Abstract
Lipodystrophy and obesity are opposites in terms of a deficiency versus excess of adipose tissue mass, yet these conditions are accompanied by similar metabolic consequences, including insulin resistance, dyslipidemia, hepatic steatosis, and increased risk for diabetes and atherosclerosis. Hepatic and myocellular steatosis likely contribute to metabolic dysregulation in both states. Inflammation and macrophage infiltration into adipose tissue also appear to participate in the pathogenesis of obesity-induced insulin resistance, but their contributions to lipodystrophy-induced insulin resistance have not been evaluated. We used aP2-nSREBP-1c transgenic (Tg) mice, an established model of lipodystrophy, to ask this question. Circulating cytokine elevations suggested systemic inflammation but even more dramatic was the number of infiltrating macrophages in all white and brown adipose tissue depots of the Tg mice; in contrast, there was no evidence of inflammatory infiltrates or responses in any other tissue including liver. Despite there being overt evidence of adipose tissue inflammation, antiinflammatory strategies including salicylate treatment and genetic suppression of myeloid NF-κB signaling that correct insulin resistance in obesity were ineffective in the lipodystrophic mice. We further showed that adipose tissue macrophages (ATMs) in lipodystrophy and obesity are very different in terms of activation state, gene expression patterns, and response to lipopolysaccharide. Although ATMs are even more abundant in lipodystrophy than in obesity, they have distinct phenotypes and likely roles in tissue remodeling, but do not appear to be involved in the pathogenesis of insulin resistance.
Keywords: diabetes, insulin resistance, obesity
Lipodystrophy is a condition in which adipose tissue mass is reduced, either regionally or in more general patterns. The etiology of adipose tissue loss may be linked in different individuals to genetic abnormalities, autoimmunity, or to certain drugs. Lipodystrophy is most commonly seen today in patients treated with retroviral agents (1). Given that they are opposites in terms of adipose tissue mass, it seems paradoxical that lipodystrophy and obesity are accompanied by similar pathological sequelae, including insulin resistance and heightened risk for diabetes, dyslipidemia, and hepatic steatosis (2). At least two consequences of diminished adipose tissue mass in lipodystrophy may contribute to the clinical picture. First is a decrease in circulating leptin, which leads to persistent hyperphagia and chronic overnutrition. Second is the loss of adipose tissue as the normal repository for lipid storage, which leads to triglyceride deposition elsewhere. The liver is an obvious site of lipid redistribution, as it is often enlarged, but other organs and tissues are also affected, including skeletal muscle (3). The insulin resistance seen in patients with lipodystrophy is often severe and has been attributed to both hepatic and myocellular steatosis.
Obesity is also accompanied by dyslipidemia and the ectopic deposition of triglyceride outside of the adipocyte (4). In obesity, adipocytes are enlarged and often increased in number, yet with such an excess of lipid some also “spills over” into other tissues. Shulman and colleagues (5) have hypothesized that the accumulation of hepatic and intramyocellular lipid causes insulin resistance through activation of protein kinase C (PKC) enzymes. Obesity is also associated with a heightened inflammatory state in adipose tissue (6). As adipose tissue expands during periods of nutritional excess, at least two inflammatory pathways are activated, the stress kinase, JNK, mediates one and the transcription factor, NF-κB, mediates the other (7, 8). Potential initiators of the inflammatory activation include ER and oxidative stress, ceramides, and other lipids, possibly by activating toll-like receptors (TLRs) (9–12). Once activated, the downstream consequences include the production of proinflammatory cytokines, chemokines, and cellular adhesion molecules that recruit and localize immune cells including monocytes and macrophages (13, 14); accumulating macrophages in expanding adipose tissue potentially participate in the pathophysiology of insulin resistance (15). In contrast, T regulatory cells (Tregs), which normally suppress inflammation and autoimmunity, decrease in number as adipose tissue expands (16).
Inflammation has not been shown to contribute to the dysmetabolic consequences of lipodystrophy, although increases in the expression of proinflammatory cytokines and increased macrophage numbers have been found in the s.c. adipose tissue of patients with HIV-associated lipodystrophy (17, 18). We therefore tested whether inflammation is associated with lipodystrophy and its metabolic consequences in aP2-nSREBP-1c transgenic (Tg) mice, an established model of the disease with characteristics that match many of those seen in humans (19). Preclinical studies with these mice formed the basis for ultimately using leptin to treat patients with lipodystrophy (20, 21), further suggesting that the pathophysiology and pharmacological responsiveness of these mice is relevant to related human conditions.
