Abstract
Increased nutrient mobilization by human activities represents one of the greatest threats to global ecosystems, but its effects on ecosystem productivity can differ depending on food web structure. When this structure facilitates efficient energy transfers to higher trophic levels, evidence from previous large-scale enrichments suggests that nutrients can stimulate the production of multiple trophic levels. Here we report results from a 5-year continuous nutrient enrichment of a forested stream that increased primary consumer production, but not predator production. Because of strong positive correlations between predator and prey production (evidence of highly efficient trophic transfers) under reference conditions, we originally predicted that nutrient enrichment would stimulate energy flow to higher trophic levels. However, enrichment decoupled this strong positive correlation and produced a nonlinear relationship between predator and prey production. By increasing the dominance of large-bodied predator-resistant prey, nutrient enrichment truncated energy flow to predators and reduced food web efficiency. This unexpected decline in food web efficiency indicates that nutrient enrichment, a ubiquitous threat to aquatic ecosystems, may have unforeseen and unpredictable effects on ecosystem structure and productivity.
Keywords: ecosystem enrichment, energy flow, food web efficiency, predator resistance, body size
By shifting species dominance and energy pathways, nutrient enrichment from human activities represents one of the greatest threats to global ecosystems with significant consequences for ecosystem structure and function (1). However, these effects are difficult to predict because of few large-scale experimental manipulations (2, 3) and the potential difficulties in predicting ecosystem-level responses from small-scale experimental approaches (4). Despite this uncertainty and limited knowledge of how aquatic ecosystems respond to nutrients, many restoration projects artificially enrich streams and rivers to stimulate fish production (5, 6). These practices are largely based on early food web models and empirical studies showing that the positive bottom-up effects of nutrient enrichment can extend to top predators (2, 7). Thus, when the entire primary consumer assemblage is equally vulnerable to predators (7), increased primary consumer production is predicted to be efficiently transferred to higher trophic levels (i.e., high trophic efficiency) where it stimulates predator production (2).
However, mounting evidence indicates that nutrient enrichment can frequently have unintended consequences as resources are diverted into alternate food web pathways that are relatively unavailable to higher trophic levels. For instance, nutrient enrichment of coastal zones can reduce trophic transfer efficiencies between algae and primary consumers, generating excess algal production that is not consumed by primary consumers and is ultimately decomposed by heterotrophic microbes (8). In extreme cases, nearly 100% of primary productivity may be diverted to microbial respiration, resulting in increasingly prevalent anoxic “dead zones” (8). Food web models also predict that nutrient enrichment can decrease food web stability as it can amplify variability in predator–prey cycles and even extirpate predator populations (i.e., “the paradox of enrichment”) (9).
More recent models predict that nutrient enrichment can further alter predator–prey interactions by increasing the dominance of predator-resistant primary consumers, diverting energy flow to predator-resistant pathways that are relatively inaccessible to top predators (10, 11). Small-scale mesocosm experiments have shown that such a reduction in trophic efficiency can ultimately decrease predator production, even with sustained increases in primary consumer productivity (i.e., resulting in a trophic decoupling) (12, 13). Thus, if nutrient enrichment disproportionately stimulates predator–resistant prey, it may reduce positive nutrient effects on predators and inhibit predator production.
Despite these results from small-scale manipulations using species-depauperate food webs, there is no ecosystem-level evidence that enrichment can decouple predator production from primary consumers. Whereas nutrient enrichment of coastal zones can reduce the production of higher trophic levels, this effect results not from a decoupling of primary consumer and predator production, but rather from a diversion of energy flow between basal resources and primary consumers that result in anoxic conditions (8). In fact, other large-scale experimental nutrient enrichments have largely stimulated both primary consumer and predator production (2, 5, 14), suggesting that trophic decouplings may be unlikely in diverse natural food webs. Because the effectiveness of antipredator defenses depend on the foraging strategies used by predators (15), food webs with a diversity of predators and foraging strategies may increase the predation risk of all prey types, maintain efficient energy flow to higher trophic levels, and reduce the likelihood of an enrichment-induced trophic decoupling.
Here we report the results from an ecosystem-level manipulation of a detritus-based headwater stream that is dominated by approximately 20 taxa of macroinvertebrate and salamander predators. Primary consumer production in these stream food webs is based on seasonal inputs of terrestrial leaf detritus because stream algal production is light limited by a dense forest understory (16). As both the macroinvertebrate and salamander predators in these stream food webs predominantly eat small-bodied primary consumers, these two predator groups occupy a similar trophic position (17–19).
