Abstract
Erythropoietin (Epo) treatment increases hematocrit (Htc) and, consequently, arterial O2 content. This in turn improves exercise performance. However, because elevated blood viscosity associated with increasing Htc levels may limit cardiac performance, it was suggested that the highest attainable Htc may not necessarily be associated with the highest attainable exercise capacity. To test the proposed hypothesis that an optimal Htc in acute and chronic Epo-treated mice exists—i.e., the Htc that facilitates the greatest O2 flux during maximal exercise—Htc levels of wild-type mice were acutely elevated by administering novel erythropoiesis-stimulating protein (NESP; wtNESP). Furthermore, in the transgenic mouse line tg6 that reaches Htc levels of up to 0.9 because of constitutive overexpression of human Epo, the Htc was gradually reduced by application of the hemolysis-inducing compound phenylhydrazine (PHZ; tg6PHZ). Maximal cardiovascular performance was measured by using telemetry in all exercising mice. Highest maximal O2 uptake 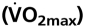 and maximal time to exhaustion at submaximal exercise intensities were reached at Htc values of 0.58 and 0.57 for wtNESP, and 0.68 and 0.66 for tg6PHZ, respectively. Rate pressure product, and thus also maximal working capacity of the heart, increased with elevated Htc values. Blood viscosity correlated with
and maximal time to exhaustion at submaximal exercise intensities were reached at Htc values of 0.58 and 0.57 for wtNESP, and 0.68 and 0.66 for tg6PHZ, respectively. Rate pressure product, and thus also maximal working capacity of the heart, increased with elevated Htc values. Blood viscosity correlated with 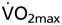 Apart from the confirmation of the Htc hypothesis, we conclude that tg6PHZ adapted better to varying Htc values than wtNESP because of the higher optimal Htc of tg6PHZ compared to wtNESP. Of note, blood viscosity plays a critical role in limiting exercise capacity.
Apart from the confirmation of the Htc hypothesis, we conclude that tg6PHZ adapted better to varying Htc values than wtNESP because of the higher optimal Htc of tg6PHZ compared to wtNESP. Of note, blood viscosity plays a critical role in limiting exercise capacity.
Keywords: blood viscosity, doping, excessive erythrocytosis, exercise performance, hemolysis
In a normal physiological hematocrit (Htc) range, erythropoietin (Epo) treatment or red blood cell retransfusion that increases hemoglobin concentration ([Hb]) improves maximal O2 uptake 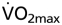 and enhances endurance performance (1–6). Little, however, is known regarding the impact of [Hb] alteration over a wide-ranging Htc. Augmented [Hb] values are associated with a rise in blood viscosity and, consequently, with a higher peripheral vascular resistance that may reduce
and enhances endurance performance (1–6). Little, however, is known regarding the impact of [Hb] alteration over a wide-ranging Htc. Augmented [Hb] values are associated with a rise in blood viscosity and, consequently, with a higher peripheral vascular resistance that may reduce  because of the falling cardiac output (7, 8). Accordingly, the exercise capacity of polycythemic patients with chronic obstructive pulmonary disease (COPD) is improved after hemodilution (9). Because of these counteracting effects, it was suggested that the optimal Htc may be lower than expected because of limitations induced by a higher blood viscosity (8, 10–12). Although the role of Htc on exercise performance seems obvious, there is only one ex vivo study available addressing the above hypothesis in isolated higher vertebrate muscles (11). It should be noted, however, that this may vary under different circumstances because of the blood's non-Newtonian behavior (13). Factors affecting this variation include the species, the organs involved, and whether the organism is in resting or exercising conditions (11, 12, 14–16). Thus, the above mentioned observations do not always reflect the general situation in exercising mammals and humans.
because of the falling cardiac output (7, 8). Accordingly, the exercise capacity of polycythemic patients with chronic obstructive pulmonary disease (COPD) is improved after hemodilution (9). Because of these counteracting effects, it was suggested that the optimal Htc may be lower than expected because of limitations induced by a higher blood viscosity (8, 10–12). Although the role of Htc on exercise performance seems obvious, there is only one ex vivo study available addressing the above hypothesis in isolated higher vertebrate muscles (11). It should be noted, however, that this may vary under different circumstances because of the blood's non-Newtonian behavior (13). Factors affecting this variation include the species, the organs involved, and whether the organism is in resting or exercising conditions (11, 12, 14–16). Thus, the above mentioned observations do not always reflect the general situation in exercising mammals and humans.
The optimal Htc hypothesis is in disagreement with several studies (17, 18). All of these authors provide evidence that O2 delivery and thus exercise performance, including 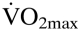 remained relatively constant with chronic excessive erythrocytosis. These findings indicate that adaptive mechanisms to excessive erythrocytosis exist.
remained relatively constant with chronic excessive erythrocytosis. These findings indicate that adaptive mechanisms to excessive erythrocytosis exist.