Results
Systemic Inflammation in Lipodystrophic Mice.
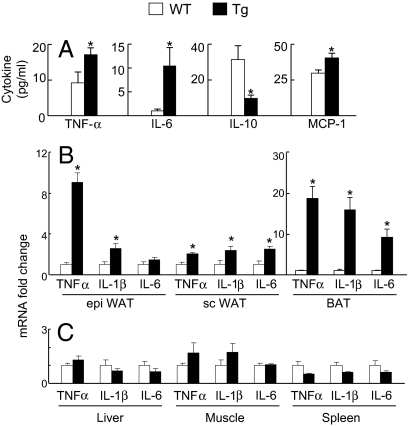
Our first studies looked for circulating evidence of inflammation in the Tg mice (Fig. 1A). Proinflammatory cytokines were elevated 1.9-fold for TNF-α and 10.2-fold for IL-6 compared to WT littermates (P < 0.05), whereas the antiinflammatory cytokine IL-10 was 3.3-fold lower in Tg mice (P < 0.03). MCP-1 was concordantly elevated 1.4-fold relative to WT (P < 0.03). Consistent with substantially reduced white adipose tissue (WAT) mass, circulating adipokines, including leptin and resistin, were lower in Tg vs. WT littermates (Fig. S1).
Fig. 1.
Circulating cytokines and gene expression. (A) Circulating cytokine and chemokine concentrations WT control (open bars) vs. Tg (filled bars) littermates. (B, C) mRNA expression in epididymal (epi) WAT; s.c. (sc) WAT; interscapular BAT, liver, muscle, and spleen. (n = 6) *P < 0.05 WT vs. Tg.
Adipose Tissue Inflammation.
To identify the source of circulating inflammatory mediators we conducted a whole-body histological examination of the Tg mice. After total body fixation and en bloc sectioning, we examined essentially every tissue and found that although there was little evidence for inflammation in other tissues, the adipose tissue of Tg mice was highly abnormal and heavily infiltrated with mononuclear cells (Fig. S2). The affected WAT included epididymal, perirenal, retroperitoneal, mesenteric, cervical, and all s.c. depots (e.g., axillary, inguinal, and popliteal). Brown adipose tissue (BAT) was also affected, including the interscapular, cervical, mediastinal, and retroperitoneal regions. Despite severe steatosis there was no evidence for an enhanced inflammatory response in the liver.
Consistent with the histological observations, mRNA expression levels for the proinflammatory cytokines TNFα, IL-1β, and IL-6 were elevated in Tg vs. WT WAT and BAT, but unchanged in other tissues such as liver, muscle, and spleen (Fig. 1 B and C; Fig. S3 A and B). In epididymal WAT, mRNA levels from TNF-α and IL-1β were elevated 9.1- and 2.6-fold, respectively, in Tg mice vs. WT controls (P < 0.02). mRNA levels for TNF-α, IL-1β, and IL-6 were increased 2.0-, 2.4-, and 2.5-fold, respectively, in inguinal s.c. WAT and 18.8-, 15.9-, and 9.2-fold respectively in interscapular BAT in Tg mice relative to WT littermates (P < 0.05).
Mononuclear cells infiltrated the atrophic visceral and s.c. WAT (Fig. 2 A–D), as well as the hypertrophic interscapular BAT (Fig. 2 E and F) of the Tg mice. The size distribution of Tg white adipocytes is markedly heterogeneous, comprising mixtures of what appear to be smaller, immature cells and larger, more mature adipocytes (Fig. 2 A–D). Tg brown adipocytes are enlarged, compared to WT counterparts, and contain large, unilocular vacuoles resembling WT white adipocytes; visually, this appears to be a BAT to WAT transition. Cytokines produced selectively by adipocytes include leptin, adiponectin, and possibly resistin. mRNA expression levels for each of these adipokines was correspondingly reduced in epididymal and s.c. Tg WAT (Fig. S3 C and D), consistent with the degree of lipoatrophy. Leptin, adiponectin, and resistin mRNA expression levels were also reduced in the Tg BAT, suggesting that although Tg BAT is hypertrophic and resembles WT WAT, the histological appearance is not accompanied by corresponding biochemical changes. A dedifferentiated phenotype for Tg BAT was further demonstrated by a strikingly diminished expression of UCP-1 (370-fold lower, P < 0.001, Fig. S3E), again suggesting that the Tg BAT lacks characteristics of both mature WT WAT and mature WT BAT. We conclude that the sources for elevated circulating inflammatory mediators in the Tg mice include both WAT and BAT and that potential contributions from other organs are likely negligible.
