Abstract
Chromosome translocations between Ig (Ig) and non-Ig genes are frequently associated with B-cell lymphomas in humans and mice. The best characterized of these is c-myc/IgH translocation, which is associated with Burkitt’s lymphoma. These translocations are caused by activation-induced cytidine deaminase (AID), which produces double-strand DNA breaks in both genes. c-myc/IgH translocations are rare events, in part because ATM, p53, and p19 actively suppress them. To further define the mechanism of protection against the accumulation of cells that bear c-myc/IgH translocation, we assayed B cells from mice that carry mutations in cell-cycle and apoptosis regulator proteins that act downstream of p53. We find that PUMA, Bim, and PKCδ are required for protection against c-myc/IgH translocation, whereas Bcl-XL and BAFF enhance c-myc/IgH translocation. Whether these effects are general or specific to c-myc/IgH translocation and whether AID produces dsDNA breaks in genes other than c-myc and Ig is not known. To examine these questions, we developed an assay for translocation between IgH and Igβ, both of which are somatically mutated by AID. Igβ/IgH, like c-myc/IgH translocations, are AID-dependent, and AID is responsible for lesions on IgH and the non-IgH translocation partners. However, ATM, p53, and p19 do not protect against Igβ/IgH translocations. Instead, B cells are protected against Igβ/IgH translocations by a BAFF- and PKCδ-dependent pathway. We conclude that AID-induced double-strand breaks in non-Ig genes other than c-myc lead to their translocation, and that at least two nonoverlapping pathways protect against translocations in primary B cells.
Keywords: B lymphocyte, c-myc, cancer, immunoglobulin
Mature B-cell lymphomas are frequently associated with chromosomal translocations between Ig loci and non-Ig genes. The latter include c-myc in Burkitt’s lymphoma, bcl-2 in follicular lymphoma, bcl-6 in diffuse large-cell lymphoma, and FGFR in multiple myeloma (1–3). These translocations are believed to be important for malignant transformation because they can deregulate the transcription of oncogenes by placing them under the control of the Ig transcriptional elements.
Translocations are thought to be particularly prevalent in B-cell lymphomas because B cells undergo a series of DNA remodeling reactions that involve obligate double-strand breaks (DSBs) in Ig loci. V(D)J recombination is the first of these reactions, and is mediated by the RAG1/2 endonuclease (4, 5). This enzyme produces paired hairpin and blunt DNA ends when it cuts recombination signal sequences during antigen receptor gene assembly in developing B-cell precursors (6, 7). Class switch recombination (CSR) and somatic hypermutation (SHM) remodel Ig genes in mature B cells activated during immune responses. CSR is a DNA recombination reaction that alters the effector function of the antibody by replacing one constant region with another without altering the antigen-binding variable region. In contrast to V(D)J recombination, CSR is not sequence-specific but instead region-specific, resulting in recombination between repetitive DNA elements found 5′ of Ig constant region genes (8). SHM changes the affinity of the antibody by introducing nontemplated nucleotide changes in the variable region of the antibody gene. Although CSR and SHM are mechanistically distinct, both processes are initiated by activation-induced cytidine deaminase (AID) (9–11). AID deaminates cytosine residues in ssDNA that are exposed during transcription, thereby producing U:G mismatches in target DNA (12–16). The resulting lesion is processed by base excision repair and/or mismatch repair enzymes to create mutations or DSBs (8, 17, 18).
Unlike RAG1/2, which targets well-defined recombination signal sequences embedded in Ig variable, diversity, and joining gene segments, AID has a preference for the RGYW motif but can mutate nearly any cytosine residue (18, 19). This lack of specificity is essential to AID’s function because it allows the enzyme to introduce mutations in multiple locations in large families of Ig heavy-chain, κ, and λ variable genes, thereby maximizing the probability of mutations that enhance antibody affinity. However, this lack of strong nucleotide specificity also makes AID a dangerous enzyme, because of its potential to damage DNA throughout the genome.