To explore the consequences of excessive erythrocytosis in vivo we developed a transgenic mouse line (termed tg6) that reaches Htc values of 0.8–0.9 as a result of a constitutive overexpression of human Epo cDNA (19). Adaptive mechanisms to excessive erythrocytosis include increased plasma nitric oxide levels and enhanced erythrocyte flexibility (19, 20). These data suggest that a shift of the optimal Htc for a maximal endurance performance to a higher Htc value occurs in tg6 mice.
The present study tested the hypothesis that there is an optimal Htc value that allows for maximal systemic endurance performance. The effect of chronically elevated Htc on exercise capacity may differ from that of acutely elevated Htc. We propose that mice with excessive erythrocytosis adapt better to varying Htc levels than animals that experience an acute increase in Htc level. For this purpose, wild-type mice were injected with novel erythropoiesis stimulating protein (NESP; wtNESP) to increase Htc, tg6 mice were treated with the hemolysis-inducing compound phenylhydrazine (PHZ; tg6PHZ), and both wild-type and tg6 mice that did not receive treatment served as controls. Metabolic and cardiovascular measurements were obtained at rest and during endurance performance, whereas whole-blood analysis including rheology was carried out at rest.
Results
Male wild-type and tg6 mice were approximately 8 and 9 weeks old, respectively, when beginning their respective treatments. As expected, differences in Htc were initially observed (Table S1): whereas wild-type males had an Htc of 0.46 ± 0.03, the constitutively Epo-overexpressing transgenic tg6 males suffered from excessive erythrocytosis expressed Htc values of 0.78 ± 0.06. No differences in age, resting mean arterial blood pressure, heart rate, O2 uptake 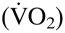 or respiratory exchange ratio (RER) were observed between wild-type and tg6 animals at the beginning of the incremental exercise test.
or respiratory exchange ratio (RER) were observed between wild-type and tg6 animals at the beginning of the incremental exercise test.
The Impact of Htc Manipulation.
The results of wild-type/wtNESP and tg6/tg6PHZ after approximately 4 and 3 weeks of treatment, respectively, are depicted in Fig. S1 and Fig. S2. Both figures show [Hb], arterial O2 saturation (SaO2), plasma, and blood volume in relation to the Htc level of each individual mouse used in this study. As shown in Fig. S1 a, there was a linear increase in [Hb] with greater Htc values in all wild-type and tg6 mice used. Whereas SaO2 (Fig. S1 b) and plasma volume (Fig. S2 a) did not significantly change in any animal, irrespective of treatment and/or genotype, changes in blood volumes paralleled those seen in Htc levels (Fig. S2 b). As is defined via the (degree two) polynomial equation, alterations at lower Htc values had a lesser impact on blood volume changes than those that were observed at higher Htc levels. Indeed, the increment of blood volume at Htc levels from 0.4 to 0.5 was approximately 19 mL/kg for wtNESP and 10 mL/kg for tg6PHZ, whereas the increment between 0.6 and 0.7 was approximately 37 and 50 mL/kg, respectively.
Optimal Htc for Maximal Endurance Performance.
Endurance performance consists of the product of an individual's  and duration of exercise at a certain percentage of that
and duration of exercise at a certain percentage of that  until exhaustion (21). Thus, to investigate the impact of varying Htc levels on endurance performance, individual data of
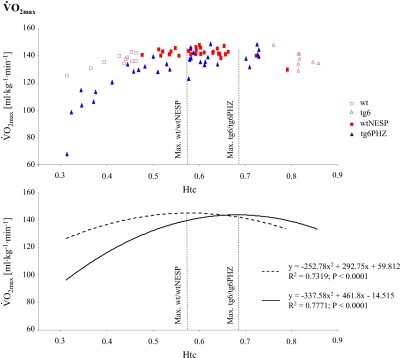
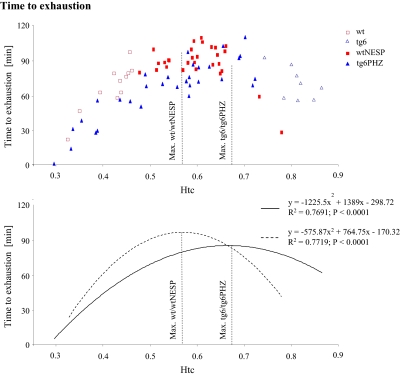
until exhaustion (21). Thus, to investigate the impact of varying Htc levels on endurance performance, individual data of  (Fig. 1) and time to exhaustion (Fig. 2) were plotted against Htc. Time to exhaustion and
(Fig. 1) and time to exhaustion (Fig. 2) were plotted against Htc. Time to exhaustion and  behaved as a polynomial second-degree function in both wild-type and tg6 mice. As such, greater changes in time to exhaustion and
behaved as a polynomial second-degree function in both wild-type and tg6 mice. As such, greater changes in time to exhaustion and  were observed at lower and higher Htc levels. Furthermore, time to exhaustion was more sensitive to Htc alterations than
were observed at lower and higher Htc levels. Furthermore, time to exhaustion was more sensitive to Htc alterations than 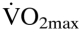 Calculations showed that greatest
Calculations showed that greatest  and time to exhaustion values were obtained at Htc values of 0.58 and 0.57 for wtNESP mice, respectively, and 0.68 and 0.66 for tg6PHZ mice, respectively.