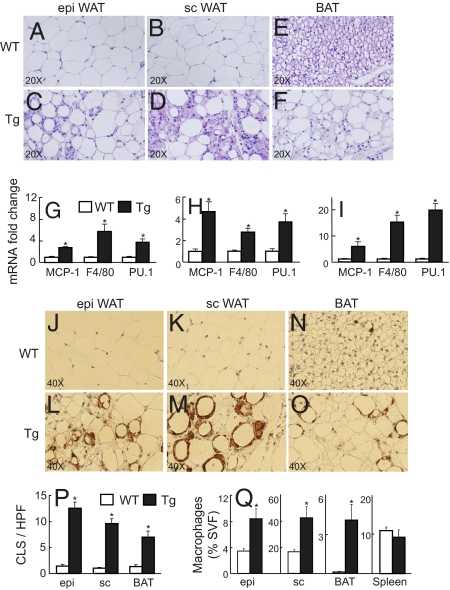
Fig. 2.
Adipose tissue histology and FACS analysis. (A–F) Histological sections from epi (A, C) and s.c. (B, D) WAT, and BAT (E, F) from representative 24-week-old WT control and Tg littermates stained with H&E. (G–I) mRNA expression from epi (G) and sc (H) WAT, and BAT (I) from WT control (open bars) vs. Tg (filled bars) littermates (n = 6). (J–O) Immunohistochemical localization of macrophages in epi (J, L), sc (K, M), and BAT (N, O) of a representative WT control vs. Tg littermates. (P) Counting of crown-like structures (CLS) per high power field (HPF) from the above male WT control and Tg littermates (10 HPF counted per mouse, n = 5 mice per genotype). (Q) Flow cytometry analyses of macrophage number from epi, sc, BAT, and spleen WT controls vs. Tg littermates. n = 4, *P < 0.05 WT vs. Tg.
Evidence for inflammatory cell infiltration of the adipose tissue of Tg mice was further evaluated using quantitative RT-PCR. mRNA expression levels corresponding to macrophage-related proteins were elevated in epididymal WAT from Tg relative to WT mice, including the chemokine MCP-1 (2.7-fold, P < 0.0002), the surface antigen Emr1 recognized by F4/80 antibody (5.8-fold, P < 0.008), and the macrophage transcription factor PU.1 (3.8-fold, P < 0.003) (Fig. 2G). mRNA expression levels for MCP-1 (4.7-fold, P < 0.004), F4/80 (2.7-fold, P < 0.002), and PU.1 (3.7-fold, P < 0.01) were similarly elevated in inguinal s.c. WAT (Fig. 2H) and to an even greater degree in the intrascapular BAT of Tg mice relative to WT littermates: MCP-1 (5.9-fold, P < 0.05), F4/80 (12-fold, P < 10−5), and PU.1 (16-fold, P < 0.0001) (Fig. 2I). Up-regulated expression of F4/80(Emr1) and PU.1 most likely reflect macrophage recruitment, whereas TNF-α, IL-1β, IL-6, and MCP-1 may be expressed by adipocytes as well as macrophages and other leukocytes.
Adipose Tissue Macrophages.
Immunohistochemistry further identified cell types involved in the inflammatory changes in Tg adipose tissue. Mac2-staining macrophages were abundant in both epididymal and s.c. WAT from lipodystrophic Tg mice and almost absent in control littermate WAT (Fig. 2 J–M). The ATMs occurred both individually and enveloping selected adipocytes to form crown-like structures (Fig. 2P; five mice of each genotype and tissue, 10 high power fields examined). ATMs were also present but infrequent in control BAT (Fig. 2N), but here too the number of infiltrating ATMs in the hypertrophic Tg BAT increased dramatically (Fig. 2 O and P). Macrophages were present diffusely but also surrounding adipocytes, suggesting accelerated adipocyte dropout as well in lipodystrophic Tg BAT.
Flow cytometry was used to further characterize and quantify the infiltrating ATMs in Tg WAT and BAT (Fig. S4 A–F). ATM numbers were increased 2.5-fold in epididymal Tg WAT (P < 0.02), 2.6-fold in s.c. Tg WAT (P < 0.02), and 41.0-fold in Tg BAT (P < 0.02) compared with WAT and BAT from WT littermates (Fig. 2Q). Macrophage numbers in the spleens of Tg and WT mice were equivalent, in contrast to the differences seen in WAT and BAT.