AID targets Igs preferentially; however, it produces low levels of mutations in multiple non-Ig loci including several important B-cell cancer-associated genes such as Pim1, Pax5, Rhoh, Bcl-6, and fas (20–24). Indeed, a recent survey suggested that up to 25% of the genes expressed in B cells may be mutated by AID, but the mutational load in these genes is 20- to 100-fold lower than those seen in Igs (25).
In addition to somatic mutations, AID also produces double-strand breaks in IgH and at c-myc (26). As a result, AID creates substrates for c-myc/IgH translocations (27–29). These translocations are infrequent events under physiologic circumstances because genomic caretakers and checkpoint regulators such as ATM, p19, and p53 actively suppress the emergence of cells carrying deregulated c-myc (28, 30). Whether AID is responsible for dsDNA breaks that lead to translocation by genes other than c-myc, and how these other translocations might be suppressed, has not been determined.
Here we show that AID mediates the formation of lesions that result in translocations between IgH and Igβ. These novel translocations are not suppressed by ATM, p53, or p19, but instead by a PKCδ-dependent pathway that can be activated by BAFF. The PKCδ-dependent pathway also suppresses c-myc/IgH translocation.
Results
Protection Against c-myc/IgH Translocations.
c-myc/IgH translocation is an infrequent event in activated B cells that is increased in the absence of ATM or p53 (28). ATM can phosphorylate p53 directly or through activation of Chk2 (31–36). To evaluate the role of Chk2 in protecting cells from c-myc/IgH translocations, we analyzed the frequency of these events in lipopolysacharide (LPS)- and IL4-stimulated Chk2−/− B cells (36). Chk2-deficient B cells were indistinguishable from controls in terms of cell division as measured by CFSE [5- (and 6-) carboxyfluorescein diacetate succinyl ester] dye dilution and switching to IgG1 (Fig. 1A). However, loss of Chk2 increased the frequency of c-myc/IgH translocations 6-fold compared to wild type (P = 0.0237), which is ?30% of the level found in p53−/− B cells (Fig. 1B). We conclude that Chk2 is required for optimal protection of B cells from c-myc/IgH translocations and, like p53, it has no significant effect on class switch recombination.
Fig. 1.
CSR and c-myc/IgH translocation in mutant B cells. (A) Flow cytometric analysis of CSR to IgG1 and cell division by dilution of CFSE in p53S18A/23A mutant, Chk−/−, Puma−/−, and p21−/− B cells activated with LPS and IL4 for 72 h. Graphs show percentage of switching relative to matched wild-type controls and/or relative to B cells stimulated with RP105 to induce cell division but not CSR. Each bar represents the mean value of two or more independent experiments. (B) Graph shows the number of Southern blot-confirmed c-myc/IgH translocations per 106 cells. Each value was obtained from independent samples from at least two mice in which a total of 10–20 million cells were assayed.
ATM and Chk2 stabilize p53 by serine phosphorylation (37). Phosphorylation of p53S18 is required for maximum activation of p53 and plays a role in activating DNA damage-induced apoptosis (38–40), whereas phosphorylation of p53S23 is believed to be required for p53 function in only certain cell types (41). Finally, combined phosphorylation of p53S18/S23 is essential for p53-dependent IR-induced apoptosis and tumor suppression (38). To determine whether these p53 phosphorylation sites are required for protection against the accumulation of cells that bear c-myc/IgH translocations, we analyzed B cells from mutant mice in which serines 18 and 23 have been replaced by alanines (p53S18A/23A) (38). These mutations did not alter B-cell division as measured by CFSE dye dilution, but p53S18A/23A B cells showed a small decrease in CSR and increased c-myc/IgH translocation to a level that was comparable to loss of Chk2 (P = 0.0068) (Fig. 1B). Thus, phosphorylation of p53 residues S18 and/or S23 is required for protection against the accumulation of cells that bear newly arising c-myc/IgH translocations in stimulated B cells in vitro, but phosphorylation of these residues does not account for the full effect of p53.
