of the CD8+ T cell response WT cells were predominantly found to have a SLEC phenotype (KLRG1high CD127low), although CD25−/− pathogen-specific CD8+ T cells failed to accumulate the SLEC population and had increased frequencies of EEC and MPEC populations (Fig. 4 A–C and Fig. S7). Furthermore, the subset skewing of the antigen-specific CD25−/− was maintained into the early memory stages. Thus, although IL-2 signaling affected the overall expansion and survival of the responding CD8+ T cells, it appeared that IL-2 was most critical for differentiation and expansion of the SLEC subset.
IL-2 Drives Maximal Proliferation of Effector Cells Late in the Response.
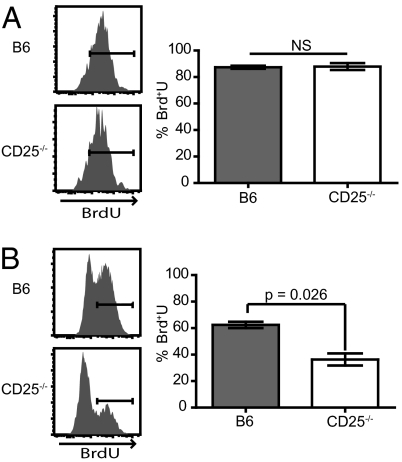
To determine the reason for the impairment of CD25−/− CD8+ T cells we measured cell proliferation by BrdU incorporation. Mixed bone marrow chimeras (WT:CD25−/−) were infected with LM-ova and the mice were treated with BrdU on either day 4 or day 8. One day later, mice were killed and BrdU incorporation into the splenic Ova/Kb-specific CD8+ T cells was analyzed. WT and CD25−/− antigen-specific CD8+ T cells incorporated similar levels of BrdU from days 4 to 5 of the response (Fig. 5A), at a time when CD25 expression is being up-regulated. However, as the peak of the response approached on days 8 and 9, WT antigen-specific CD8+ T cells incorporated significantly more BrdU than their CD25-deficient counterparts (Fig. 5B). Thus, in agreement with earlier studies (32), initial CD8+ T cell expansion was IL-2 independent, although sustained proliferative expansion and/or survival required CD25 expression.
Fig. 5.
CD25-deficient effector CD8+ T cells do not maintain a high proliferative level late in the immune response. Mice were infected with Lm-ova then treated i.p. with 1mg of BrdU every 12 h either on day 4 (A) or day 8 (B) and harvested one day later. Representative histograms display BrdU incorporation in Ova/Kb-specific CD8+ T cells and the right graph shows the mean ± 1 SD for each time point. Statistical significance was determined using a Student's t test.
CD25-Deficient CD8+ T Cells Retain the Ability to Respond to Secondary Challenges.
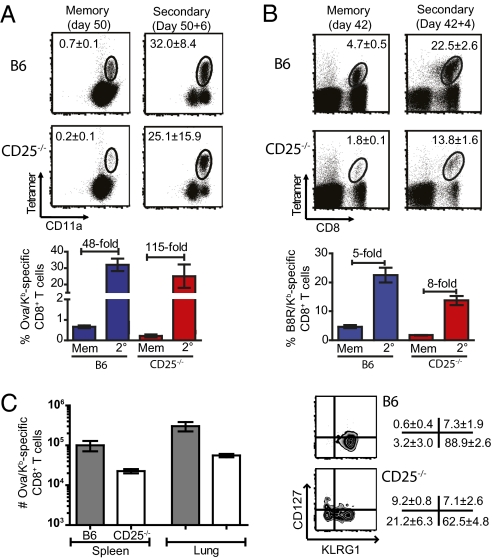
To elucidate whether the CD25−/− memory population was capable of a secondary response, LM-ova memory mice were restimulated with LM-ova, whereas VV-WR memory chimeras were challenged with an attenuated LM-B8R. Although fewer CD25−/− memory cells were present, due to the reduced primary response, in both infections the CD25−/− CD8+ T cells were able to undergo a robust secondary expansion, which was similar when compared to WT cells (Fig. 6 A and B).
Fig. 6.