AntiInflammatory Strategies.
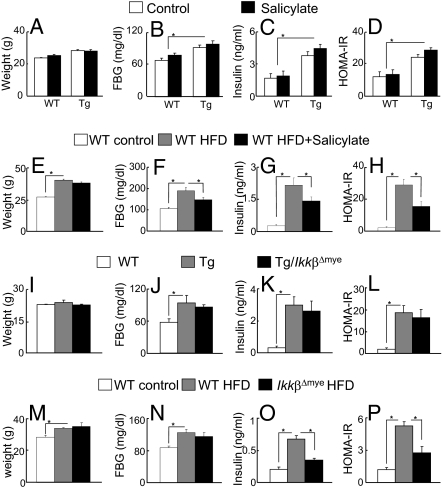
Tg adipose tissue was infiltrated with a striking number of macrophages, reminiscent of obesity but to a more exaggerated extent (Fig. S4G). We therefore wondered whether antiinflammatory strategies being used to treat insulin resistance in obesity could similarly be used in lipodystrophic mice. Two methods are available to conveniently target inflammation in obese mice as a way of improving insulin resistance. The first is the antiinflammatory drug salicylate, an effective pharmacologic inhibitor of NF-κB and insulin sensitizer in rodents and humans (8, 22–25). The other uses Cre-Lox technology to genetically delete IκB kinase β (IKKβ) and thus inhibit NF-κB, in myeloid cells (IkkβΔmye), including macrophages (26). We asked whether either of these antiinflammatory approaches would be effective in lipodystrophy.
Salicylate was incorporated into the diet (4 g salicylate/kg chow) as is often done to treat obesity-induced insulin resistance (8, 23). Despite the fact that Tg mice were treated with the drug at doses and periods of time sufficient to decrease insulin resistance in both high fat diet (HFD) and genetic (ob/ob) obesity models, salicylate had no impact on insulin resistance in lipodystrophic mice. Fasting glucose and insulin levels and HOMA-IR values were identical to Tg littermates treated with the same diet lacking salicylate (Fig. 3 A–D); in contrast, each of these parameters was improved by salicylate treatment of mice fed a HFD (Fig. 3 E–H). In the second set of studies Tg mice were crossed with IkkβFlox/Flox and LyzM-Cre mice to generate lipodystrophic Tg mice lacking IKKβ in myeloid lineages. NF-κB activation is defective in macrophages and granulocytes of IkkβΔmye mice, which protects against the development of insulin resistance in diet-induced obesity (26) (Fig. 3 M–P). This was not the case in Tg/IkkβΔmye mice, as body weights, fasting glucose, insulin levels, and HOMA-IR values were essentially identical to Tg/IkkβFlox/Flox littermate controls (Fig. 3 I–L). Measurements of TG content in insulin-responsive tissues such as liver and muscle showed no change between Tg and salicylate-treated or Tg/IkkβΔmye mice (Fig. S5). These data strongly indicate that inflammation in lipodystrophic mice does not induce insulin resistance as it does in obesity, despite there being substantial evidence of inflammation both systemically and in Tg adipose tissue.
Fig. 3.
Antiinflammatory strategies. (A–D) Sodium salicylate treatment of lipodystrophic mice. WT control (open bars) and Tg (filled bars) littermates were fed normal chow (control) or chow containing 4 g/kg sodium salicylate for 5 weeks. (E–H) C57BL/6J male mice treated with chow (open bars) and HFD (gray bars) for 10 weeks or HFD for 5 weeks followed by HFD plus salicylate for another 5 weeks (black bars). (I–L) Myeloid-selective deletion of IKKβ in Tg male mice. WT control (open bars), Tg (gray bars), and Tg/IkkβΔmye (black bars) littermates were fed normal chow. (M–P) WT control male mice treated with chow (open bars) or with HFD (gray bars) and IkkβΔmye mice treated with HFD (black bars) for 6 weeks. Body weights (A, E, I, M), fasting blood glucose (B, F, J, N), fasting insulin (C, G, K, O), and the resistance index, HOMA-IR (D, H, L, P) (homeostasis model assessment-insulin resistance: fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5) were measured. n = 7, *P < 0.05.