Activation of p53 induces apoptosis through PUMA, and produces cell-cycle arrest by activating the cell-cycle inhibitor p21 (42–44). To determine whether the protective effects of p53 on c-myc/IgH translocations are achieved through apoptosis or cell-cycle arrest or both, we analyzed CSR and c-myc/IgH translocation in LPS- and IL4-activated PUMA- and p21-deficient B cells. PUMA−/− and p21−/− B cells were similar in that neither altered CSR or cell division significantly (Fig. 2A). However, loss of PUMA increased the frequency of translocation whereas loss of p21 did not (Fig. 1B). We conclude that PUMA is required for optimal protection against c-myc/IgH translocation in activated B cells.
Fig. 2.
Class switch recombination and c-myc/IgH translocations in Bcl-2 family mutant B cells. (A) Flow cytometric analysis of cell division by CFSE dye dilution and CSR in LPS and IL4 stimulated B cells from wild-type, Bcl-2 transgenic, Bcl-XL transgenic, and Bim−/− mice. Graphs show percentage of CSR relative to matched controls. (B) Graph represents the number of c-myc/IgH translocations per 106 cells. Each value was obtained from three independent samples with a total of 10–20 million cells assayed.
PUMA is a Bcl-2 family member that induces apoptosis by binding to and inhibiting prosurvival Bcl-2 family members (45). To determine whether persistent activation of Bcl-2 or Bcl-XL is sufficient to protect against c-myc/IgH translocation, we analyzed activated B cells from Bcl-2 and Bcl-XL transgenic mice (46, 47). Consistent with their prosurvival function, deregulated expression of Bcl-2 or Bcl-XL increased the number of live cells that failed to divide or underwent a small number of cell divisions in response to LPS + IL4 (Fig. 2A). In addition, we found a decrease in switching to IgG1 in Bcl-2 but not Bcl-XL transgenic B cells. However, only Bcl-XL increased the frequency of c-myc/IgH translocations (Fig. 2B). We conclude that deregulated expression of Bcl-XL can counteract the normal protective effects of p53 and enhance the survival of B cells that undergo c-myc/IgH translocation.
Like PUMA, Bim is a proapoptotic Bcl-2 family member whose deletion accelerates lymphomagenesis in Eμ-myc transgenic mice (48). To determine whether loss of Bim also enhances c-myc/IgH translocation, we assayed LPS- and IL4-activated Bim−/− B cells. Loss of Bim produced a decrease in CSR to IgG1 and increased the frequency of c-myc/IgH translocations to the similar degree as loss of PUMA or transgenic expression of Bcl-XL (P = 0.0023) (Fig. 2B). Our results suggest that c-myc/IgH translocation activates p53-induced apoptosis through PUMA and Bim possibly by inhibition of Bcl-XL and Bcl-6 (49).
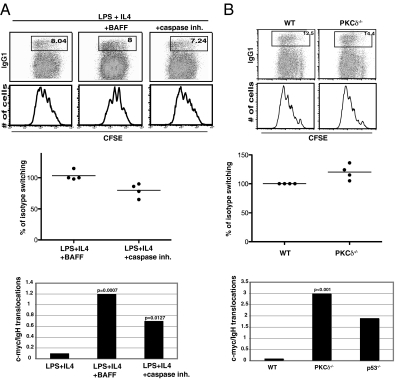
BAFF is an important B-cell cytokine that promotes B-cell survival and whose deregulated expression is associated with autoimmunity and increased incidence of lymphoma (50–52). To determine whether BAFF enhances translocation, we added this cytokine to cultures of LPS- and IL4-stimulated B cells. BAFF did not alter cell division or switching to IgG1, but like transgenic Bcl-2 and Bcl-XL enhanced survival of cells that had undergone fewer divisions (Fig. 3A). BAFF increased the frequency of c-myc/IgH translocation 10-fold (P = 0.0007). Similar to BAFF, addition of caspase inhibitor II resulted in a 7-fold increase in translocation (P = 0.0127) (Fig. 3A). We conclude that BAFF enhances the survival of B cells that carry c-myc/IgH translocation.
Fig. 3.