For 5 years, we experimentally enriched a treatment stream with moderate levels of dissolved nitrogen and phosphorus and compared the food web response in the treatment stream to a reference stream. Previous work in these streams showed that nutrient enrichment increased microbial production at the base of the food web, where it subsequently stimulated primary consumer and predator production (3, 20). There was also a strong linear relationship between predator and prey production under reference conditions (16), which suggested a relatively efficient flow of energy between heterotrophic microbes, primary consumers, and predators. Therefore, on the basis of our earlier results from the first 2 years of enrichment (3) and from a similar long-term enrichment that showed positive effects of nutrient enrichment on predators and primary consumers (2, 14), we hypothesized a priori that in subsequent years of nutrient enrichment (years 4 and 5), primary consumer and macroinvertebrate predator production would continue to be positively correlated.
Results
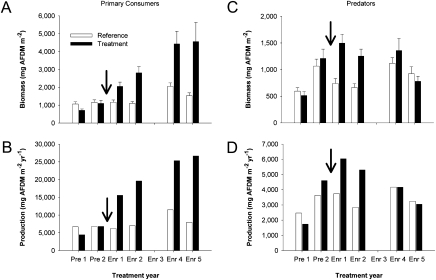
Unexpectedly, during the fourth and fifth years of the experiment, nutrient enrichment produced a trophic decoupling whereby enrichment continued to stimulate primary consumer production with no concomitant increase in macroinvertebrate predators (Fig. 1 A–D, Table S1). In addition, this primary consumer and predator response varied with time. Two years of nutrient enrichment stimulated the production and biomass of both primary consumers and predators (3), which agreed with other nutrient enrichment manipulations (2, 14). However, this short-term response contrasted sharply with our longer-term results showing that predator biomass and production did not respond positively to nutrient enrichment, despite continued stimulation of primary consumer biomass (P < 0.001) and production relative to the pretreatment period (Fig. 1 A–D, Table S1). Thus, predators initially increased with short-term enrichment, but then declined to pretreatment levels with a longer-term enrichment, even as primary consumer production continued to increase in the treatment stream (Fig. 1 A–D).
Fig. 1.
Average annual biomass (mean ± SE) and secondary production of primary consumers (A and B) and predators (C and D) during the 7-year experiment. The arrow indicates the beginning of nutrient enrichment. Each year represents an average of 12 monthly samples with 4 samples per stream. Note difference in scales between primary consumers and predators. AFDM is ash-free dry mass.
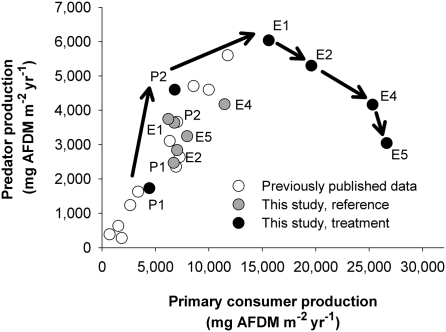
This trophic decoupling reduced overall food web efficiency during the long-term enrichment (Fig. 2) and contrasted with previous studies showing evidence of highly efficient energy transfer from primary consumers to predators in similar stream food webs (16, 21). Because of this reduction, we observed dramatically different relationships between primary consumer and predator production in the treatment and reference streams. Predator production varied linearly and steeply with primary consumer production during all years in the reference stream (ref. 3 and this study) and in similar streams (21, 22). Primary consumer and predator production were similarly related in the treatment stream during the pretreatment period (Fig. 2) (3). These strong linear relationships suggested efficient energy transfer between primary consumers and predators under reference conditions. Conversely, during the enrichment period, primary consumer production continued to respond positively to long-term enrichment, but predators declined to pretreatment levels in a nonlinear trajectory over time (Fig. 2). In the fourth year of enrichment, the reference and treatment streams (represented by E4 in Fig. 2) had comparable levels of predator production, despite approximately 2.2 times greater primary consumer production in the treatment stream relative to the reference stream. These contrasting responses of predators and primary consumers strongly reduced the contribution of predators to overall macroinvertebrate biomass. Before enrichment, predators and primary consumers each contributed ≈50% to total biomass (Fig. 1 A and C). However, during the final 2 years of enrichment, predator contribution declined to 18% in the treatment stream, but remained at ≈40% in the reference stream. Taken together, our results provide evidence that long-term nutrient enrichment did not stimulate predator production and reduced the efficiency of energy flow from primary consumers to predators.
Fig. 2.
Relationship between primary consumer and predator secondary production for the reference stream (gray circles), the treatment stream (black circles), and previously published data (open circles). The arrows represent the temporal trajectory of the treatment stream starting with the 2 years of pretreatment (P1 and P2) and ending with the fifth year of enrichment (E5). The data labels correspond to the sampling year for the reference and treatment streams. The previously published data include 5 years of production data from the reference stream (C53) and a similar Coweeta stream (C55) that had experimentally reduced terrestrial leaf inputs during 4 of those years (21). It also includes previously published data from an unmanipulated year that compared our current reference (C53) and treatment (C54) streams (22). AFDM is ash-free dry mass.