CD25-deficient memory CD8+ T cells are able to mount a robust secondary response. CD25−/−/WT mixed chimera mice were primed with 103 CFU of LM-ova (A) or 2 × 106 PFU of VV-WR (B) and left for more than 42 days and challenged with 5 × 104 CFU of LM-ova or 106 CFU of LM-B8R, respectively. Mice were subsequently bled to monitor the magnitude of the pathogen-specific CD8+ T cell response. Representative dot plots show the prechallenge frequency of antigen-specific CD8+ T cells and the peak recall response. The bar graph shows the average expansion of the pathogen-specific CD8+ T cells at the indicated times and relative fold expansion of each population. (C) WT (CD45.1+) and CD25−/− (CD45.2+) CD8+ T cells were purified by flow cytometry and then 1,000 Ova/Kb-specific CD8+ T cells mixed together and injected i.v. into naïve CD45.1+/CD45.2+ recipient mice. One day later mice were challenged with 5 × 103 PFU of Lm-ova and the number of Ova/Kb-specific CD8+ T cells was enumerated in the spleen and lungs on day 8 postchallenge. Zebra plots are gated on the Ova/Kb-specific CD8+ T cells of WT or CD25−/− origin and secondary effector cell subpopulations were determined. Values represent the mean ± 1 SD from four mice and data are representative of two independent experiments.
To further examine the recall potential of the CD25-deficient pathogen-specific CD8+ T cells, memory CD8+ T cells from chimeric mice were sorted into CD25−/− (CD45.2+) and WT (CD45.1+) populations. After sorting, 1,000 Ova/Kb-specific CD8+ T cells of each were mixed and transferred into naïve C57BL/6 mice (CD45.1+ CD45.2+). One day after transfer, mice were challenged with 2 × 103 CFU of LM-ova and 8 days later the mice were killed and the absolute number of Ova/Kb-specific CD8+ T cells of CD25−/− and WT origin was enumerated. Both the WT and CD25-deficient memory cells underwent substantial secondary expansion, greater than 359-fold (WT) and greater than 122-fold (CD25−/−) in the spleen, respectively (Fig. 6C). Furthermore, when the secondary effector cells were divided based on KLRG1 and CD127 expression, the SLEC population was depressed within the CD25−/− cells, whereas the EEC and MPEC subsets were increased, as was observed in the primary response (Fig. 6C).
Discussion
CD4+ T cell help has been shown to be pivotal for primary or secondary CD8+ T cell responses in numerous infection models. However, perplexing discrepancies remain between models as to the nature of the mechanisms that dictate the level of CD4+ T cell dependency of a particular response. In addition, the CD4+ T cell derived factor(s) that provide help to the CD8+ T cells may be varied. Although CD40-mediated activation of DC may be one mechanism to promote CD8+ T cell responses, IL-2 production may also augment the CD8+ T cell response either alone or in concert with CD40 signaling. For instance, no defect has been observed for memory CD8+ T cells developing in the absence of CD40 signaling during LM infection (33). In contrast, CD40 signaling appears to be critical for full differentiation of memory CD8+ T cells following influenza A virus, murine γ-herpesvirus, and intranasal vaccinia virus infections (23, 29). Our results showed that the CD8+ T cell response to i.p. VV infection was CD40 independent but required CD4+ T cells, IL-2, and CD28-mediated costimulation (Fig. 1). CD4+ T cells and IL-2 were also critical for driving normal primary CD8+ T cell responses to LM and VV-WR infections (Figs. 2 and 3). Interestingly, CD4+ T cell help and IL-2 were needed relatively late in the primary response to promote proliferation and perhaps survival because early CD8+ T cell expansion occurred normally in the absence of CD4+ T cells or CD25 but was followed by a more dramatic contraction phase (Fig. 3). Thus, it is important to consider the overall kinetics of a response when analyzing potentially controlling factors.