CSR and c-myc/IgH translocations from BAFF-stimulated wild-type B cells or PKCδ−/− B cells. (A) Flow cytometric analysis of CSR to IgG1, and cell division and c-myc/IgH translocation in wild-type B-cell cultures supplemented with BAFF or caspase inhibitor II (50 μM; Calbiochem). (B) CSR to IgG1 and c-myc/IgH translocations in LPS- and IL4-stimulated PKCδ−/− B cells. Each value was obtained from four independent samples (10 million cells were assayed).
BAFF promotes B-cell survival by activating antiapoptotic responses through NF-κB and by preventing nuclear localization of protein kinase Cδ (PKCδ) (53). Because nuclear accumulation of PKCδ is essential for induction of apoptosis, we analyzed CSR and c-myc/IgH translocations in PKCδ−/− B cells. Although CSR was not affected by the absence of PKCδ, the frequency of c-myc/IgH translocation was dramatically increased (Fig. 3B). We conclude that apoptotic signals that depend on PKCδ play an important role in elimination of B cells with c-myc/IgH translocations.
Igβ/IgH Translocations.
Up to 25% of the 118 genes expressed in germinal center B cells are mutated by AID, but at levels that are 20- to 100-fold lower than Ig (25). For example, mutations in Igβ, an essential signaling component of the B-cell receptor, are found at a rate of 1 × 10−4 whereas mutations in Ig JH4 were present at 1.6 × 10−2 in the same experiments (22, 25).
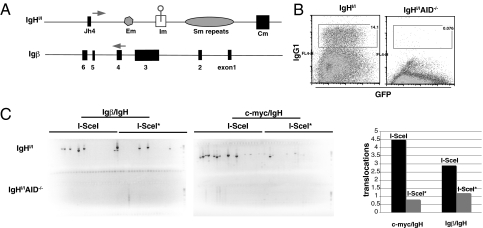
To determine whether AID produces DSBs in Igβ that might lead to chromosomal translocations, we designed a PCR assay for detecting translocations between Igβ and IgH (Fig. 4A). Amplified products were confirmed as authentic Igβ/IgH translocations by sequencing. Translocation breakpoints showed small amounts of microhomology (1–4 bp) and a few nucleotide additions (Table S1). Igβ/IgH translocations appeared at a frequency of 0.4 × 10−6 in wild-type B cells stimulated with LPS and IL4 but were not detected in the absence of AID (Fig. 5A). Thus, in addition to c-myc/IgH translocations, activated B cells also suffer AID-dependent translocations between Igβ and IgH.
Fig. 4.
Igβ/IgH translocations. (A) Schematic representation of Igβ and IgH locus showing the position of the primers used to detect Igβ/IgH translocations. (B) Flow cytometric analysis of CSR to IgG1 from IgHI/I and IgHI/I AID−/− B cells stimulated with LPS and IL4 and infected with a retrovirus carrying I-SceI or I-SceI* and GFP as a marker (26). GFP+ cells were sorted and analyzed for translocations. (C) Translocations by retroviral I-SceI or I-SceI*, which is an inactive mutant form of the enzyme. Ethidium bromide-stained agarose gels with PCR products corresponding to Igβ/IgH and c-myc/IgH translocations as verified by sequencing. Graph represents the comparison of translocation frequencies from two independent experiments in which a total of 10 million cells were assayed.
Fig. 5.
Frequency of Igβ/IgH translocations in mutant B cells. (A) Graphs represent number of translocations per 106 cells from wild-type, AID−/−, ATM−/−, p53−/−, and p19−/− B cells. c-myc/IgH (left) and Igβ/IgH (right) translocation frequency obtained from the same DNA samples. (B) Igβ/IgH translocations in wild-type B cells supplemented with BAFF or PKCδ−/− B cells. Graph represents the comparison of translocation frequencies from three independent samples in which a total of 10 million cells were assayed.