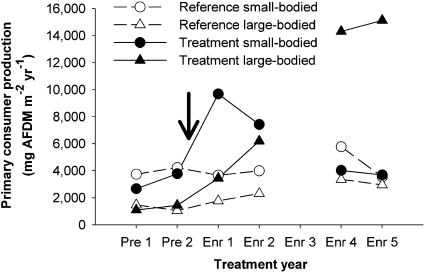
The alteration of the predator–prey relationship during the long-term enrichment was largely driven by changes in the relative dominance of large- vs. small-bodied primary consumers (Fig. 3). Because predators from our study streams primarily eat small-bodied primary consumers and seldom eat large-bodied prey (17–19), large-bodied primary consumers are likely more resistant to predation by these instream predators. Thus, as the predation risk of prey can decline with increased body size (11, 23), the wide variation in primary consumer body sizes in our streams (<1 mm to 65 mm) likely increased the variation in the relative predation risk of primary consumers. The reference and treatment streams initially did not differ in the production of large- or small-bodied primary consumers during the pretreatment period (Fig. 3). Enrichment increased the production of both large- and small-bodied primary consumers in the first 2 years of enrichment, but only increased the production of large-bodied primary consumers in the treatment stream during years 4 and 5 (Fig. 3). Because small-bodied prey declined to pretreatment levels by the fourth year of enrichment, long-term enrichment primarily stimulated those primary consumers that were relatively resistant to predation (Fig. 3). Thus, enrichment did not stimulate predator production because the increase in predator-resistant (i.e., large-bodied) taxa likely did not benefit instream predators.
Fig. 3.
Size-specific secondary production of the 10 dominant primary consumers in the reference and treatment streams. For any given year, the displayed secondary production represented 70–90% of total primary consumer production. Each individual within these 10 taxa was classified as either small-bodied individuals (body length ≤ 10 mm; circles) or large-bodied individuals (body length > 10 mm; triangles), and their production was subsequently summed. Large-bodied individuals were relatively predator-resistant compared to small-bodied primary consumers. The arrow indicates the beginning of nutrient enrichment. AFDM is ash-free dry mass.
Discussion
Our results provide strong evidence that nutrient enrichment reduced energy flow to predators and decreased the trophic transfer efficiency between primary consumers and predators. Thus, even within a diverse food web with 20 predator taxa, long-term nutrient enrichment decoupled primary consumer and predator production, as most primary consumer production was relatively unavailable to predators. Nutrient enrichment of natural food webs may not always increase predator production, but instead can produce unintended “ecological surprises” as ecosystem-level nutrient responses are likely context dependent. Our results further demonstrate our limited ability to predict how higher trophic levels in aquatic ecosystems will respond to nutrient enrichment and highlight the difficulties in predicting long-term food web responses from few large-scale experimental manipulations.
The lack of a significant positive predator response to nutrient enrichment suggests that the majority of the increased ecosystem productivity in our study was confined to the lower trophic levels. This suggests that increased nutrient supplies do not always propagate up food webs to increase the productivity or biomass of higher trophic levels. These findings largely agree with an earlier regional comparison of food chain lengths showing that increased ecosystem size, but not productivity, lengthened food chains (24). However, as we fully quantified changes in energy flow within these trophic levels, our results indicate a likely mechanism explaining why increased ecosystem productivity does not increase the trophic position of predators or add additional trophic levels. Specifically, nutrient enrichment resulted in inefficiencies between primary consumers and predators that limited the transfer of energy to higher trophic levels and attenuated the positive effects of nutrients. This occurred despite increased trophic efficiencies between basal resources and primary consumers due to increased resource nutrient content (e.g., reduced C:N and C:P) (25). At higher trophic positions, other factors (e.g., resistance to predation) may limit further trophic transfers. Therefore, if increased ecosystem productivity is confined to lower trophic levels and does not stimulate predator production, it likely diminishes the ability of enrichment to support additional trophic levels regardless of any increase in basal resource productivity. Overall, these results provide additional evidence that the positive effects of nutrient enrichment can attenuate with increasing trophic distance (26).
These results suggest that trophic decouplings due to nutrient enrichment, as well as other types of natural or anthropogenic disturbance, may be more likely in food webs dominated by gape-limited predators. When predators are gape limited, primary consumers may obtain predator size refugia and divert production away from predators. However, a long-term nutrient enrichment of the Kuparuk River in Alaska did not lead to a trophic decoupling of the top fish predator in this ecosystem, Arctic grayling (Thymallus arcticus). Nutrient enrichment of this ecosystem continued to stimulate arctic grayling production even after 16 years of seasonal enrichment (2). Although they are gape-limited predators, Arctic grayling are substantially larger than predators found in our study and could more easily consume larger prey (27). Thus, they could maintain a positive nutrient response (2).