and time to exhaustion values were obtained at Htc values of 0.58 and 0.57 for wtNESP mice, respectively, and 0.68 and 0.66 for tg6PHZ mice, respectively.
Fig. 1.
Relationship between hematocrit (Htc) and maximal O2 uptake 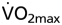 in wtNESP and tg6PHZ mice. Single prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ. Also depicted are maximal
in wtNESP and tg6PHZ mice. Single prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ. Also depicted are maximal 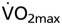 values of wt/wtNESP (Max. wt/wtNESP) and tg6/tg6PHZ (Max. tg6/tg6PHZ).
values of wt/wtNESP (Max. wt/wtNESP) and tg6/tg6PHZ (Max. tg6/tg6PHZ).
Fig. 2.
Relationship between hematocrit (Htc) and time to exhaustion in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ. Also depicted are maximal time to exhaustion values of wt/wtNESP (Max. wt/wtNESP) and tg6/tg6PHZ (Max. tg6/tg6PHZ).
Effect of Varying Blood Volume, Total Hemoglobin Mass, and Viscosity on Endurance Performance.
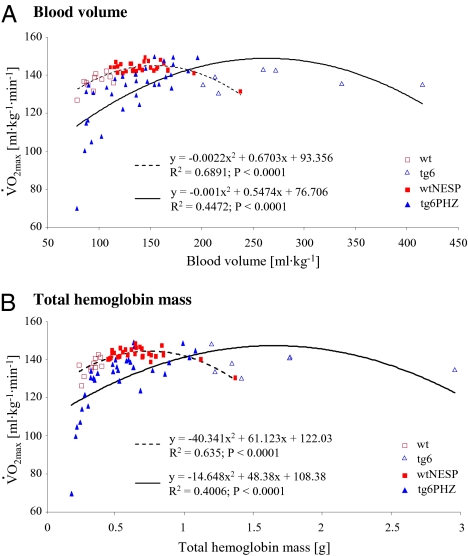
Besides Htc, blood volume and total hemoglobin mass play an important role in cardiovascular performance (2, 3, 22). To determine the blood volume and total hemoglobin mass that are required to reach maximal endurance performance, both parameters were correlated with 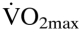 As shown in Fig. 3, when
As shown in Fig. 3, when  of wtNESP and tg6PHZ were expressed as a function of blood volume or total hemoglobin mass, again a polynomial second-degree relation was observed. The graphs illustrate that approximately twice as much blood volume or total hemoglobin mass is necessary to reach maximal
of wtNESP and tg6PHZ were expressed as a function of blood volume or total hemoglobin mass, again a polynomial second-degree relation was observed. The graphs illustrate that approximately twice as much blood volume or total hemoglobin mass is necessary to reach maximal  in tg6PHZ compared to the levels required in wtNESP.
in tg6PHZ compared to the levels required in wtNESP.
Fig. 3.
Relationship between maximal O2 uptake 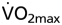 and blood volume (a) and total hemoglobin mass (b) during terminal determination in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ.
and blood volume (a) and total hemoglobin mass (b) during terminal determination in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ.
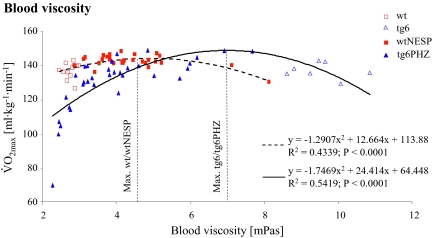
Excessive erythrocytosis is known to impair blood flow because of an Htc-dependent elevation of blood viscosity, and consequently less O2 is transported to the tissue (10). In turn, this reduces exercise capacity. We observed a correlation between  and blood viscosity (Fig. 4). Compared to wtNESP, Epo-overexpressing tg6 mice reached maximal
and blood viscosity (Fig. 4). Compared to wtNESP, Epo-overexpressing tg6 mice reached maximal  at a higher blood viscosity. The association between blood viscosity and Htc in wild-type and tg6 mice was similar with our previous findings (20).
at a higher blood viscosity. The association between blood viscosity and Htc in wild-type and tg6 mice was similar with our previous findings (20).