To determine whether AID is the source of dsDNA breaks in Igβ that lead to Igβ/IgH translocations, we made use of mice that carry an I-SceI site in IgH (IgHI/I) (26). I-SceI is an endonuclease that recognizes an 18-bp site that is not normally present in the mouse genome (54). Expression of I-SceI in IgHI/I B cells produces DSBs 5′ of Sμ irrespective of AID expression (26). Similar to c-myc/IgH translocation, I-SceI expression in IgHI/I B cells resulted in high levels of Igβ/IgH translocations (3 × 10−6 Igβ/IgH versus 4.5 × 10−6 c-myc/IgH) (Fig. 4C). In contrast, AID-deficient IgHI/I B cells (IgHI/IAID−/−) assayed under the same conditions were devoid of both c-myc/IgH and Igβ/IgH translocations (Fig. 4C). Thus, AID is required for the DSBs in the Igβ gene that lead to Igβ/IgH translocations in activated B cells.
ATM, p53, and p19 Do Not Protect Against Igβ/IgH Translocations.
ATM, p53, and p19 protect B cells against c-myc/IgH translocation. These factors can be activated directly by any event that is associated with DNA damage before translocation or by c-myc overexpression, which induces the oncogenic stress response after aberrant chromosome joining (55, 56). In contrast, Igβ/IgH translocation should not activate the oncogenic stress pathway. To determine whether ATM, p53, and p19 suppress Igβ/IgH translocations, we compared c-myc/IgH and Igβ/IgH translocations in activated ATM−/−, p53−/−, and p19−/− B cells. Whereas the frequency of c-myc/IgH translocation was increased in the absence of ATM or p53 or p19, we found no increase in the rate of Igβ/IgH translocations in the same samples (Fig. 5A). We conclude that dsDNA breaks that are obligate intermediates in Igβ/IgH translocations fail to activate ATM-p53-p19-dependent cellular responses. The difference between the two types of translocations suggests that activation of ATM, p53, and p19 is mediated by overexpression of c-myc and that the degree of DNA damage incurred during CSR is not sufficient to induce p53-mediated cell death.
Protection Against Translocation by PKCδ.
BAFF promotes B-cell survival by PKCδ- and NF-κB-dependent pathways that are not known to intersect with ATM or the oncogenic stress response (52). To determine the role of this pathway in Igβ/IgH translocations, we analyzed PKCδ−/− or BAFF-treated wild-type B cells. Similar to c-myc/IgH, the frequency of Igβ/IgH translocation was increased by addition of BAFF or in the absence of PKCδ (Fig. 5B). We conclude that PKCδ protects against and that BAFF enhances the survival of B cells that carry either c-myc/IgH or Igβ/IgH translocations.
Discussion
B-cell transformation is the end product of a multistep process that frequently includes chromosomal translocations that activate oncogenes (56). An additional common lesion is inactivation of tumor suppressors such as p53, either directly by mutation or through alterations in its regulators like ATM or Bcl-6 (1). The experiments presented here provide additional insights into molecular requirements for translocation reactions in B lymphocytes and the cellular pathways that protect against these events.
Chromosomal translocations between c-myc and IgH are signature features of Burkitt’s lymphoma (57–64). These translocations, like all others, require formation of double-strand breaks on both partner chromosomes. In the case of c-myc/IgH translocation, AID creates the DSBs on both partners, but the DSBs on c-myc are rare and limit the frequency of translocation (26, 65). This is consistent with the rather low rate of AID-mediated mutation in c-myc and this gene’s propensity for high-fidelity repair (24, 25). But c-myc is only one of many non-Ig genes that are translocated in lymphoma or mutated by AID (1–3, 25). Our experiments show that in addition to c-myc, AID also produces lesions in Igβ that can serve as substrates for translocation to IgH. Thus, AID-induced DSBs are not limited to c-myc or IgH. We speculate that in addition to Igβ, such lesions may occur at many of the other known AID target genes and account for a substantial fraction of the DSBs that lead to lymphoma-associated translocations in Ig and non-Ig genes.
