Abstract
Over 170 million people are chronically infected by the hepatitis C virus (HCV) and at risk for dying from liver cirrhosis and hepatocellular carcinoma. Current therapy is expensive, associated with significant side effects, and often ineffective. Discovery of antiviral compounds against HCV traditionally involves a priori target identification followed by biochemical screening and confirmation in cell-based replicon assays. Typically, this results in the discovery of compounds that address a few predetermined targets and are prone to select for escape variants. To attempt to identify antiviral compounds with broad target specificity, we developed an unbiased cell-based screening system involving multiple rounds of infection in a 96-well format. Analysis of a publicly available library of 446 clinically approved drugs identified 33 compounds that targeted both known and previously unexplored aspects of HCV infection, including entry, replication, and assembly. Discovery of novel viral and cellular targets in this manner will broaden the therapeutic armamentarium against this virus, allowing for the development of drug mixtures that should reduce the likelihood of mutational escape.
Keywords: cell-based assay, antivirals, entry, replication, assembly
Hepatitis C virus (HCV) (Flaviviridae) is an enveloped, positive-stranded RNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma (1). HCV establishes persistent infection, and more than 170 million people are chronically infected worldwide (2). Chronic infection is associated with chronic hepatitis, cirrhosis, and hepatocellular carcinoma (3). Although the mechanisms by which HCV causes liver disease are not entirely understood, immunologically mediated events play an important role in HCV clearance and pathogenesis (4).
The HCV plus-stranded RNA genome (9.6 kb) encodes a single polyprotein that is cleaved into structural (core, E1, E2, and p7) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins (5). Infection is initiated by virus-particle binding to cellular receptors, internalization through receptor-mediated endocytosis, and delivery of the viral genome to the cytosol after endosomal acidification (6). Delivery and/or translation of incoming viral genomes depends on cellular autophagy-related factors (7), enabling viral gene expression, replication (8), and production of progeny virus, which depends on VLDL biosynthesis (9–11).
Currently, there is no vaccine against HCV, and the standard therapy (pegylated IFN-alfa plus ribavirin) is associated with significant side effects and is only effective in a fraction of the patients (12). Establishment of HCV replicons (13, 14) greatly contributed to the discovery of antiviral compounds that target the viral NS3-4A serine protease and NS5B RNA polymerase (15). Although they are extremely potent, these agents select for resistant variants because of the error-prone RNA polymerase activity of HCV (16).
The development of HCV infection models (17–19) that reproduce the entire life cycle of HCV in vitro created the opportunity to discover novel antiviral compounds that target every step in the viral life cycle in an unbiased manner. Using this system (19), we screened the National Institutes of Health Clinical Collection (NCC) (20), a set of 446 compounds that have been clinically tested for a wide variety of indications, for the ability to inhibit HCV infection in vitro. Thirty-three compounds displayed antiviral activity in the absence of cytotoxicity at low-micromolar and submicromolar concentrations. Many of the candidates were lysosomotropic compounds that inhibited HCV entry with differential efficacy against genotype 1a (H77) and genotype 2a (JFH-1) envelope glycoproteins. Two compounds, MK886 and pterostilbene, were potent inhibitors of persistent HCV infection: MK886 reduced intracellular HCV RNA levels, and pterostilbene inhibited infectious particle assembly and secretion without altering HCV RNA levels. Toremifene blocked viral entry but also displayed antiviral activity in persistently infected cells; it has a small impact on intracellular infectivity and HCV RNA accumulation but strongly inhibited progeny virus secretion. Finally, rabeprazole inhibited HCV infection by targeting a yet uncharacterized aspect of the HCV life cycle downstream of entry and upstream of replication.
These results illustrate the ability of our screening system to identify inhibitors that target known and unknown aspects of the viral life cycle, providing a set of chemical tools to dissect the HCV life cycle, and identifying interesting candidates for the development of novel therapeutic strategies against HCV.
Results
Screening for Antiviral Compounds at Low Multiplicity of Infection.
We have previously shown that the number of HCV-positive cells increases as HCV infection spreads in cell culture after inoculation of Huh-7 cells at low multiplicity (19). Therefore, if viral infection occurs in the presence of a given antiviral molecule, the number of positive cells and the accumulation of viral antigen per cell should be reduced proportionally to the antiviral activity of the compound. Thus, by measuring the amount of viral protein in cells that have been inoculated at low multiplicity in the presence of an antiviral compound, one will determine its impact on the ability of the virus to infect target cells, replicate, produce progeny virus, and infect new target cells in subsequent rounds of infection.