However, even in food webs dominated by fish predators, large-bodied primary consumers can reduce their predation risk through predator-size refugia or other antipredator defenses (28, 29) and potentially lead to a similar diversion of resources under nutrient-enriched conditions. For instance, larval gizzard shad (Dorosoma cepedianum) eat primarily small-bodied zooplankton; thus, the increased dominance of large-bodied zooplankton may reduce prey availability and threaten the recruitment of this common lake fish (28). In addition, during drought years on the South Fork Eel River, CA, the increased dominance of a large-bodied, case-building caddisfly (Dicosmoecus gilvipes) reduced energy flow to steelhead (Oncorhynchus mykiss) as algal production was diverted into a predator-resistant Dicosmoecus pathway (29, 30). Because increased light availability can stimulate algal production and likely accelerates Dicosmoecus dominance (31), any increase in primary productivity associated with nutrient enrichment could potentially strengthen this energetic diversion. Given the prevalence of gape-limited predators and predator-resistant prey in a variety of aquatic ecosystems, our results suggest that trophic decouplings due to enrichment, as well as other types of natural and anthropogenic disturbances, may potentially be widespread occurrences.
The mechanism by which large-bodied taxa became dominant in this ecosystem is likely a function of several factors. Differential predation pressure likely contributed to the lower production of small- vs. large-bodied macroinvertebrates, as small-bodied primary consumers are the preferred prey of both macroinvertebrate and vertebrate predators in these streams (17–19). Declines in leaf litter standing crop and habitat complexity during the enrichment (32) likely altered this predation risk. Large-bodied primary consumers may reduce their predation risk via body-size refugia (11, 23) and be less dependent on leaf litter habitat for spatial refugia. Thus, the decline in spatial refugia provided by leaf litter may have disproportionately increased the predation risk of small-bodied prey and counteracted their potential positive nutrient response.
Although several large-bodied taxa responded positively to enrichment, the relative dominance of a large-bodied caddisfly, Pycnopsyche spp., steadily increased throughout the experimental enrichment (Fig. S1). This suggests that enrichment beyond our 5-year manipulation would have likely continued to decouple predator production because of several factors that would have maintained conditions conducive to this common consumer's dominance. Pycnopsyche spp. are competitive dominants in these stream ecosystems (33) and eat leaf detritus (25), which exhibited larger increases in resource quality than other basal resources during our experimental enrichment (34, 35). Pycnopsyche’s period of peak production is earlier than many other leaf-eating taxa and occurs before periods of low resource availability (32, 36). They also construct rigid stone cases and obtain a larger maximum body size than other leaf-eating taxa (22 mm vs. 14 mm), which may reduce their predation risk. The combination of these traits likely allowed this taxon to better exploit the positive enrichment effects on resource quality. Because prolonged enrichment would likely strengthen, not weaken, these benefits, the observed trophic decoupling is unlikely to be easily reversed with continued enrichment. However, this decoupling would have likely reversed when enrichment ceased because of the strong donor-controlled aspects of these ecosystems: both basal resources via seasonal litterfall and larval aquatic insect populations are renewed annually to a large extent.
This decoupling of the predator–prey relationship observed in our study may have ecosystem-level effects that extend beyond our particular study streams. Headwater streams similar to our study streams dominate overall stream miles and are a common landscape feature within an ecosystem type that has a worldwide distribution (37). Thus, our results indicate an important nutrient enrichment response that is applicable to globally distributed aquatic food webs and helps increase our understanding of how such stream networks may respond to enrichment. Streams similar to our study streams are also important sites for carbon and nutrient transformations within river networks (37, 38) and are directly linked to downstream food webs through material, energy, and macroinvertebrate transport (39). As macroinvertebrate consumers are important drivers of many of these processes (40), our observed decoupling of the predator–prey relationship has the potential to alter the functioning of overall river networks through changes in these downstream subsidies. In fact, a concurrent study showed that nutrient enrichment increased organic matter processing and downstream carbon export because of associated changes in consumer production (35, 41).
Although enrichment decoupled primary consumer and predator production in this headwater stream, other predators may have still benefited from this increased primary consumer production. Macroinvertebrate production not consumed by headwater stream predators can represent an important subsidy to terrestrial and downstream food webs (42, 43). Thus, enrichment may have increased the export of prey production to terrestrial predators as adult emergence or to downstream predators as drift. It is also possible that this greater prey production could eventually facilitate the introduction of a new predator taxon that could use this increased prey production. Although the ultimate fate of the primary consumer production is not known, these various cross-boundary linkages suggest that our nutrient-induced trophic decoupling may indirectly affect a variety of food webs not directly experiencing enrichment.