B cells are normally protected against developing AID-induced c-myc/IgH translocations by ATM, p53, and p19, or genes such as Bcl-6 that interfere with p53 induction (28, 30, 49, 66) (Fig. 2C). It is therefore not surprising that the expression of these genes is frequently altered in translocation-bearing lymphomas (67). The p53 pathway might be triggered directly by the DSBs created by AID, or alternatively indirectly by deregulated expression of translocated genes such as c-myc (56). DNA damage-dependent activation of p53 would be expected to suppress any translocation irrespective of the site of the DNA break. In contrast, the oncogenic stress response would only be engaged by the subset of translocations that involve oncogene deregulation. Our experiments show that loss of ATM, or p53 or p19, is not sufficient to alter the frequency of Igβ/IgH translocations, and therefore AID-induced DNA damage alone is insufficient to activate the p53 pathway. In contrast to p53 and p19, ATM is activated during CSR and is required for efficient completion of the reaction (68, 69). Therefore, despite ATM’s activation during CSR, AID-induced DSBs alone fail to prevent accumulation of Igβ/IgH translocations. This disconnect between the ATM-mediated DNA damage response and p53-induced cell death is likely to be necessary for successful CSR; otherwise, all cells that activate ATM while attempting CSR would be eliminated. We conclude that B cells are protected from c-myc/IgH translocations primarily by the p53-mediated oncogenic stress response.
The idea that p53-induced apoptosis is an important mechanism for elimination of B cells that overexpress c-myc as a result of translocation is supported by the observation that deregulated expression of antiapoptotic factors like Bcl-2 or Bcl-XL accelerates lymphoma development in c-myc transgenic mice (70–72). Our experiments demonstrate that cells that develop AID-mediated c-myc/IgH translocations are eliminated by a pathway that requires PUMA, Bim, and caspase activation and can be reversed by overexpression of Bcl-XL but not Bcl-2. The surprising difference between Bcl-XL and Bcl-2 might in part be attributed to a reciprocal expression pattern of these two factors in developing and activated B cells. Whereas Bcl-XL is expressed in pre-B cells, Bcl-2 is expressed in and is crucial for the survival of immature and mature naïve B cells (47, 73), and only Bcl-XL is significantly up-regulated in activated B cells (47). Thus, enforced transgenic expression of Bcl-2 under the control of Ig regulatory elements in the Bcl-2 transgene may account in part for its interference with CSR and inability to protect against accumulation of cells with c-myc/IgH translocation. The p53 pathway is only one of many molecular pathways that regulate survival by inducing apoptosis (30). One of the other key survival pathways in mature B cells is mediated by the tumor necrosis factor (TNF) family of cytokines, which includes BAFF (52). Like loss of p53, deregulated expression of BAFF is associated with lymphomas that carry chromosomal translocations in humans and mice (50, 74, 75). This effect is likely due to the enhanced survival of translocation-bearing B cells when they are stimulated with BAFF (Fig. 3A). More intriguing is the observation that PKCδ, a key mediator of the BAFF signaling pathway, is essential to prevent the survival of B cells that carry both c-myc/IgH and Igβ/IgH translocations. Therefore, in contrast to the p53-mediated pathway, the apoptosis pathway triggered by PKCδ does not require oncogene activation.
Finally, deregulated expression of BAFF is associated with autoimmune diseases such as Sjogren’s syndrome, rheumatoid arthritis, and systemic lupus erythematosus that also show an increase in the incidence of lymphoma (50, 76). BAFF is believed to promote autoimmunity by promoting the survival of autoreactive B cells. We speculate that BAFF-dependent increase in survival of B cells with chromosome translocation may in part account for the link between autoimmunity and lymphoma.
Methods
Mice.
IgHI/I (26); Chk2−/− (36); p53S18A/23A (38); p21−/− (42); PUMA−/− (44); Bcl-XL (47); Bcl-2 (46); PKCδ−/− (77) p19−/− (78); ATM−/− (79) (obtained from the Mouse Models of Human Cancer Consortium); and p53−/− (purchased from Taconic Laboratories) were previously described. All experiments with mice were performed according to protocols approved by the Rockefeller University Animal Care and Use Committee.