We have developed a colorimetric assay to quantitate viral antigen levels in infected cell cultures in a 96-well format. This assay allows quantification of the amount of E2 protein in a given well, which reflects the efficiency of HCV infection and spread in a moderate throughput format. This approach was used to interrogate the NIH Clinical Collection library, composed of 446 clinically approved small molecules (20), for antiviral activity against HCV infection. Screening was performed at a single dose of 10 μM in duplicate wells. This analysis revealed 33 nontoxic molecules displaying >85% reduction in HCV infection, as compared to the vehicle (DMSO) control (Table 1 and Table S1). In addition to these strong inhibitors, 11 compounds displayed intermediate activity (75–85% inhibition; Table S2.1) and 30 compounds had slight antiviral activity (50–75% inhibition; Table S2.2). Thirty-six of the 446 compounds were cytotoxic and could not be evaluated for antiviral activity (Table S2.3). Most of the analyzed compounds (336) were inactive (Table S2.4). As expected, some of the active antiviral compounds were already known to have antiviral activity against HCV [e.g., nelfinavir (21), nitazoxanide (22), and MK886 (23)]; others are known to be active against other viruses {e.g., haloperidol [EBV (24)] and amiodarone [SARS (25)]}. However, the ability of most of the compounds to inhibit HCV infection was unexpected.
Table 1.
Primary screening hits
| Compound name | NCC compound number | HCV infection, % of control | Toxicity, biomass | EC50, μM | Error | EC90, μM | Error | LD50, μM | Error | LD50/EC50 | LD50/EC90 | Reported antiviral activity |
| Cyproheptadine | CPD000058431 | 0.3 | 72.2 | 0.7 | 0.2 | 3.0 | 0.0 | 33.5 | 1.5 | 51.5 | 11.2 | |
| Toremifene | CPD000469213 | 0.6 | 86.2 | 0.5 | 0.0 | 1.8 | 0.2 | 19.7 | 0.3 | 40.7 | 11.1 | |
| Fluphenazine | CPD000058411 | 0.0 | 76.5 | 0.5 | 0.2 | 1.4 | 0.6 | 14.5 | 5.5 | 29.0 | 10.4 | |
| Trifluoperazine | CPD000059133 | 0.3 | 86.9 | 0.6 | 0.3 | 1.7 | 0.5 | 16.5 | 1.5 | 30.0 | 10.0 | EBV entry (24) |
| Pizotyline | CPD000466272 | 0.0 | 69.8 | 0.7 | 0.1 | 3.0 | 0.0 | 28.0 | 2.0 | 39.1 | 9.3 | |
| Pterostilbene | CPD000440694 | 0.0 | 68.6 | 1.7 | 0.1 | 3.0 | 0.0 | 28.0 | 2.0 | 16.5 | 9.3 | |
| CGS 12066B | CPD000468733 | 0.3 | 106.9 | 1.0 | 0.0 | 3.5 | 0.5 | 32.5 | 2.5 | 32.5 | 9.3 | |
| Prochlorperazine | CPD000466275 | 0.0 | 72.8 | 1.3 | 0.7 | 3.3 | 0.8 | 29.3 | 12.3 | 22.3 | 9.0 | |
| Rabeprazole | CPD000469174 | 4.0 | 113.6 | 1.6 | 0.4 | 7.5 | 2.5 | 66.5 | 13.5 | 40.6 | 8.9 | |
| Doxepin | CPD000449270 | 14.7 | 99.2 | 4.0 | 2.0 | 9.5 | 5.5 | 65.0 | 15.0 | 16.3 | 6.8 | |
| Ketotifen | CPD000058462 | 11.1 | 105.9 | 4.9 | 3.1 | 12.3 | 0.3 | 72.3 | 27.8 | 14.8 | 5.9 | |
| MK886 | CPD000466278 | 5.4 | 110.3 | 2.1 | 0.1 | 6.5 | 0.5 | 37.5 | 12.5 | 17.9 | 5.8 | HCV infection (23) |
| Amiodarone | CPD000058296 | 1.3 | 95.7 | 0.9 | 0.3 | 4.4 | 0.6 | 21.8 | 6.8 | 23.3 | 4.9 | SARS-CV entry (25) |
| Imatinib | CPD000469175 | 0.1 | 76.8 | 2.8 | 0.2 | 5.5 | 0.5 | 25.5 | 4.5 | 9.2 | 4.6 | |
| Lacidipine | CPD000466342 | 0.0 | 72.4 | 1.8 | 0.3 | 4.3 | 0.3 | 17.5 | 7.5 | 10.0 | 4.1 | |
| SB 205607 | CPD000466296 | 13.3 | 109.3 | 4.5 | 1.5 | 12.5 | 2.5 | 49.5 | 0.5 | 11.1 | 4.0 | |
| Lofepramine | CPD000469292 | 7.8 | 104.3 | 2.3 | 1.3 | 9.5 | 5.5 | 37.5 | 2.5 | 16.7 | 3.9 | |
| Rimcazole | CPD000466293 | 0.0 | 81.0 | 1.5 | 0.0 | 5.0 | 0.0 | 19.7 | 10.7 | 13.0 | 3.9 | |
| Mifepristone | CPD000058481 | 1.8 | 91.5 | 2.3 | 0.3 | 7.0 | 3.0 | 27.5 | 7.5 | 12.2 | 3.9 | |