Adipose Tissue Transplantation to Reverse Insulin Resistance.
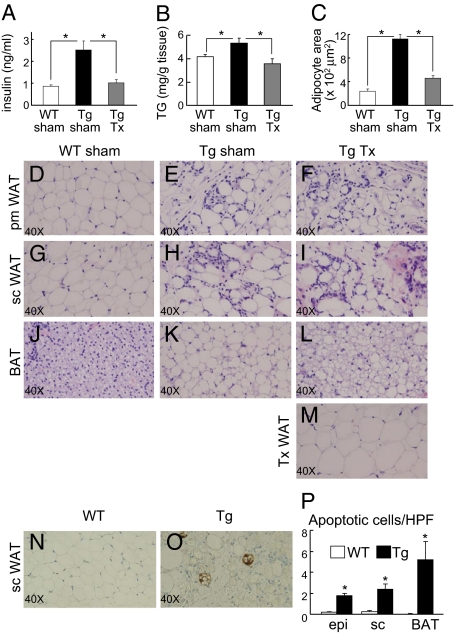
We next asked the converse question, what effect would reversal of insulin resistance have on the leukocyte infiltrates in Tg adipose tissue? To accomplish this we used methods for adipose tissue transplantation very similar to those originally developed by Gavrilova and Reitman (27). Parametrial fat pads from WT female littermate donors were implanted s.c. into the recipients’ backs. Four weeks after surgery, transplanted Tg mice had normalized fasting insulin levels (Fig. 4A). The restoration of insulin sensitivity was accompanied by a redistribution of triglyceride from ectopic sites, including liver (Fig. 4B) and BAT (Fig. 4 C and J–L), to the transplanted donor WAT, which appeared healthy and well-vascularized, without overt signs of inflammation (Fig. 4M). Presumably in response to lipid redistribution, the adipocytes in the transplanted donor parametrial WAT were larger on average than adipocytes in control (sham) parametrial WAT (Fig. 4 D and M). The restoration of insulin sensitivity was likely contributed to by both redistribution of triglyceride from ectopic sites (e.g., liver, muscle) into transplanted adipose tissue and increased circulating leptin to reduce hyperphagia (WT sham, 2.19 ± 0.40 ng/mL; Tg sham, 0.61 ± 0.10 ng/mL; and transplanted Tg, 1.03 ± 0.18 ng/mL).
Fig. 4.
Fat transplantation and adipose tissue apoptosis. (A–M) Normal WT fat was transplanted into Tg female littermate recipients. (A–C) Fasting insulin, liver triglyceride (TG) content, and brown adipocyte area were measured four weeks after the surgery for sham-operated WT controls (open bars), sham-operated Tg mice (black bars), and Tg mice transplanted with WT fat (Tg Tx, gray bars) (n = 6). *P < 0.05. (D–L) Histological sections (H&E staining) from parametrial (pm) WAT (D–F), sc (G–I) and BAT (J–L) of representative WT sham-operated, Tg sham-operated, and Tg transplanted littermates. (M) Histological section (H&E) of transplanted fat pad removed from the recipient 4 weeks after the surgery. (N–O) Histological sections stained for apoptosis of sc WAT from a representative WT control and Tg littermates. (P) Cell counting per HPF from the above WT control (open bars) vs. Tg (filled bars) littermates epi, sc and BAT (10 HPF counted per mice, n = 5 mice per genotype). *P < 0.05 WT vs. Tg.
Despite positive changes in multiple metabolic parameters, endogenous WAT in the transplant recipients was unaltered following surgery, with degrees of mononuclear cell infiltration and heterogeneity of adipocyte size that were indistinguishable from the Tg sham controls (Fig. 4 D–I). On the other hand, brown adipocyte size decreased substantially (Fig. 4C) and there was reversion from unilocular lipid vacuoles to more normal appearing multilocular vacuoles, undoubtedly due to redistribution of triglyceride into the WT donor transplanted fat (Fig. 4 C and J–L). Thus, insulin sensitivity and adipose tissue inflammation coexisted in the transplanted Tg mice, consistent again with the notion that ATMs in the Tg mice do not contribute to insulin resistance, as they appear to in obesity.
Adipocyte Apoptosis.