Fig. 4.
Relationship between maximal O2 uptake 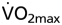 and blood viscosity during terminal determination in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ. Also depicted are maximal
and blood viscosity during terminal determination in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ. Also depicted are maximal 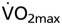 values of wt/wtNESP (Max. wt/wtNESP) and tg6/tg6PHZ (Max. tg6/tg6PHZ).
values of wt/wtNESP (Max. wt/wtNESP) and tg6/tg6PHZ (Max. tg6/tg6PHZ).
Increasing Mean Arterial Blood Pressure, Constant Heart Rate, and Altered Stroke Volume with Rising Htc Levels at 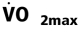
Mean arterial blood pressure, heart rate, and stroke volume were all quantified at 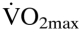 Mean arterial blood pressure rose with increasing Htc levels in wtNESP and tg6PHZ (Fig. S3 a). Overall, wtNESP reached higher mean arterial blood pressure values compared to those in tg6PHZ. Heart rate did not change with increasing Htc levels and also was not different from group to group (Fig. S3 b).
Mean arterial blood pressure rose with increasing Htc levels in wtNESP and tg6PHZ (Fig. S3 a). Overall, wtNESP reached higher mean arterial blood pressure values compared to those in tg6PHZ. Heart rate did not change with increasing Htc levels and also was not different from group to group (Fig. S3 b).
Previous studies have identified a correlation between stroke volume and O2 pulse during exercise in humans (23, 24). Fig. S3 c shows O2 pulse as a function of Htc at 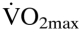 The O2 pulse of wtNESP and tg6PHZ rises slightly with increasing Htc levels, plateaus at respective maximum Htc values of 0.58 for wild-type mice and 0.68 for tg6 mice, and then continues to decreases with higher values. Fitted curves display polynomial second-degree characteristics. In comparison with wtNESP, tg6PHZ showed a greater impact of varying Htc levels on O2 pulse.
The O2 pulse of wtNESP and tg6PHZ rises slightly with increasing Htc levels, plateaus at respective maximum Htc values of 0.58 for wild-type mice and 0.68 for tg6 mice, and then continues to decreases with higher values. Fitted curves display polynomial second-degree characteristics. In comparison with wtNESP, tg6PHZ showed a greater impact of varying Htc levels on O2 pulse.
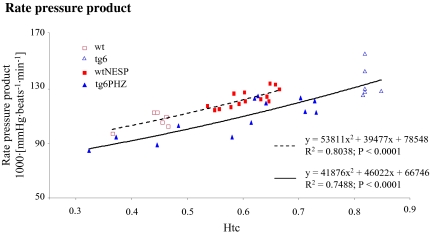
Increasing Myocardial 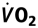 with Rising Htc Levels at
with Rising Htc Levels at 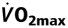
To study the impact of myocardial 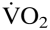 on
on 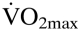 the rate pressure product was also correlated with Htc levels. Myocardial
the rate pressure product was also correlated with Htc levels. Myocardial 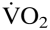 increased with increasing Htc values in both groups (Fig. 5). Both graphs showed a similar slope, but wtNESP mice had higher rate pressure product values at corresponding Htc levels than did tg6PHZ, indicating that the hearts of wtNESP mice had a higher requirement of myocardial O2 supply.
increased with increasing Htc values in both groups (Fig. 5). Both graphs showed a similar slope, but wtNESP mice had higher rate pressure product values at corresponding Htc levels than did tg6PHZ, indicating that the hearts of wtNESP mice had a higher requirement of myocardial O2 supply.
Fig. 5.
Relationship between hematocrit (Htc) and rate pressure product pulse at maximal O2 uptake 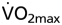 in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ.
in wtNESP and tg6PHZ mice. Singles prints represent individual values. ---, regression plot of wild-type (wt)/wtNESP; —, regression plot of tg6/tg6PHZ.
Discussion
The present study demonstrates that optimal Htc values maximizing systemic endurance performance in mammals exist. In doing so we observed that NESP-treated wild-type animals maximized exercise performance at a lower optimal Htc than hemolysed tg6PHZ mice constitutively overexpressing Epo. Furthermore, the data demonstrated that (i) the optimal Htc levels for maximal systemic exercise performance and maximal stroke volume were similar; and (ii) blood volumes were dramatically increased at higher Htc levels.