In summary, nutrient enrichment dramatically shifted the primary consumer assemblage in this stream food web to larger-bodied, predator-resistant taxa. As this shift decoupled predator and prey production, nutrient enrichment ultimately diverted energy flow into predator-resistant pathways that reduced overall food web efficiency. Humans are intentionally (e.g., salmon restoration) and unintentionally (e.g., land-use change and agricultural run-off) increasing nutrient inputs to a variety of aquatic ecosystems (1, 5); thus, nutrient enrichments similar to our experimental manipulation are a frequent global occurrence. Given the prevalence of this environmental change in a diversity of ecosystems that include predator-resistant prey and gape-limited predators, our results suggest that nutrient-stimulated resource flows can be diverted into predator-resistant pathways and thereby truncate predator responses. As we did not originally predict a decline in trophic efficiency, our results also show our current inability to fully assess a priori how ecosystems will respond to enrichment. Therefore, even in ecosystems where energy flow is predicted to be relatively efficient, nutrient enrichment may still increase the production of nontarget taxa (e.g., predator/grazer resistant prey), decrease the production of higher trophic levels, or lead to unintended consequences that may compromise the productivity of freshwater ecosystems.
Materials and Methods
We conducted this study at the US Department of Agriculture Forest Service Coweeta Hydrologic Laboratory, a long-term ecological research site in Macon County, NC. Coweeta is a heavily forested experimental watershed (2,185 ha) located in the southern Appalachians. The forest is dominated by mixed hardwoods (oak, maple, and tulip poplar) with a dense understory dominated by Rhododendron maximum that results in heavy stream shading. This light limitation decreases autotrophic production and increases the food web’s reliance on heterotrophic microbes that colonize inputs of terrestrial leaves (16, 25).
To test the long-term effects of nutrient enrichment on macroinvertebrate food webs, we used a paired-watershed approach in two forested headwater catchments (C53 and C54) with similar physiochemical properties (i.e., catchment area, slope, elevation, discharge, temperature, and pH). Both streams were fishless and were dominated by over 20 taxa of macroinvertebrate [e.g., Beloneuria (Plecoptera), Ceratopogonidae (Diptera), Cordulegaster (Odonata), Hexatoma (Diptera]), and Lanthus (Odonata)] or vertebrate [e.g., Eurycea wilderae (Plethodontidae) and Desmognathus quadramaculatus (Plethodontidae)] predators. Further descriptions of the study sites are reported elsewhere (22).
The reference (C53) and treatment (C54) streams did not differ in nutrient concentrations before the experimental enrichment (mean ± SE, C53: DIN: 23.2 ± 8.5 μg L−1, SRP: 6.8 ± 3.0 μg L−1; C54: DIN: 29.3 ± 4.9 μg L−1, SRP: 9.5 ± 2.3 μg L−1). From July 2000 to August 2005 (approximately 1,877 days), we experimentally enriched a 150-m reach of the treatment stream with nitrogen (NH4NO3) and phosphorus (K2HPO4 and KH2PO4). We dripped nutrients continuously along a 150-m reach of the treatment stream using an irrigation line running down the center of the stream. Details of the nutrient-delivery system have been previously reported (20). This flow-proportional delivery system increased nutrient concentrations in the treatment stream to a realistic, moderate-level enrichment (DIN: 506.2 ± 36.3 μg L−1, SRP: 80.0 ± 5.6 μg L−1), whereas the reference stream concentrations during this same time period were comparable to the pretreatment period (DIN: 31.0 ± 3.4 μg L−1, SRP: 8.0 ± 1.3μg L−1). We monitored stream nutrient concentrations every 2 weeks at three points along the 150-m reach of the treatment stream and at the weir of the reference stream (44). Our enriched dissolved nutrient concentrations were within the range of those measured at sites experiencing agricultural and urban watershed land uses (45). Water temperature was measured every 30 min with temperature probes (Onset Computer). We measured stream discharge at 5-min intervals with an ISCO data logger.
We sampled the benthic macroinvertebrate fauna in both streams during an initial pretreatment period (September 1998 to June 2000) followed by a 5-year experimental period (July 2000 to August 2005). On a monthly basis, we collected four mixed-cobble substrate samples per stream using a stovepipe corer (400 cm2) and processed them according to established protocols (3). We identified most taxa to genus; however, we only identified Chironomidae to either Tanypodinae (predators) or non-Tanypodinae (nonpredators), and noninsects (e.g., oligochaetes, nematodes, copepods, etc.) to the lowest possible taxonomic level. We measured the length of each individual to the nearest millimeter and then applied previously published length-mass regressions (46) to quantify ash-free dry mass (AFDM). For most taxa, we calculated secondary production with the size-frequency method corrected for cohort production intervals (21, 47). However, we used the instantaneous growth rate method to calculate secondary production of non-Tanypodinae chironomids (48). For a few taxa that lacked sufficient data to calculate secondary production with these two methods, we estimated their annual production by multiplying their annual standing stock biomass by their average production to biomass ratio (e.g., oligochaetes, nematodes, copepods). We classified all taxa as predators or primary consumers on the basis of literature values (49) and on previous research (21).