B-Cell Cultures and Retroviral Transduction.
B lymphocytes were cultured and transduced with retroviruses as described (28). The I-SceI and mutant control I-SceI* retroviruses were as described (26). BAFF (R&D Systems) was used at a concentration of 50 ng/mL caspase inhibitor II (Calbiochem) was used at a concentration of 50 μM. Cells were analyzed after 3 days of culture unless otherwise indicated.
Flow Cytometry and Cell Sorting.
For flow cytometric analysis, cell suspensions were stained with fluorochrome-conjugated anti-IgG1 (Pharmingen). Data were acquired on a FACSCalibur (Becton Dickinson) and analyzed with FlowJo software (Treestar). CFSE labeling for cell division was at 37°C for 10 min in 5 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes). Lymphoid populations were sorted to >95% purity with a FACSVantage SE with DiVa option or FACSAria instruments (Becton Dickinson).
PCR Assay for Translocations.
PCR reactions for c-myc/IgH translocations were performed as described (28). For Igβ/IgH translocations, nested PCR was done using the following primers. For the Igβ side, the following primers (Igβ exon 4) were used for nested PCR: 5′-AAGTAGCAGGAAGATGGGCAC AATG-3′; 5′-TGAAGAGGATGATGAGGAGGGTCTG-3′. For the IgH side, the following primers upstream of Sm were used for nested PCR: 5′-TGAGGACCAGAGAGGGAT AAAAGAGAA-3′ and 5′-GGGGAGGGGGTGTCAAATAATAAGA-3′; Igβ/IgH translocations were confirmed by direct cloning and sequencing.
Supplementary Material
Acknowledgments
We thank Klara Velinzon for FACSorting and David Bosque for help in managing the mouse colonies. The work was supported in part by an NIH grant to M.C.N. (AI037526). A.N. was supported by the Intramural Research Program of the NIH, National Cancer Institute and the Center for Cancer Research. M.C.N. is an HHMI Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908946107/DCSupplemental.
References
- 1.Küppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 2.Leder P, et al. Translocations among antibody genes in human cancer. Science. 1983;222:765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- 3.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 4.Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- 5.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 6.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 7.Sleckman BP. Lymphocyte antigen receptor gene assembly: Multiple layers of regulation. Immunol Res. 2005;32:253–258. doi: 10.1385/IR:32:1-3:253. [DOI] [PubMed] [Google Scholar]
- 8.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu M, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 11.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 15.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 16.Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 17.Rada C, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 18.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 19.Rogozin IB, Pavlov YI, Bebenek K, Matsuda T, Kunkel TA. Somatic mutation hotspots correlate with DNA polymerase η error spectrum. Nat Immunol. 2001;2:530–536. doi: 10.1038/88732. [DOI] [PubMed] [Google Scholar]
- 20.Shen HM, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- 21.Pasqualucci L, et al. BCL-6 mutations in normal germinal center B cells: Evidence of somatic hypermutation acting outside Ig loci. Proc Natl Acad Sci USA. 1998;95:11816–11821. doi: 10.1073/pnas.95.20.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon MS, Kanegai CM, Doerr JR, Wall R. Somatic hypermutation of the B cell receptor genes B29 (Igβ, CD79b) and mb1 (Igα, CD79a) Proc Natl Acad Sci USA. 2003;100:4126–4131. doi: 10.1073/pnas.0735266100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müschen M, et al. Somatic mutation of the CD95 gene in human B cells as a side-effect of the germinal center reaction. J Exp Med. 2000;192:1833–1840. doi: 10.1084/jem.192.12.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 26.Robbiani DF, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovalchuk AL, et al. AID-deficient Bcl-xL transgenic mice develop delayed atypical plasma cell tumors with unusual Ig/Myc chromosomal rearrangements. J Exp Med. 2007;204:2989–3001. doi: 10.1084/jem.20070882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramiro AR, et al. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramiro AR, et al. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. doi: 10.1038/onc.2008.312. [DOI] [PubMed] [Google Scholar]