 as well as time to exhaustion as well as initially rose, reached a maximum value, and then decreased with increasing Htc values. Maximal
as well as time to exhaustion as well as initially rose, reached a maximum value, and then decreased with increasing Htc values. Maximal  and time to exhaustion values were found at Htc levels of 0.58 and 0.57 for wtNESP, and 0.68 and 0.66 for tg6PHZ, respectively. Our data are in agreement with the classical optimal Htc hypothesis (8, 10–12, 25), where it was speculated that the optimal systemic Htc of mammals would be much higher than the commonly observed 0.45. These calculations were based on the conditions present in the circulatory system at rest, as exercise induces changes in vessel diameters, blood flow, internal temperature, and blood distribution. However, before this study there was no experimental proof of this concept in living mammals. Gaethgens and coworkers (11) studied the effect of blood perfusion creating various Htc levels in isolated dog muscle during rhythmic isotonic exercise. Maximal
and time to exhaustion values were found at Htc levels of 0.58 and 0.57 for wtNESP, and 0.68 and 0.66 for tg6PHZ, respectively. Our data are in agreement with the classical optimal Htc hypothesis (8, 10–12, 25), where it was speculated that the optimal systemic Htc of mammals would be much higher than the commonly observed 0.45. These calculations were based on the conditions present in the circulatory system at rest, as exercise induces changes in vessel diameters, blood flow, internal temperature, and blood distribution. However, before this study there was no experimental proof of this concept in living mammals. Gaethgens and coworkers (11) studied the effect of blood perfusion creating various Htc levels in isolated dog muscle during rhythmic isotonic exercise. Maximal 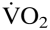 and contractile power reached a plateau at Htc levels between 0.4 and 0.7. The authors concluded that these results may not be transferable to whole-body systemic exercise because each organ may present with its own individual optimal Htc (11, 16). Therefore, the systemic Htc value must be interpreted as an average of all organ-specific optimal Htc values, thereby providing the whole organism with adequate O2 supply. During exercise more O2 is required by the working skeletal muscles and thus a shift from the optimal physiological to the optimal Htc value for maximal endurance performance is observed. To cover their high O2 demand during strenuous exercise, some terrestrial vertebrate species, such as horses and dogs, but not mice, release stored erythrocytes into the circulation by splenic contraction (26, 27). This mechanism elevates the blood O2-carrying capacity during exercise within seconds. As a consequence, Htc increases from ≈0.4 at rest to ≈0.6 at exercise (28, 29) facilitating an improvement in exercise performance. These hematopoietic parameters return quickly to resting values when the animals stop exercising, preventing a constant overload of the cardiovascular system (28–30). In light of our data, it is tempting to speculate that horses and dogs temporarily elevate their Htc levels close to the optimal value Htc to reach maximal systemic endurance performance. Indeed, splenic contraction has also been observed in humans during performing a maximal exercise test, as evidenced by a reduction in its volume and two-thirds decrease in splenic erythrocyte content, but Htc levels increased only a very small percentage from rest to maximal working levels (31).
and contractile power reached a plateau at Htc levels between 0.4 and 0.7. The authors concluded that these results may not be transferable to whole-body systemic exercise because each organ may present with its own individual optimal Htc (11, 16). Therefore, the systemic Htc value must be interpreted as an average of all organ-specific optimal Htc values, thereby providing the whole organism with adequate O2 supply. During exercise more O2 is required by the working skeletal muscles and thus a shift from the optimal physiological to the optimal Htc value for maximal endurance performance is observed. To cover their high O2 demand during strenuous exercise, some terrestrial vertebrate species, such as horses and dogs, but not mice, release stored erythrocytes into the circulation by splenic contraction (26, 27). This mechanism elevates the blood O2-carrying capacity during exercise within seconds. As a consequence, Htc increases from ≈0.4 at rest to ≈0.6 at exercise (28, 29) facilitating an improvement in exercise performance. These hematopoietic parameters return quickly to resting values when the animals stop exercising, preventing a constant overload of the cardiovascular system (28–30). In light of our data, it is tempting to speculate that horses and dogs temporarily elevate their Htc levels close to the optimal value Htc to reach maximal systemic endurance performance. Indeed, splenic contraction has also been observed in humans during performing a maximal exercise test, as evidenced by a reduction in its volume and two-thirds decrease in splenic erythrocyte content, but Htc levels increased only a very small percentage from rest to maximal working levels (31).