| Clobenpropit | CPD000469632 | 10.2 | 89.0 | 3.8 | 0.8 | 9.0 | 3.0 | 35.0 | 0.0 | 9.3 | 3.9 | |
| Salmeterol | CPD000466295 | 1.4 | 88.5 | 3.5 | 0.5 | 8.5 | 2.5 | 33.0 | 17.0 | 9.4 | 3.9 | |
| Azelastine | CPD000469183 | 0.5 | 82.1 | 2.9 | 1.1 | 6.3 | 2.8 | 24.1 | 5.9 | 8.3 | 3.9 | |
| Desloratadine | CPD000149358 | 1.4 | 88.4 | 2.3 | 0.2 | 6.5 | 1.0 | 24.0 | 6.0 | 10.4 | 3.7 | |
| AM-251 | CPD000466284 | 12.9 | 94.2 | 3.2 | 1.4 | 8.3 | 1.8 | 30.0 | 0.0 | 9.5 | 3.6 | |
| Rifabutin | CPD000466322 | 12.7 | 87.0 | 6.0 | 3.0 | 13.0 | 7.0 | 42.5 | 7.5 | 7.1 | 3.3 | |
| Indatraline | CPD000449273 | 0.7 | 68.1 | 1.1 | 0.1 | 3.0 | 0.0 | 9.2 | 0.8 | 8.1 | 3.1 | |
| Nelfinavir | CPD000469186 | 0.8 | 95.3 | 3.7 | 0.3 | 8.5 | 0.5 | 23.9 | 1.9 | 6.4 | 2.8 | HCV replication (21) |
| Haloperidol | CPD000449283 | 2.4 | 89.8 | 3.3 | 1.3 | 10.5 | 4.5 | 28.0 | 4.0 | 8.6 | 2.7 | EBV entry (24) |
| Benproperine | CPD000469294 | 3.4 | 98.0 | 4.0 | 2.0 | 8.5 | 2.5 | 22.5 | 2.5 | 5.6 | 2.6 | |
| M-paroxetine | CPD000469181 | 7.8 | 99.0 | 3.0 | 1.0 | 10.5 | 4.5 | 24.6 | 4.6 | 8.3 | 2.3 | |
| Carvedilol | CPD000449280 | 0.7 | 76.0 | 4.5 | 0.5 | 8.0 | 2.0 | 16.5 | 1.5 | 3.7 | 2.1 | |
| Calcipotriol | CPD000466353 | 14.1 | 93.3 | 5.5 | 0.5 | 14.0 | 2.0 | 26.6 | 6.4 | 4.8 | 1.9 | |
| Nitazoxanide | CPD000466367 | 6.3 | 69.5 | 2.3 | 0.5 | 10.0 | 0.0 | 14.7 | 4.7 | 6.3 | 1.5 | HCV replication(22) |
Displayed are NCC library compounds showing antiviral activity (>15% HCV infection) at 10 μM with no associated toxicity (>70% cell biomass). NCC compound number permits access to extensive compound information through PubChem (http://pubchem.ncbi.nlm.nih.gov). Data are shown as average and mean error of a minimum of two independent experiments performed in duplicate, except for original screening values, which represent average values of duplicate wells.
Once the antiviral activity of the compounds was confirmed, we determined their potency (EC50) and toxicity (LD50) by determining the antiviral activity of serial dilutions at concentrations ranging from 50 μM to 0.4 nM. Table 1 shows the EC50 and EC90 values for these compounds, as well as their cytotoxicity (LD50). To reduce the selected compounds to a manageable number and to minimize the contribution of cytotoxicity in their characterization, we arbitrarily selected 12 compounds showing a LD50/EC90 ratio of >5 for further analysis [Table 1 (selected compounds)].
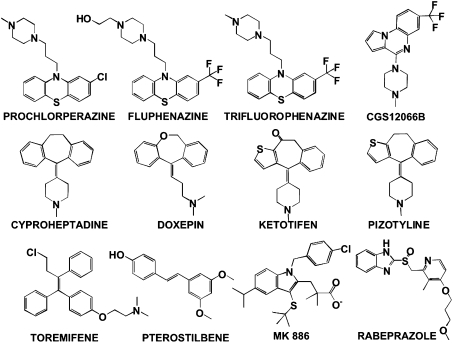
Several of the 12 compounds are tricyclic with a tertiary amine in their structure (Fig. 1), characteristic of compounds known to interfere with endosomal maturation (26, 27). Indeed, chlorpromazine, a phenothiazine that is nearly identical to prochlorperazine and very similar to trifluoperazine and fluphenazine, inhibits clathrin-dependent endocytosis (27) and the entry of several viruses, including HCV, in cell culture (28). Thus, it is possible that these phenothiazines inhibit HCV entry, as shown in other viral systems (24, 29). Other antiviral compounds, like pizotyline, CGS12066B, cyproheptadine, doxepin, and ketotifen (Fig. 1), also display a tricyclic lipophilic moiety and a tertiary amine (Fig. 1), suggesting that they might also interfere with this process. Rabeprazole (Fig. 1), an acid-activated proton pump H+/K+-ATPase inhibitor (PPI) (30), may also inhibit viral entry because PPIs have been shown to impair endosomal acidification (31), which is required for HCV entry (6).