- 31.Canman CE, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 32.Banin S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa K, Taya Y, Tamai K, Yamaizumi M. Requirement of ATM in phosphorylation of the human p53 protein at serine 15 following DNA double-strand breaks. Mol Cell Biol. 1999;19:2828–2834. doi: 10.1128/mcb.19.4.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair (Amst) 2004;3:1039–1047. doi: 10.1016/j.dnarep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Hirao A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai H, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 38.Chao C, Herr D, Chun J, Xu Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J. 2006;25:2615–2622. doi: 10.1038/sj.emboj.7601167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borges HL, Chao C, Xu Y, Linden R, Wang JY. Radiation-induced apoptosis in developing mouse retina exhibits dose-dependent requirement for ATM phosphorylation of p53. Cell Death Differ. 2004;11:494–502. doi: 10.1038/sj.cdd.4401366. [DOI] [PubMed] [Google Scholar]
- 40.Sluss HK, Armata H, Gallant J, Jones SN. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol Cell Biol. 2004;24:976–984. doi: 10.1128/MCB.24.3.976-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, et al. Mutation of mouse p53 Ser23 and the response to DNA damage. Mol Cell Biol. 2002;22:2441–2449. doi: 10.1128/MCB.22.8.2441-2449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 44.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 45.Adams JM, Cory S. Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonnell TJ, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 47.Grillot DA, et al. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J Exp Med. 1996;183:381–391. doi: 10.1084/jem.183.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasqualucci L, et al. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008;40:108–112. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 50.Batten M, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172:812–822. doi: 10.4049/jimmunol.172.2.812. [DOI] [PubMed] [Google Scholar]
- 51.Chiu A, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109:729–739. doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 53.Mecklenbräuker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCδ-mediated nuclear signalling. Nature. 2004;431:456–461. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 54.Stoddard BL. Homing endonuclease structure and function. Q Rev Biophys. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- 55.Cleveland JL, Sherr CJ. Antagonism of Myc functions by Arf. Cancer Cell. 2004;6:309–311. doi: 10.1016/j.ccr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 57.Adams JM, Gerondakis S, Webb E, Corcoran LM, Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci USA. 1983;80:1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams JM, et al. Transcriptionally active DNA region that rearranges frequently in murine lymphoid tumors. Proc Natl Acad Sci USA. 1982;79:6966–6970. doi: 10.1073/pnas.79.22.6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crews S, Barth R, Hood L, Prehn J, Calame K. Mouse c-myc oncogene is located on chromosome 15 and translocated to chromosome 12 in plasmacytomas. Science. 1982;218:1319–1321. doi: 10.1126/science.7146913. [DOI] [PubMed] [Google Scholar]
- 60.Dalla-Favera R, Martinotti S, Gallo RC, Erikson J, Croce CM. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983;219:963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- 61.Erikson J, Ar-Rushdi A, Drwinga HL, Nowell PC, Croce CM. Transcriptional activation of the translocated c-myc oncogene in Burkitt lymphoma. Proc Natl Acad Sci USA. 1983;80:820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamlyn PH, Rabbitts TH. Translocation joins c-myc and immunoglobulin γ 1 genes in a Burkitt lymphoma revealing a third exon in the c-myc oncogene. Nature. 1983;304:135–139. doi: 10.1038/304135a0. [DOI] [PubMed] [Google Scholar]
- 63.Marcu KB, et al. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci USA. 1983;80:519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taub R, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci USA. 1982;79:7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang JH, et al. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature. 2009;460:231–236. doi: 10.1038/nature08159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito M, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lumsden JM, et al. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J Exp Med. 2004;200:1111–1121. doi: 10.1084/jem.20041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J Exp Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28:1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 72.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 73.Chao DT, Korsmeyer SJ. BCL-2 family: Regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 74.Moreaux J, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He B, et al. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–3279. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 76.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 77.Leitges M, et al. Exacerbated vein graft arteriosclerosis in protein kinase Cδ-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamijo T, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 79.Barlow C, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.