At first glance, the observed relationship between  and Htc in our study is in contradiction to others described in human studies. Several investigators have shown that there is a strong correlation between
and Htc in our study is in contradiction to others described in human studies. Several investigators have shown that there is a strong correlation between  and total hemoglobin mass or blood volume (2, 3, 22), but not between
and total hemoglobin mass or blood volume (2, 3, 22), but not between  and Htc (32). It is of note, however, that most human studies are carried out with Htc levels between 0.4 and 0.5. Thus, the ergogenic effect of total hemoglobin mass and blood volume as the primary limiting factors of performance in endurance sports seems only to be valid in the physiologically occurring Htc range. At higher Htc levels, other factors, such as blood viscosity, may counteract the positive effects of enhanced arterial O2 content because the total hemoglobin mass, unlike to Htc or [Hb], cannot be masked by other factors. In agreement with this hypothesis, we found that the closest relationship between
and Htc (32). It is of note, however, that most human studies are carried out with Htc levels between 0.4 and 0.5. Thus, the ergogenic effect of total hemoglobin mass and blood volume as the primary limiting factors of performance in endurance sports seems only to be valid in the physiologically occurring Htc range. At higher Htc levels, other factors, such as blood viscosity, may counteract the positive effects of enhanced arterial O2 content because the total hemoglobin mass, unlike to Htc or [Hb], cannot be masked by other factors. In agreement with this hypothesis, we found that the closest relationship between  and total hemoglobin mass in wtNESP animals is within the Htc range of 0.4 and 0.55 (R2 = 0.513; P < 0.001).
and total hemoglobin mass in wtNESP animals is within the Htc range of 0.4 and 0.55 (R2 = 0.513; P < 0.001).
The augmented blood volume at higher Htc values observed in tg6 mice is caused by the dramatically increased number of erythrocytes, whereas plasma volume remains unchanged (19). An elevation in blood volume enhances end-diastolic volume (preload) and results in increased stroke volume via the Starling mechanism (33), which will lead to the enhancement of  as long as heart rate is not altered. However, after reaching a maximum of 0.57 for wtNESP and 0.68 for tg6PHZ, the blood viscosity may have a negative contribution to cardiac performance. High blood viscosity increases arterial blood pressure and diminishes venous return because of the increased peripheral resistance (12, 34), a fact that may reduce stroke volume and thus exercise performance. A converse observation is made in patients suffering from polycythemic COPD after phlebotomy (9). In these patients, an improvement in exercise tolerance appears to be due to an improvement in cardiac function as evidenced, primarily, by an increased stroke volume. The close correlation between
as long as heart rate is not altered. However, after reaching a maximum of 0.57 for wtNESP and 0.68 for tg6PHZ, the blood viscosity may have a negative contribution to cardiac performance. High blood viscosity increases arterial blood pressure and diminishes venous return because of the increased peripheral resistance (12, 34), a fact that may reduce stroke volume and thus exercise performance. A converse observation is made in patients suffering from polycythemic COPD after phlebotomy (9). In these patients, an improvement in exercise tolerance appears to be due to an improvement in cardiac function as evidenced, primarily, by an increased stroke volume. The close correlation between  and blood viscosity, as well as between stroke volume, arterial blood pressure, and Htc, confirms this explanation. Moreover, the fact that rate pressure product and arterial blood pressures were increasing with incremental elevation of Htc levels shows that
and blood viscosity, as well as between stroke volume, arterial blood pressure, and Htc, confirms this explanation. Moreover, the fact that rate pressure product and arterial blood pressures were increasing with incremental elevation of Htc levels shows that  was unaffected by the maximal work capacity of the heart at optimal Htc.
was unaffected by the maximal work capacity of the heart at optimal Htc.
The different optimal Htc levels obtained by increasing the normal physiological Htc of wild-type mice and reducing the elevated Htc of tg6 mice could be resolved by at least two mechanisms: (i) enhancement of endothelial nitric oxide synthase activity, which results in peripheral vasodilation despite concomitant increased endothelial-1 levels (19, 35), and (ii) regulated elevation of blood viscosity by increasing erythrocyte flexibility in tg6 mice compared to their controls (20). Both mechanisms would induce a shift to a higher optimal Htc value in the tg6 mice. Thus, tg6PHZ might be able to adapt better to varying Htc levels than wtNESP. Physiological adaptations to excessive erythrocytosis are also observed in dogs and humans (17, 18). Moreover, one case report in sport medicine describes a successful Finnish cross-country skier with an autosomal dominant mutation in Epo receptor that resulted in increased sensitivity of erythroid progenitors to Epo that ultimately led to Htc levels of 0.68 (17). That endurance athlete won several Olympic gold medals. Based on our study, we conclude that his Htc may be very close to the optimal Htc for maximal endurance performance.
In summary, the results of the present study confirm the optimal Htc hypothesis during systemic exercise in mice. The reason for this is that blood viscosity increases with rising Htc levels, limiting the blood's O2 transport capacity. Furthermore, animals with chronic excessive erythrocytosis adapted better to different Htc levels than did acutely NESP-injected animals. At normoxia, the heart can tolerate higher rate pressure product at higher Htc levels. Thus, the optimal Htc values for maximal endurance performance were not caused by heart failure or attainment of the maximal working capacity of the heart,  is mainly limited by O2 delivery.
is mainly limited by O2 delivery.
Materials and Methods
Experimental Animals and Set-up.