Fig. 1.
Chemical structure of primary hits selected for further analysis: Compounds displaying therapeutic indexes above five (TI90; LD50/EC90 > 5) that were selected for further analysis.
In addition, we identified three compounds (Fig. 1) that target cellular proteins known to be required for efficient HCV RNA replication. From these, pterostilbene and MK886 are ligands [agonist (32) and antagonist (33)] of the peroxisome proliferator-activated receptor α (PPAR-α), which is known to be required for HCV RNA replication (23, 34). The third compound is toremifene, which modulates primarily the estrogen receptor (35), which in turn modulates HCV replication (36). Therefore, these compounds could inhibit HCV RNA replication if they exert their antiviral activity through their known cellular targets.
To study the mode of action of the selected molecules, we examined their ability to inhibit viral entry by studying their impact on HCV-pseudotype (HCVpp) infection (37), and their ability to interfere with downstream steps by studying their effect on persistent HCV infections.
Analysis of Viral Entry by Using HCV Pseudotypes.
We used HCVpp infection to recapitulate HCV particle adsorption, internalization, and viral envelope-mediated fusion to evaluate the impact of these compounds on viral entry. The selected compounds were mixed with retroviral particles pseudotyped with glycoproteins from HCV genotypes 2a (JFH-1pp) and 1a (H77pp), or VSV glycoprotein (VSVpp) as a control, and added to target Huh-7 cells at a final concentration two times the EC90 (2× EC90). All of the selected compounds except MK886, pterostilbene, and rabeprazole inhibited JFH-1pp entry (Fig. 2A), suggesting that they inhibit HCV entry. In contrast, none of the compounds inhibited VSVpp entry, indicating that VSVg-mediated entry and retrovirus-driven GFP expression were not altered by any of these inhibitors at the assayed concentrations (Fig. 2A). Strikingly, all of the active compounds displayed reduced activity (prochlorperazine, trifluoperazine, fluphenazine, and CGS12066B) or almost no activity (pizotyline, cyproheptadine, doxepin, and ketotifen) against genotype 1a (H77) pseudotyped particle entry at the assayed concentrations. This preferential JFH-1 inhibition was also evident for toremifene that displayed unexpected antiviral activity against HCVpp (Fig. 2A). To rule out the possibility that differential entry kinetics might be responsible for the apparent selectivity of these drugs, we pretreated the cells with the inhibitors for 2 h before inoculation with the HCVpp with results identical to those shown in Fig. 2A. H77pp entry was selectively inhibited at higher concentrations (4× EC90) of cyproheptadine, pizotyline, CGS12066b, and toremifene without affecting VSVpp entry (Fig. S1), suggesting that, despite its reduced susceptibility to this class of inhibitors, H77pp entry is more sensitive than VSVpp to this class of compounds.
Fig. 2.
Analysis of viral entry using HCVpp. (A) Huh-7 cells were inoculated with JFH-1, H77, and VSV pseudotypes in the presence of 2× EC90 concentrations of the selected compounds. Infection efficiency was estimated by the number of GFP-positive cells and expressed as percentage of the DMSO control. (B) Impact of the selected compounds on JFH-1 and H77C3JFH1 virus infection. Data are shown as average and mean error values of a minimum of two independent experiments in duplicate.
This differential inhibition could be reproduced by using bona fide virus comparing the apparent infectivity titers of the parental JFH-1 and a chimeric virus bearing the structural region from genotype 1a strain H77 [H77C3JFH-1 (38)] in the absence or presence of the inhibitors (2× EC90; Fig. 1B). These results confirm that JFH-1 and H77 glycoprotein-mediated entry differ in their susceptibility to these lysosomotropic compounds.
Neither rabeprazole nor MK886 nor pterostilbene displayed significant activity against the HCV-pseudotypes (Fig. S2), suggesting that they target an aspect of the infection downstream of viral entry.
Analysis of the Effect of Inhibitors on Persistent Infection.