Our findings indicated that one mechanism by which CD4+ T cells may provide help was through the control of the up-regulation of CD25 expression by recently activated CD8+ T cells (Fig. 1). The decreased CD25 expression could be a direct result of CD28:CD80/86 signals but might also be a consequence of decreased IL-2 production in the absence of CD4+ T cells because IL-2 is known to regulate CD25 expression (34). Both are likely the case as engagement of the CD28:CD80/86 pathway causes IL-2 expression (30) and the provision rIL-2 in the absence of CD4+ T cell help resulted in up-regulation of CD25 expression (Fig. S2 and ref. 34). Either way, in the absence of CD4+ T cells, CD25 was poorly up-regulated on responding CD8+ T cells after VV, VSV, and LM infections. This is in line with previous studies that have demonstrated that CD4+ T cell help regulates CD25 expression on CD8+ T cells after HSV-1 infection (35) and DC vaccination (36) and correlated with the CD4+ T cell dependence of these responses. In contrast, previous studies reported no CD4+ T cell or CD25 involvement in the primary CD8+ T cell response to LCMV and LM infections (18, 19, 27, 28), although other studies support a role for CD4+ T cells in primary CD8+ T cell responses to these and other immunogens (21, 22). Interestingly, the route of infection may also determine the necessity of CD4+ T cell help because primary CD8+ T cell responses were “help” independent when mice were infected i.n. with VV-WR (23), but our current study demonstrated the primary response to i.p. infection with VV-WR was dependent on CD4+ T cell help (Fig. 2). Interestingly, our data indicated that the early CD8+ T cell response was independent of CD4+ T cell help and IL-2, whereas the continued differentiation and expansion typically required both (Figs. 3 and 5). Whether the necessary IL-2 for sustained CD8+ T cell expansion is derived solely from CD4+ T cells is not known. Our previous studies showed that autocrine CD8+ T cell produced IL-2 is not required for maximal expansion but, in fact, appears to dampen the overall magnitude of the response (32). Furthermore, work by Livingstone and colleagues demonstrated that CD4+ T cells provided the major source of IL-2 for responding antigen-specific CD8+ T cells (26). In either case, CD25 expression would be required to mediate IL-2 effects and therefore CD4+ T cells would be involved in the regulation of the CD8+ T cell response at multiple levels.
Recent work illustrates that substantial heterogeneity exists within the effector CD8+ T cell population. Originally, memory-precursors were identified by their retention of CD127 expression (37, 38), which has now been refined to use KLRG1 and CD127 expression to identify MPEC and SLEC (12–14), and, in this report, EEC. Only recently have some of the factors regulating the differentiation of the MPEC and SLEC populations been identified. Although earlier work demonstrated that TCR or cytokine signaling alone were insufficient to up-regulate KLRG1 on T cells (39), recent work has shown that IL-12 signaling in the antigen-specific CD8+ T cells is important for SLEC differentiation and this process is T-bet mediated (12). Furthermore, inflammatory and IL-12 signaling events are temporally linked with TCR engagement (16). The transcription factor Blimp1 has been found to be important in the generation of SLEC (40, 41). Interestingly, Blimp1 expression is regulated by IL-2 in vitro (42) and our data demonstrated that IL-2 mediated signals played an important role in regulating the formation of the SLEC population (Fig. 4). Thus, IL-12 likely mediates early events in the initial differentiation of the SLEC, although IL-2, perhaps through enhancement of Blimp1 expression, acts at later times to further promote the differentiation of more SLECs, perhaps from EEC that express the highest levels of CD25.
Fig. 4.
SLEC-development late in the response requires CD25-mediated signals. CD25−/− mixed chimera mice were infected with 103 CFU of Lm-ova and effector CD8+ T cells were monitored in the blood. (A) Representative zebra plots show the differentiation of Ova/Kb-specific CD8+ T cells after Lm-ova infection in the WT and CD25-deficient cell populations. The values represent the mean ± 1 SD for each effector cell population. (B and C) Graphical representation of different effector CD8+ T cells within the pathogen-specific population at the indicated times following LM-ova (B) and VV-WR (C) infections. The bars display the mean percentage ± one SD: WT pathogen-specific CD8+ T cells (blue) and CD25−/− pathogen-specific CD8+ T cells (red). The asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, and ***P < 0.001).
Our studies further demonstrated that CD25-deficient pathogen-specific CD8+ T cells generated an MPEC population during the effector stage, and these cells went on to form a stable, long-lived memory pool (Figs. 3 and 4). Moreover, in the absence of IL-2 driven signals, memory CD8+ T cells from LM and VV infected mice responded vigorously to a secondary challenge (Fig. 6). However, SLEC development was again dampened just as we observed in the primary response implicating IL-2 in modulation of the primary and secondary responses through enhancing the late differentiation of the SLEC population. Nevertheless, we found little evidence for a helpless phenotype of memory CD8+ T cells that developed in the absence of CD4+ T cells or CD25. Previous studies performed with LCMV-specific P14 TCR transgenic CD8+ T cells lacking CD25 indicate that although cells are capable of proliferating during a secondary response, their overall accumulation is impaired. The authors attributed these findings to a potential increase in cell death (27). In contrast, we observed robust expansion of CD25−/− CD8+ T cells during the secondary response at multiple time points. It is possible that LCMV secondary responses are dependent upon IL-2 signals, although the infectious models we studied are not because the LCMV results were confirmed by a separate group (28). However, Williams et al. (27) also observed a defect in recall responses to LM-ova in experiments that were very similar to our own. Although we cannot explain these discrepancies, the reproducibility of our results in three separate infection models suggested that imprinting of proliferative capacity during the primary CD8+ T cell response for the derived memory cells is not a generalizable phenomenon. Therefore, we conclude that CD4+ T cell help drives CD25 up-regulation and this in turn allows IL-2 to enhance SLEC differentiation during both primary and secondary CD8+ T cell responses, rather than acting as a “programmer” of the downstream memory response.