The constitutively Epo-overexpressing tg6 mouse line was generated as described in ref. 19. Compared to wild-type control, the tg6 mouse line had a 10- to 12-fold increase in plasma Epo-levels, resulting in Htc levels of up to 0.9 (19, 20). Approximately half of the offspring were hemizygous for the transgene and were used for the hemolysis-inducing experiments, whereas the other half were used as wild type for the hemoconcentration experiments. Males were 12 weeks old during the first exercise test (Table S1). In total, 41 wild-type mice and 40 tg6 mice were analyzed. No weight loss occurred during the study period. Mice were kept in standard rodent cages (T3) with food and water supplied ad libitum in a 12 h/12 h light-dark cycle. The experimental protocols were approved by the Kantonales Veterinäramt Zürich and were performed in accordance with the Swiss animal protection laws and institutional guidelines.
The experimental design is shown in Fig. S4. At an age of 3 weeks, only tg6 mice were splenectomized to keep Htc levels low, because extramedullary erythropoiesis occurs in the spleen (20). One week later, telemetric blood pressure transmitters were implanted in 20 wild-type mice and 19 tg6 mice that were 4 weeks old. In the remaining animals, dummy transmitters were implanted (wild type, n = 21; tg6, n = 21). Adjustments of the Htc levels were started in 8- and 9-week-old animals, respectively. At an age of 12 weeks, the main experiments were conducted, including incremental as well as constant workload exercise tests (see below) followed by measurements of the SaO2, [Hb], Htc, blood viscosity, plasma, and blood volume. To exclude the impact of circadian rhythm, all measurements were performed at the identical time of day.
Other detailed methods are provided in SI Materials and Methods.
Statistics.
All data were analyzed by using StatView software (Version 4.57; Abacus Concepts). The relationship between the two parameters was analyzed with linear or polynomial regression. Significances were performed by a one-way analysis of variance (ANOVA). Results are expressed as mean ± SD. Statistical difference was set at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by the Forschungskredit (University of Zurich) and the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912924107/DCSupplemental.
References
- 1.Ekblom B, Berglund B. Effect of erythropoietin administration on mammal aerobic power. Scand J Med Sci Sports. 1991;1:88–93. [Google Scholar]
- 2.Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol. 1968;25:619–625. doi: 10.1152/jappl.1968.25.5.619. [DOI] [PubMed] [Google Scholar]
- 3.Kanstrup IL, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc. 1984;16:256–262. [PubMed] [Google Scholar]
- 4.Lundby C, et al. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RJ, et al. Effect of simulated altitude erythrocythemia in women on hemoglobin flow rate during exercise. J Appl Physiol. 1988;64:1644–1649. doi: 10.1152/jappl.1988.64.4.1644. [DOI] [PubMed] [Google Scholar]
- 6.Turner DL, et al. Limitations to VO2max in humans after blood retransfusion. Respir Physiol. 1993;92:329–341. doi: 10.1016/0034-5687(93)90017-5. [DOI] [PubMed] [Google Scholar]
- 7.Connes P, Yalcin O, Baskurt O, Brun JF, Hardeman M. In health and in a normoxic environment, VO2 max is/is not limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:2099. doi: 10.1152/japplphysiol.00279.2006. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC, Richardson TQ. Effect of hematocrit on venous return. Circ Res. 1961;9:157–164. doi: 10.1161/01.res.9.1.157. [DOI] [PubMed] [Google Scholar]
- 9.Chetty KG, Brown SE, Light RW. Improved exercise tolerance of the polycythemic lung patient following phlebotomy. Am J Med. 1983;74:415–420. doi: 10.1016/0002-9343(83)90960-9. [DOI] [PubMed] [Google Scholar]
- 10.Crowell JW, Smith EE. Determinant of the optimal hematocrit. J Appl Physiol. 1967;22:501–504. doi: 10.1152/jappl.1967.22.3.501. [DOI] [PubMed] [Google Scholar]
- 11.Gaehtgens P, Kreutz F, Albrecht KH. Optimal hematocrit for canine skeletal muscle during rhythmic isotonic exercise. Eur J Appl Physiol Occup Physiol. 1979;41:27–39. doi: 10.1007/BF00424466. [DOI] [PubMed] [Google Scholar]
- 12.Villafuerte FC, Cárdenas R, Monge-C C. Optimal hemoglobin concentration and high altitude: a theoretical approach for Andean men at rest. J Appl Physiol. 2004;96:1581–1588. doi: 10.1152/japplphysiol.00328.2003. [DOI] [PubMed] [Google Scholar]