We evaluated trophic level responses to enrichment with community biomass and secondary production. However, because it integrates multiple metrics in assessing taxonomic response to enrichment (i.e., abundance, biomass, growth rate, survivorship, and development time) (50), secondary production was the best metric to quantify the overall response.
Because large-bodied primary consumers were relatively predator resistant (17–19), we also conducted an additional size-specific comparison of the primary consumer production response. This comparison helped distinguish between the response of preferred prey (small-bodied individuals) and predator-resistant primary consumers (large-bodied individuals). First, we selected the 10 most dominant taxa in both streams, which represented 70–90% of total primary consumer production in a given year [Pycnopsyche spp. (Trichoptera), Tipula sp. (Diptera), Fattigia sp. (Trichoptera), Lepidostoma spp. (Trichoptera), Tallaperla spp. (Plecoptera), Molophilus sp. (Diptera), Leuctra spp. (Plecoptera), Diplectrona sp. (Trichoptera), non-Tanypodinae Chironomidae (Diptera), Copepoda]. We then categorized each individual within these 10 taxa on the basis of body size. We did not assign a single body size to each taxon (i.e., an average or maximum body size) because early instars of typically large-bodied prey are likely more vulnerable to predation than later instars of the same taxon. Instead, we classified each individual within a given taxon as either large-bodied (>10 mm in total length) or small-bodied primary consumers (≤10 mm) because this delineation categorized the preferred prey taxa (i.e., non-Tanypodinae chironomids and copepods) as small-bodied individuals. On the basis of this body size grouping, we then summed the secondary production of all individuals within both of these body size categories, regardless of taxonomic affiliation. We repeated this process for each year and stream and compared the trends graphically.
To assess short- vs. long-term macroinvertebrate responses, we divided the study into three time periods: pretreatment (PRE 1 and PRE 2; July 1998–August 2000), short-term response (ENR 1 and ENR 2; September 2000–August 2002), and long-term response (ENR 4 and ENR 5; September 2003–August 2005). Within this notation, the number following the abbreviations (PRE: pretreatment year or ENR: enrichment year) corresponded with treatment year (e.g., ENR 1 represented the first year of nutrient enrichment). The third year of enrichment (ENR 3; September 2002–August 2003) was not included in the analysis because samples were lost due to inadequate preservation. Bias due to the exclusion of ENR 3 is extremely unlikely because any trends associated with ENR 3 would be captured by the final 2 years of enrichment (ENR 4 and ENR 5). These time periods were selected because ≥2 years of enrichment allowed many of the taxa to reach new population levels, as 90% of these taxa have life cycles of 1 year or less, and only two taxa have larval periods longer than 2 years [Anchytarsus (1,095 days), Cordulegaster (1,140 days)] (21). Thus, by the fourth and fifth year of enrichment, 90% of taxa would have produced >4 generations under nutrient-enriched conditions. Moreover, those taxa with a life cycle of 1 year or less represent approximately 90–95% of the total secondary production in any given year.
We used randomized intervention analysis (RIA) to analyze the effects of enrichment on macroinvertebrate biomass (Table S1). By comparing the differences in the treatment (C54) and reference stream (C53) during the pretreatment and nutrient enrichment periods, RIA assessed the null hypothesis that macroinvertebrate biomass in the treatment stream did not change relative to the reference stream during the enrichment (51). To isolate the long-term and short-term responses over time, we conducted three separate RIA analyses on both primary consumer and predator biomass (Table S1). Short-term responses of macroinvertebrate biomass to nutrient enrichment were assessed by comparing the short-term response (ENR 1 and 2; n = 26 months) to the pretreatment period (PRE 1 and 2; n = 22 months) (3). To evaluate the longer-term responses, we compared ENR 4 and 5 (n = 24 months) to the pretreatment period (PRE 1 and 2). Comparing the long-term (ENR 4 and 5) and short-term (ENR 1 and 2) responses tested whether the effects of long-term enrichment had leveled off after an initial short-term response. We calculated probabilities of change for each pairwise comparison using 1,000 random permutations of interstream differences (51).
Supplementary Material
Acknowledgments
We thank S. Dye, N. Taylor, R. Hilten, J. Benstead, and C. Tant for help in the field. We also thank all laboratory technicians, but especially E. Baker, A. Ely, J. Hoehn, and J. Holland. This experiment was made possible by the contributions of our collaborators, D. Conners, V. Gulis, K. Suberkropp, and C. Tant. Logistical support was provided by staff at the Coweeta Hydrologic Laboratory. Water chemistry analyses were conducted at the University of Georgia (UGA) Analytical Chemistry Lab. The Rosemond lab group, S. Dye, R. Hall, J. Hutchens, and E. Rosi-Marshall provided insightful comments on an early draft of this manuscript. Funding was provided by National Science Foundation (NSF) (DEB-9806610, DEB-0318063, DEB-9629268, and DEB-0212315). J. Davis was supported by a NSF graduate research fellowship and a UGA presidential fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908497107/DCSupplemental.