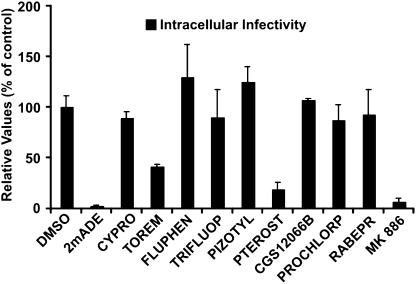
HCV efficiently establishes persistent infection in cell culture, where constant viral RNA translation, replication, particle assembly, and secretion occur (39). Thus, using that system, the effect of a compound on intracellular and extracellular infectivity and HCV RNA levels can be measured. All 12 compounds [Table 1 (selected compounds)] were added (2× EC90) to persistently infected cells for 48 h to monitor their impact on the intracellular content of infectious virus. Of all of the selected compounds, only MK886, pterostilbene, and toremifene caused reduction of intracellular infectivity levels after treatment (Fig. 3), indicating that these compounds interfere with persistent infection. Because active HCV RNA replication is required for maintenance of normal intracellular infectivity levels, as shown by the strong reduction in intracellular infectivity levels in cells treated with the polymerase inhibitor 2′-C-methyladenosine (Fig. 3), we also measured intracellular and extracellular HCV RNA and extracellular infectivity levels to differentiate between inhibitors that reduce intracellular infectivity levels by reducing HCV RNA levels and inhibitors that target steps downstream.
Fig. 3.
Impact of the selected compounds on persistent JFH-1 infections. Persistently infected Huh-7 cells were treated for 48 h with the indicated inhibitors (2× EC90). Data are shown as average and mean error intracellular infectivity 48 h posttreatment and expressed as percentage of control of a minimum of two experiments performed in triplicate.
Treatment with MK886 reduced proportionally both intra- and extracellular infectivity and HCV RNA levels in persistently JFH-1 infected cells (Fig. 4A), suggesting that MK886 interferes with HCV infection by reducing intracellular HCV RNA levels, and, consequently, production of progeny virus. This intracellular HCV RNA reduction was also observed (albeit less efficiently) in cells persistently infected with the genotype 1a infectious clone H77S and treated with the same dose of MK886 (Fig. S3). These results suggest that MK886 HCV inhibits HCV infection by interfering with HCV RNA replication, a notion that is supported by its comparable antiviral activity on a full-length HCV replicon (Fig. S4).
Fig. 4.
Inhibitors of persistent infection display different mechanisms of action. Intracellular and extracellular infectivity and HCV RNA levels after a 48-h treatment of persistently infected Huh-7 cells with 2′-C-methyladenosine (1 μM) or MK886 (14 μM) (A), pterostilbene (6 μM) (B), or toremifene (4 μM) (C). Data are presented as average and mean error of two experiments performed in triplicate (n = 6) and are expressed as percentage of the values obtained in the vehicle-treated (DMSO) cells.
When added to persistently infected cells, pterostilbene reduced intracellular as well as extracellular infectivity and HCV RNA levels (Fig. 4B; infectivity) without altering intracellular viral RNA content (Fig. 4B; HCV RNA), suggesting that it targets a process downstream of RNA replication that leads to infectious particle assembly.
Toremifene slightly reduced intracellular infectivity and HCV RNA content in persistently infected cells and caused a disproportionate reduction in extracellular infectivity and HCV RNA levels (Fig. 4C), suggesting that, in addition to its effect on JFH-1 entry (Fig. 2), it may also interfere with late steps in the viral life cycle, especially the secretion of progeny viral particles (Fig. 4C).
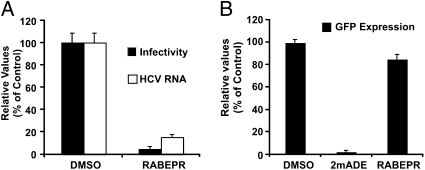
Intriguingly, rabeprazole did not display any antiviral activity in persistently infected cells (Fig. 3) nor did it inhibit HCVpp infection (Fig. S2), suggesting that it targets a step of the viral life cycle downstream of viral envelope fusion and upstream of viral RNA replication. To test this hypothesis, we evaluated its impact (2× EC90) on the accumulation of HCV RNA and intracellular infectivity in single cycle (m.o.i. of 10) infection experiments. This analysis revealed that rabeprazole reduced the accumulation of both intracellular infectivity and HCV RNA (Fig. 5A), suggesting that it targets an early step of the infection preceding HCV RNA replication, because the same dose of rabeprazole had no effect on persistent HCV infections (Fig. 3). Therefore, we asked whether rabeprazole blocks primary translation and establishment of replication complexes by examining the impact of antiviral doses of rabeprazole on the establishment of viral replication after electroporation (7) of a subgenomic HCV replicon. Huh-7 cells were transfected with a subgenomic HCV RNA bearing a GFP reporter gene and treated with a vehicle control, 2′-C-methyladenosine (1μM), or rabeprazole (20 μM). Analysis of GFP expression levels by flow cytometry revealed that rabeprazole did not interfere with the initiation of HCV RNA replication or GFP expression (Fig. 5B), suggesting that rabeprazole blocks the infection upstream of initial HCV RNA translation and replication. The lack of activity of rabeprazole on HCVpp indicates that it either targets a step downstream of glycoprotein-mediated fusion or that it targets an aspect of viral entry that is not recapitulated in the HCVpp system—e.g., capsid disassembly or trafficking of the viral RNA.