- 13.El-Sayed MS, Ali N, El-Sayed Ali Z. Haemorheology in exercise and training. Sports Med. 2005;35:649–670. doi: 10.2165/00007256-200535080-00001. [DOI] [PubMed] [Google Scholar]
- 14.Connes P, et al. Is hemoglobin desaturation related to blood viscosity in athletes during exercise? Int J Sports Med. 2004;25:569–574. doi: 10.1055/s-2004-821118. [DOI] [PubMed] [Google Scholar]
- 15.Kusunoki M, et al. Effects of hematocrit variations on cerebral blood flow and oxygen transport in ischemic cerebrovascular disease. J Cereb Blood Flow Metab. 1981;1:413–417. doi: 10.1038/jcbfm.1981.45. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Heros RC, Mullan JC, Korosue K. Optimum degree of hemodilution for brain protection in a canine model of focal cerebral ischemia. J Neurosurg. 1994;80:469–475. doi: 10.3171/jns.1994.80.3.0469. [DOI] [PubMed] [Google Scholar]
- 17.Juvonen E, Ikkala E, Fyhrquist F, Ruutu T. Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood. 1991;78:3066–3069. [PubMed] [Google Scholar]
- 18.Lindenfeld J, Weil JV, Travis VL, Horwitz LD. Hemodynamic response to normovolemic polycythemia at rest and during exercise in dogs. Circ Res. 1985;56:793–800. doi: 10.1161/01.res.56.6.793. [DOI] [PubMed] [Google Scholar]
- 19.Ruschitzka FT, et al. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci USA. 2000;97:11609–11613. doi: 10.1073/pnas.97.21.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel J, et al. Transgenic mice overexpressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102:2278–2284. doi: 10.1182/blood-2003-01-0283. [DOI] [PubMed] [Google Scholar]
- 21.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Åstrand P-O. Experimental Studies of Physical Working Capacity in Relation to Sex and Age. Copenhagen: Munksgaard; 1952. [Google Scholar]
- 23.Bhambhani YN. Prediction of stroke volume during upper and lower body exercise in men and women. Arch Phys Med Rehabil. 1995;76:713–718. doi: 10.1016/s0003-9993(95)80524-9. [DOI] [PubMed] [Google Scholar]
- 24.Crisafulli A, et al. Estimating stroke volume from oxygen pulse during exercise. Physiol Meas. 2007;28:1201–1212. doi: 10.1088/0967-3334/28/10/006. [DOI] [PubMed] [Google Scholar]
- 25.Buick FJ, Gledhill N, Froese AB, Spriet L, Meyers EC. Effect of induced erythrocythemia on aerobic work capacity. J Appl Physiol. 1980;48:636–642. doi: 10.1152/jappl.1980.48.4.636. [DOI] [PubMed] [Google Scholar]
- 26.Dane DM, et al. Splenectomy impairs diffusive oxygen transport in the lung of dogs. J Appl Physiol. 2006;101:289–297. doi: 10.1152/japplphysiol.01600.2005. [DOI] [PubMed] [Google Scholar]
- 27.Fedde MR, Wood SC. Rheological characteristics of horse blood: significance during exercise. Respir Physiol. 1993;94:323–335. doi: 10.1016/0034-5687(93)90027-8. [DOI] [PubMed] [Google Scholar]
- 28.Wagner PD, et al. Effects of altered FIO2 on maximum VO2 in the horse. Respir Physiol. 1996;105:123–134. doi: 10.1016/0034-5687(96)00044-8. [DOI] [PubMed] [Google Scholar]
- 29.Wu EY, Ramanathan M, Hsia CC. Role of hematocrit in the recruitment of pulmonary diffusing capacity: comparison of human and dog. J Appl Physiol. 1996;80:1014–1020. doi: 10.1152/jappl.1996.80.3.1014. [DOI] [PubMed] [Google Scholar]
- 30.York EL, Jones RL, Menon D, Sproule BJ. Effects of secondary polycythemia on cerebral blood flow in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1980;121:813–818. doi: 10.1164/arrd.1980.121.5.813. [DOI] [PubMed] [Google Scholar]
- 31.Laub M, et al. Spleen emptying and venous hematocrit in humans during exercise. J Appl Physiol. 1993;74:1024–1026. doi: 10.1152/jappl.1993.74.3.1024. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt W, et al. Blood volume and hemoglobin mass in endurance athletes from moderate altitude. Med Sci Sports Exerc. 2002;34:1934–1940. doi: 10.1097/00005768-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Vatner SF, Franklin D, Higgins CB, Patrick T, Braunwald E. Left ventricular response to severe exertion in untethered dogs. J Clin Invest. 1972;51:3052–3060. doi: 10.1172/JCI107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson TQ, Guyton AC. Effects of polycythemia and anemia on cardiac output and other circulatory factors. Am J Physiol. 1959;197:1167–1170. [Google Scholar]
- 35.Quaschning T, et al. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. FASEB J. 2003;17:259–261. doi: 10.1096/fj.02-0296fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.