References
- 1.Smith VH, Tilman GD, Nekola JC. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut. 1999;100:179–196. doi: 10.1016/s0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 2.Slavik J, et al. Long-term responses of the Kuparuk River ecosystem to phosphorus fertilization. Ecology. 2004;85:939–954. [Google Scholar]
- 3.Cross WF, Wallace JB, Rosemond AD, Eggert SL. Whole-system nutrient enrichment increases secondary production in a detrital-based ecosystem. Ecology. 2006;87:1556–1565. doi: 10.1890/0012-9658(2006)87[1556:wneisp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter SR. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology. 1996;77:677–680. [Google Scholar]
- 5.Slaney PA, Ward BR, Wightman JC. In: Nutrients in Salmonid Ecosystems: Sustaining Production and Biodiversity. Stockner J, editor. Bethesda, MD: American Fisheries Society; 2003. pp. 111–126. [Google Scholar]
- 6.Compton JE, et al. Ecological and water quality consequences of nutrient addition for salmon restoration in the Pacific Northwest. Front Ecol Environ. 2006;4:18–26. [Google Scholar]
- 7.Oksanen L, Fretwell SD, Arruda J, Niemela P. Exploitation ecosystems in gradients of primary productivity. Am Nat. 1981;118:240–261. [Google Scholar]
- 8.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzweig ML. Paradox of enrichment: Destabilization of exploitation ecosystems in ecological times. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- 10.Abrams PA. Effect of increased productivity on the abundances of trophic levels. Am Nat. 1993;141:351–371. [Google Scholar]
- 11.Chase JM. Food web effects of prey size refugia: Variable interactions and alternative stable equilibria. Am Nat. 1999;154:559–570. doi: 10.1086/303260. [DOI] [PubMed] [Google Scholar]
- 12.Bohannan BJM, Lenski RE. Effect of prey heterogeneity on the response of a model food chain to resource enrichment. Am Nat. 1999;153:73–82. doi: 10.1086/303151. [DOI] [PubMed] [Google Scholar]
- 13.Stevens MHH, Steiner CE. Effects of predation and nutrient enrichment on a food web with edible and inedible prey. Freshw Biol. 2006;51:666–671. [Google Scholar]
- 14.Deegan LA, Peterson BJ. Whole-river fertilization stimulates fish production in an arctic tundra river. Can J Fish Aquat Sci. 1992;49:1890–1901. [Google Scholar]
- 15.Power ME, Marks JC, Parker MS. Variation in the vulnerability of prey to different predators: Community-level consequences. Ecology. 1992;73:2218–2223. [Google Scholar]
- 16.Wallace JB, Eggert SL, Meyer JL, Webster JR. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science. 1997;277:102–104. [Google Scholar]
- 17.Davic RD. Ontogenetic shift in diet of Desmognathus quadramaculatus. J Herpetol. 1991;25:108–111. [Google Scholar]
- 18.Hall RO, Wallace JB, Eggert SL. Organic matter flow in stream food webs with reduced detrital resource base. Ecology. 2000;81:3445–3463. [Google Scholar]
- 19.Johnson BR, Wallace JB. Bottom-up limitation of a stream salamander in a detritus-based food web. Can J Fish Aquat Sci. 2005;62:301–311. [Google Scholar]
- 20.Gulis V, Suberkropp K. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol. 2003;48:123–134. [Google Scholar]
- 21.Wallace JB, Eggert SL, Meyer JL, Webster JR. Effects of resource limitation on a detrital-based ecosystem. Ecol Monogr. 1999;69:409–442. [Google Scholar]
- 22.Lugthart GJ, Wallace JB. Effects of disturbance on benthic functional structure and production in mountain streams. J N Am Benthol Soc. 1992;11:138–164. [Google Scholar]
- 23.Crowl TA, Covich AP. Predator induced life-history shifts in a freshwater snail. Science. 1990;247:949–951. doi: 10.1126/science.247.4945.949. [DOI] [PubMed] [Google Scholar]
- 24.Post DM, Pace ML, Hairston NG. Ecosystem size determines food-chain length in lakes. Nature. 2000;405:1047–1049. doi: 10.1038/35016565. [DOI] [PubMed] [Google Scholar]
- 25.Cross WF, Wallace JB, Rosemond AD. Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology. 2007;88:2563–2575. doi: 10.1890/06-1348.1. [DOI] [PubMed] [Google Scholar]
- 26.Brett MT, Goldman CR. Consumer versus resource control in freshwater pelagic food webs. Science. 1997;275:384–386. doi: 10.1126/science.275.5298.384. [DOI] [PubMed] [Google Scholar]
- 27.Golden HE, Deegan LA. The trophic interactions of young Arctic grayling (Thymallus arcticus) in an Arctic tundra stream. Freshw Biol. 1998;39:637–648. [Google Scholar]