Fig. 5.
Rabeprazole interferes with early steps of viral infection. (A) Huh-7 cells were infected (m.o.i. of 10) with D183 JFH-1 virus in the presence of rabeprazole (20 μM). Intracellular and extracellular infectivity as well as HCV RNA levels are shown as average and mean error of a representative experiment performed in triplicate and are expressed as percentage of DMSO-treated cells. (B) GFP expression reflecting subgenomic HCV replicon replication after transfection into Huh-7 cells in the presence of DMSO or 2′-C-methyladenosine (1 μM) or rabeprazole (20 μM). Data are expressed as average and mean error of at least two independent experiments performed in duplicate.
Discussion
Using an unbiased, miniaturized cell-based screening system, we discovered the anti-HCV properties of 33 clinically approved compounds. Although some of these compounds [e.g., nelfinavir (21), nitazoxanide (22), and MK886 (23)] were known to have antiviral activity, most were unexpected because their clinical indications are unrelated to treatment of viral infection. All of the antiviral compounds displayed activity at nontoxic concentrations and therefore may have therapeutic potential, especially because nitazoxanide, the compound displaying the lowest therapeutic index, is currently under clinical evaluation and has been reported to improve the outcome of standard IFN treatment (22). Therefore, the compounds described in Table 1 may be worthy of further development. Interestingly, a literature search revealed that toremifene, rabeprazole, and MK886 display antiviral activity in vitro at concentrations that can be safely achieved in plasma of patients treated with therapeutic doses of these drugs for other indications (Table S3). It is important to note, however, that much more information than just dose comparisons is necessary before concluding that these compounds can be used to treat HCV infection in vivo.
For practical reasons, we limited our analysis to a group of 12 compounds with the highest therapeutic indices. Studies performed with HCVpp demonstrated that most of the tested compounds inhibited viral entry. These compounds display common structural features and are generically designated as lipophilic amines or cationic amphiphilic drugs (CAD) (26). Such compounds are known to be lysosomotropic and to interfere with multiple cellular processes, including clathrin-dependent endocytosis (40) and, for certain viruses, viral entry (41). Accumulation of these drugs in acidic compartments is thought to occur by protonation of their tertiary amine at low pH, which impedes the diffusion of the charged molecule through lipid membranes (40, 42). Therefore, these compounds act as mild bases and have the potential to neutralize endosomal pH and to alter the biophysical properties of the membranes in which they are inserted, resulting in the inhibition of multiple processes occurring in the targeted compartment (43, 44).
Interestingly, these inhibitors display selective anti-HCV activity relative to VSV-pseudotypes at the assayed concentrations, suggesting that HCV is particularly susceptible to inhibition by this class of compounds, some of which inhibit other virus infections, including VSV, usually at higher concentrations (41). Furthermore, JFH-1 and H77 pseudoparticles displayed differential susceptibility to these compounds, with H77 requiring higher compound concentrations for comparable inhibition. Thus, these compounds revealed intergenotypic differences at the level of glycoprotein-mediated entry and they constitute chemical tools to study physicochemical aspects of the entry process. Additional structure–activity relationship analysis of these compounds must be carried out to elucidate the basis for their differential potency against JFH-1 and H77 pseudoparticle entry to reveal potential differential intergenotypic entry requirements.
MK886 reduced intracellular HCV RNA levels and, consequently, production of progeny virus in persistently infected cells. Our results indicate that it interfered with HCV RNA replication, consistent with its previously reported antiviral activity in a different cell culture infection system (23) and with a report that suggests that PPAR-α antagonism inhibits HCV RNA replication (34).
Although pterostilbene also reduced infectious virus production in persistently infected cells, it did so without altering intracellular HCV RNA levels, indicating that it targets a different aspect of the infection, downstream of viral replication, probably at the level of particle assembly. Studies that address the precise mode of action of pterostilbene could provide new insights into the molecular mechanisms underlying infectious viral particle assembly, an aspect of HCV infection that has not yet been approached therapeutically.
Toremifene citrate, an estrogen receptor modulator similar to tamoxifen (45), inhibited multiple steps in the HCV life cycle: most notably entry and particle secretion with a minor impact on intracellular infectivity and HCV RNA content at the assayed concentrations. Its antiviral activity appears to recapitulate the estrogen receptor-independent effects of tamoxifen, which disrupts cellular endocytic and secretory pathways by accumulating in acidic subcellular compartments (46). Because of the structural similarity of toremifene and tamoxifen, we speculate that, in cell culture, toremifene accumulates in compartments such as lysosomes and trans-Golgi vesicles, and interferes with virus endocytosis and secretion.