Materials and Methods
Mice.
All mice were used between 5 and 8 weeks of age for these studies. To generate mixed bone marrow chimeras, B6-Ly5.2 recipient mice were lethally irradiated with approximately 1,000 rads and subsequently injected i.v. with a total of 106 bone marrow cells containing a mixture of CD25−/− and B6-Ly5.2 at a 2:1 ratio. Mice were left for at least 50 days before infection. All animal protocols were approved by the Animal Care Committee at University of Connecticut Health Center, Farmington, CT, and the Animal Care and Use Program at Dartmouth College, Hanover, NH.
Experimental Treatment of Mice.
Mice were infected with 105 PFU VSV-ova (i.v.), 103 CFU LM-ova (i.v), or i.p. infection with 2 × 106 VV-WR. For secondary infections VV-WR memory mice were infected i.v. with 1 × 106 CFU of attenuated (ActA- LLO-) LM expressing the B8R epitope (LM-B8R), whereas both VSV-ova and LM-ova memory mice were challenged with 5 × 104 CFU of LM-ova. CD4+ T cells were depleted as previously described (21, 23). To supply exogenous IL-2, mice were treated with 15,000 U of recombinant human IL-2 i.p., as previously performed (34).
Tissue Sample Preparation and Flow Cytometric Analysis.
Mice were either bled at the indicated time points or single cell suspensions of the indicated tissues were prepared by collagenase digestion as previously described (2), and cells were stained as previously described (1, 23). BrdU incorporation was analyzed as previously described (1).
Adoptive Transfer and Recall of Memory CD8+ T Cells.
WT (CD45.1) and CD25-deficient (CD45.2) memory CD8+ T cells were sorted on a FACSAria to >98% purity. 1000 Ova/Kb-specific memory CD8+ T cells from each cell population were mixed together and injected i.v. into naïve CD45.1/CD45.2 recipient mice. One day later mice were challenged with 5 × 103 PFU of LM-ova and the number of tetramer+ cells was enumerated in the spleen and lungs on day 8 postchallenge.
Statistical Analysis.
Statistical significance was determined by a Student's t test using Prism 5. Significance was set as any P value less than 0.05.
Supplementary Material
Acknowledgments
The authors thank Dr. Charles Surh (The Scripps Research Institute, La Jolla, CA) for providing CD25-deficient bone marrow. We also thank Diane Gran for expert assistance in cell sorting. This work was funded by National Institutes of Health grants AI041576 and AI076457 (to L.L.), AI069943 and CA103642 (to E.J.U.), T32AI07363 (to M.J.M.), and F32AI074277 (to J.J.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909945107/DCSupplemental.
References
- 1.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 4.D'Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: Transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 7.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefrançois L, Masopust D. The road not taken: Memory T cell fate ‘decisions’. Nat Immunol. 2009;10:369–370. doi: 10.1038/ni0409-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stemberger C, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 11.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 18.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 19.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzo AL, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 22.Shedlock DJ, et al. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 23.Fuse S, et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 25.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 26.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 27.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 29.Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc Natl Acad Sci USA. 2001;98:6325–6329. doi: 10.1073/pnas.101136898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahinian A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 32.D'Souza WN, Lefrançois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 33.Montfort MJ, Bouwer HG, Wagner CR, Hinrichs DJ. The development of functional CD8 T cell memory after Listeria monocytogenes infection is not dependent on CD40. J Immunol. 2004;173:4084–4090. doi: 10.4049/jimmunol.173.6.4084. [DOI] [PubMed] [Google Scholar]
- 34.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 35.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 36.Blachère NE, et al. IL-2 is required for the activation of memory CD8+ T cells via antigen cross-presentation. J Immunol. 2006;176:7288–7300. doi: 10.4049/jimmunol.176.12.7288. [DOI] [PubMed] [Google Scholar]
- 37.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 38.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 39.Robbins SH, Terrizzi SC, Sydora BC, Mikayama T, Brossay L. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J Immunol. 2003;170:5876–5885. doi: 10.4049/jimmunol.170.12.5876. [DOI] [PubMed] [Google Scholar]
- 40.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8 T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8 T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.