- 28.Bremigan MT, Stein RA. Gape-dependent larval foraging and zooplankton size: Implications for fish recruitment across systems. Can J Fish Aquat Sci. 1994;51:913–922. [Google Scholar]
- 29.Power ME, Parker MS, Dietrich WE. Seasonal reassembly of a river food web: Floods, droughts, and impacts of fish. Ecol Monogr. 2008;78:263–282. [Google Scholar]
- 30.Wootton JT, Parker MS, Power ME. Effects of disturbance on river food webs. Science. 1996;273:1558–1561. [Google Scholar]
- 31.Power ME, Parker MS, Wootton JT. In: Food Webs: Integration of Patterns and Dynamics. Polis GA, Winemiller KO, editors. New York: Chapman and Hall; 1996. pp. 286–297. [Google Scholar]
- 32.Suberkropp K, Gulis V, Rosemond AD, Benstead JP. Nutrient enrichment shifts microbial and detrital pathways in a headwater stream ecosystem. Limnol Oceanogr. 2010;55:149–160. [Google Scholar]
- 33.Creed RP, Cherry RP, Pflaum JR, Wood CJ. Dominant species can produce a negative relationship between species diversity and ecosystem function. Oikos. 2009;118:723–732. [Google Scholar]
- 34.Cross WF, Benstead JP, Frost PC, Thomas SA. Ecological stoichiometry in freshwater benthic systems: Recent progress and perspectives. Freshw Biol. 2005;50:1895–1912. [Google Scholar]
- 35.Greenwood JL, Rosemond AD, Wallace JB, Cross WF, Weyers HS. Nutrients stimulate leaf breakdown rates and detritivore biomass: Bottom-up effects via heterotrophic pathways. Oecologia. 2007;151:637–649. doi: 10.1007/s00442-006-0609-7. [DOI] [PubMed] [Google Scholar]
- 36.Huryn AD, Wallace JB. Community structure of Trichoptera in a mountain stream: Spatial patterns of production and functional organization. Freshw Biol. 1988;20:141–155. [Google Scholar]
- 37.Meyer JL, Wallace JB. In: Ecology: Achievement and Challenge. Press MC, Huntly NJ, Levin S, editors. Oxford: Blackwell Science; 2001. pp. 295–317. [Google Scholar]
- 38.Peterson BJ, et al. Control of nitrogen export from watersheds by headwater streams. Science. 2001;292:86–90. doi: 10.1126/science.1056874. [DOI] [PubMed] [Google Scholar]
- 39.Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. The river continuum concept. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 40.Cuffney TF, Wallace JB, Lugthart GJ. Experimental evidence quantifying the role of benthic invertebrates in organic matter dynamics of headwater streams. Freshw Biol. 1990;23:281–299. [Google Scholar]
- 41.Benstead JP, et al. Nutrient enrichment alters storage and fluxes of detritus in a headwater stream ecosystem. Ecology. 2009;90:2556–2566. doi: 10.1890/08-0862.1. [DOI] [PubMed] [Google Scholar]
- 42.Baxter CV, Fausch KD, Saunders WC. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zone. Freshw Biol. 2005;50:201–220. [Google Scholar]
- 43.Romaniszyn ED, Hutchens JJ, Wallace JB. Aquatic and terrestrial invertebrate drift in southern Appalachian Mountain streams: Implications for trout food resources. Freshw Biol. 2007;52:1–11. [Google Scholar]
- 44.APHA. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association; 1998. [Google Scholar]
- 45.Alexander RB, Smith RA. Trends in the nutrient enrichment of U.S. rivers during the late 20th century and their relation to changes in probable stream trophic conditions. Limnol Oceanogr. 2006;51:639–654. [Google Scholar]
- 46.Benke A, Huryn AD, Smock LA, Wallace JB. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc. 1999;18:308–343. [Google Scholar]
- 47.Benke AC. Modification of the Hynes method for estimating secondary production with particular significance for multivoltine populations. Limnol Oceanogr. 1979;24:168–171. [Google Scholar]
- 48.Cross WF, Johnson BR, Wallace JB, Rosemond AD. Contrasting response of stream detritivores to long-term nutrient enrichment. Limnol Oceanogr. 2005;50:1730–1739. [Google Scholar]
- 49.Merritt RW, Cummins KW. An Introduction to the Aquatic Insects of North America. Dubuque, IA: Kendall Hunt; 1996. [Google Scholar]
- 50.Benke AC. Concepts and patterns of invertebrate production in running waters. Verhandlungen Internationale Vereinigung für theoretische und angewandte Limnologie. 1993;25:15–38. [Google Scholar]
- 51.Carpenter SR, Frost TM, Heisey D, Kratz TK. Randomized intervention analysis and the interpretation of whole-ecosystem experiments. Ecology. 1989;70:1142–1152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.