Intriguingly, we could not identify the step of the viral life cycle targeted by rabeprazole. However, our data suggest that rabeprazole interferes with an early step in the infection, most likely downstream of entry and upstream of translation of the incoming HCV RNA—e.g., capsid disassembly or trafficking of the incoming genomes from the mature endosome to the replication site. Because trafficking of intracellular vesicles depends highly on ATPases (47), we speculate that viral infection may be aborted by inhibition of cellular ATPase(s) responsible for the sorting of incoming viral particles in a postendosomal compartment. If this is the case, rabeprazole would constitute the first example of inhibition of HCV infection at this level and reveal a new step in the infection that is not identifiable in the HCVpp and replicon systems but is potentially amenable to therapeutic intervention.
In summary, the results presented in this study describe a powerful unbiased screening system than can identify inhibitors of every aspect of HCV infection in cell culture. The main difference with other screening systems is that the enhanced replication capacity of the D183 virus (19) and the hypersusceptibility of Huh-7.5.1 c2 cells (48) enables several rounds of infection to occur within 72 h in culture, where expression of the viral antigen (E2) after infection at low multiplicity (m.o.i. of 0.01) not only depends on the ability of the virus to enter, replicate, and express viral genes, but also in its ability to spread to other cells, via either cell-to-cell spread (49) or secretion of progeny virus that initiate subsequent rounds of infection. Thus, our system extends the number of potential targets by interrogating libraries for compounds that inhibit events downstream of virus-dependent gene expression and that result in inhibition of viral spread. This is the case for pterostilbene, a compound that would not have been identified in systems where progeny virus production is not considered—i.e., single-cycle screening systems. Despite these great advantages, the strongest limitation of this assay is that it is restricted to the JFH1-related genotype 2a infection system, underscoring the need to develop efficient cell culture infection systems for the remaining HCV genotypes.
Finally, the unbiased nature of this cell-based system extends the potential targets to all of the host cell factors that contribute to efficient HCV infection and spread, enabling discovery of compounds that interfere with HCV infection by modulating these factors and are less likely to result in rapid selection of escape mutants. The discovery of compounds that, like rabeprazole, target currently unapproachable aspects of HCV infection provides the opportunity and the pharmacological tools to study events in the viral life cycle that might otherwise remain elusive.
Methods
Library Screening.
Compound stock solutions (10 mM in DMSO) were diluted to a final concentration of 20 μM in 100 μl of growth medium (DMEM-10% FCS) and mixed (1:1) with 100 μl of a virus dilution (2 × 103 infectious focus forming units (ffu) per mL) in medium. One hundred microliters of this mixture was used to inoculate 104 Huh-7.5.1-c2 cells per well [multiplicity of infection (m.o.i.) of 0.01] in a 96-well format (Falcon flat-bottom, 96-well cell culture microplate; BD Biosciences) in duplicate. The cells were incubated at 37°C for 3 days, after which they were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature (RT). PFA-fixed test wells were washed twice with 200 μl of PBS and incubated with 50 μl of blocking buffer [0.3% Triton X-100/3% BSA/10% FCS/5% hydrogen peroxide (H2O2) in PBS] for 1 h at RT. The cells were then washed twice with 200 μl of PBS, and 1 μg/mL anti-E2 antibody [AR3A (50)] was added in incubation buffer (0.3% Triton X-100/3% BSA in PBS) for 1 h at RT. The cells were washed four times with 200 μl of PBS and incubated with a 1:15,000 dilution of a goat anti-human IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch) for 1 h at RT. The cells were washed again four times with 200 μl of PBS and developed by using TMB (Pierce). The reaction was stopped by addition of 50 μl of 0.5 M H2SO4 solution. Absorbance at 450 nm (OD450) was measured directly from the test plate. Every test plate included control wells with uninfected cells that were used to subtract background values. Colorimetric values were transformed into infection efficiency values by using a standard curve generated by serial 2-fold virus dilutions starting at 200 ffu per well. Data were considered only if the standard curves displayed correlation coefficients (r2) above 0.97.
Compound toxicity was determined by evaluating remaining cell biomass at 72 h after inoculation by crystal violet staining and colorimetry at 570 nm as described in ref. 51. Compounds resulting in a reduction of the biomass below ≈70% of that of the controls were considered toxic and discarded for further analysis.
Additional methods are provided in the SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo) for kindly providing the infectious JFH-1 molecular clone and replicon constructs; Dr. S. Lemon (University of Texas, Galveston, Texas) for providing the infectious molecular clone H77S; Drs. Mansun Law and Dennis Burton (Scripps Research Institute) for providing the recombinant human IgG anti-E2; Dr. Francois-Loic Cosset (Ecole Normale Superieure, INSERM U758, Lyon, France) for providing the vectors necessary for HCVpp production; Dr. Zhong (Gilead Sciences, Foster City, CA) for providing the 2'-C-methyladenosine Drs. Marlene Dreux, Urtzi Garaigorta, and Stefan Wieland for expert advice and useful discussions; and Brian Boyd and Josan Chung for excellent technical assistance. This work was supported by National Institutes of Health Grants R01-CA108304 and R01-AI079043. This is manuscript no. 20301 from the Scripps Research Institute.
Footnotes
F.V.C. has an equity interest in Viriome, Inc., which has an exclusive option to license the technology described in this article.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912966107/DCSupplemental.
References
- 1.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1:559–568. vi–vii. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36(Suppl 1):S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 5.Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631–1648. doi: 10.1099/0022-1317-81-7-1631. [DOI] [PubMed] [Google Scholar]
- 6.Burlone ME, Budkowska A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J Gen Virol. 2009;90:1055–1070. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- 7.Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 9.Gastaminza P, et al. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahmias Y, et al. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 13.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1975. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann V, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 15.Neyts J. Selective inhibitors of hepatitis C virus replication. Antiviral Res. 2006;71:363–371. doi: 10.1016/j.antiviral.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Thompson AJ, McHutchison JG. Antiviral resistance and specifically targeted therapy for HCV (STAT-C) J Viral Hepat. 2009;16:377–387. doi: 10.1111/j.1365-2893.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 17.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindenbach BD, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 19.Zhong J, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical N. NIH Clinical Collection. 2008 [Google Scholar]
- 21.Toma S, et al. Inhibition of intracellular hepatitis C virus replication by nelfinavir and synergistic effect with interferon-alpha. J Viral Hepatitis. 2009;16:506–512. doi: 10.1111/j.1365-2893.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- 22.Rossignol JF, Elfert A, El-Gohary Y, Keeffe EB. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136:856–862. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Aly HH, Shimotohno K, Hijikata M. 3D cultured immortalized human hepatocytes useful to develop drugs for blood-borne HCV. Biochem Biophys Res Commun. 2009;379:330–334. doi: 10.1016/j.bbrc.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 24.Nemerow GR, Cooper NR. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci USA. 1984;81:4955–4959. doi: 10.1073/pnas.81.15.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadler K, et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 26.Kornhuber J, et al. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J Med Chem. 2008;51:219–237. doi: 10.1021/jm070524a. [DOI] [PubMed] [Google Scholar]
- 27.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard E, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candurra NA, Maskin L, Damonte EB. Inhibition of arenavirus multiplication in vitro by phenotiazines. Antiviral Res. 1996;31:149–158. doi: 10.1016/0166-3542(96)06956-2. [DOI] [PubMed] [Google Scholar]
- 30.Pace F, Pallotta S, Casalini S, Porro GB. A review of rabeprazole in the treatment of acid-related diseases. Ther Clin Risk Manag. 2007;3:363–379. [PMC free article] [PubMed] [Google Scholar]
- 31.Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 32.Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53:3403–3407. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- 33.Kehrer JP, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochem J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakic B, et al. Peroxisome proliferator-activated receptor alpha antagonism inhibits hepatitis C virus replication. Chem Biol. 2006;13:23–30. doi: 10.1016/j.chembiol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Kangas L, Nieminen AL, Cantell K. Additive and synergistic effects of a novel antiestrogen, toremifene (Fc-1157a), and human interferons on estrogen responsive MCF-7 cells in vitro. Med Biol. 1985;63:187–190. [PubMed] [Google Scholar]
- 36.Watashi K, et al. Anti-hepatitis C virus activity of tamoxifen reveals the functional association of estrogen receptor with viral RNA polymerase NS5B. J Biol Chem. 2007;282:32765–32772. doi: 10.1074/jbc.M704418200. [DOI] [PubMed] [Google Scholar]
- 37.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietschmann T, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong J, et al. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J Virol. 2006;80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 41.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 42.Firestone RA, Pisano JM, Bonney RJ. Lysosomotropic agents. 1. Synthesis and cytotoxic action of lysosomotropic detergents. J Med Chem. 1979;22:1130–1133. doi: 10.1021/jm00195a026. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- 44.Seelig A, Allegrini PR, Seelig J. Partitioning of local anesthetics into membranes: surface charge effects monitored by the phospholipid head-group. Biochim Biophys Acta. 1988;939:267–276. doi: 10.1016/0005-2736(88)90070-3. [DOI] [PubMed] [Google Scholar]
- 45.Harvey HA, Kimura M, Hajba A. Toremifene: an evaluation of its safety profile. Breast. 2006;15:142–157. doi: 10.1016/j.breast.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen IM, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timpe JM, et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 50.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 51.Bernhardt G, Reile H, Birnböck H, Spruss T, Schönenberger H. Standardized kinetic microassay to quantify differential chemosensitivity on the basis of proliferative activity. J Cancer Res Clin Oncol. 1992;118:35–43. doi: 10.1007/BF01192309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.