The number of infiltrating leukocytes in Tg WAT and BAT, and the envelopment of selected adipocytes by infiltrating leukocytes, suggested that adipocytes were either dying prematurely or producing chemoattractants that recruit the immune cells. Dying adipocytes were identified in Tg epididymal WAT by an increase in the number of cells that did not stain for perilipin, a protein that coats the lipid droplet, compared with littermate controls (Fig. S6 A and B). The leukocyte-encircled adipocytes appeared to lack an intact plasma membrane and were likely dead or dying as has been observed in obesity (28). We next considered potential mechanisms for cell death in the Tg mice. Immunohistochemical staining with anti-caspase-3 revealed increased numbers of apoptotic cells in both WAT and BAT from Tg mice (Fig. 4O; Fig. S6 F–H) compared with control littermates (Fig. 4N; Fig. S6 C–E). Positively stained cells, counted from multiple sections (5 mice each genotype, 10 high power fields/mouse), were increased 11-fold in epididymal WAT (Tg, 1.76 ± 0. vs. WT, 0.16 ± 0.06; P < 0.0001), 10.7-fold in s.c. WAT (Tg, 2.36 ± 0.51 vs. WT, 0.22 ± 0.1; P < 0.003), and 260-fold in BAT (Tg, 5.22 ± 1.72 vs. WT, 0.02 ± 0.02; P < 0.02) (Fig. 4P). In contrast, adipocytes from HFD treated mice did not show evidence of apoptosis (Fig. S6I), indicating potentially different mechanisms for adipocyte death, leukocyte recruitment, and adipose tissue inflammation in obesity vs. lipodystrophy.
Macrophage Phenotypes.
Macrophages that phagocytose apoptotic cells use distinct mechanisms and are differentially activated compared to macrophages recruited to sites of inflammation and necrosis (29). Adipocyte apoptosis in Tg adipose tissue thus suggested that these macrophages might differ from those present in the adipose tissue of obese mice, particularly because macrophages thought to contribute to the development of obesity-induced insulin resistance are thought to surround necrotic as opposed to apoptotic adipocytes (28). We therefore further analyzed the macrophages from obese and lipodystrophic mice (Fig. 5 A–D).
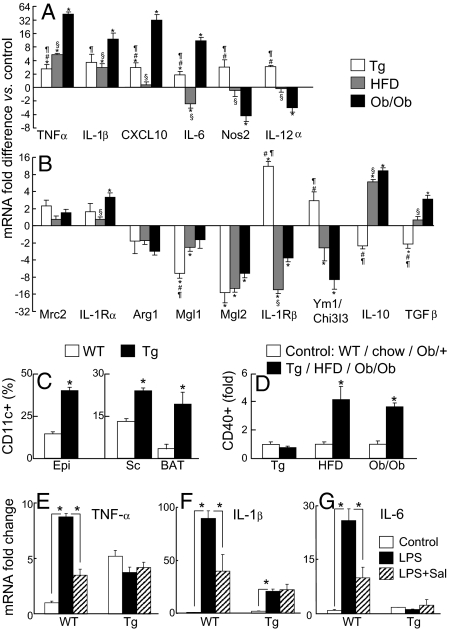
Fig. 5.
Analyses of macrophage gene expression and activation. (A–B) mRNA gene expression from epi WAT sorted macrophages (CD11b+F4/80+) from Tg (white bars), HFD (gray bars) and ob/ob (black bars) and their respective littermate controls. Fold change in gene expression over the appropriate control is plotted on a log2 scale (n = 5). P < 0.05 *Tg, HFD or ob/ob vs. the respective control, #Tg vs. HFD, ¶Tg vs. ob/ob and §HFD vs. ob/ob. (C) CD11c expression (CD11b+F4/80+CD11c+) in WT mice (open bars) vs. Tg littermates (filled bars; n = 3–4). *P < 0.05 WT vs. Tg. (D) Adipose tissue macrophage activation based on CD40 expression (CD11b+F4/80+CD40+) in control mice (open bars) vs. Tg, HFD or ob/ob littermates respectively (filled bars; n = 3–4). *P < 0.05 control vs. ob/ob or HFD. (E–G) Epididymal WAT mRNA expression from WT control and Tg littermates injected with saline (open bars), LPS (black bars) or treated with sodium salicylate before injection with LPS (striped bars) (n = 5; *P < 0.05).
Sorted macrophages (Fig. S4 A–F) were collected and analyzed for mRNA expression. The expression patterns for genes associated with classical (M1) and alternative (M2) activation are plotted in Fig. 5 A and B, respectively. As has been reported previously (15), we see trends toward the up-regulation of a subset of M1-associated genes and the down-regulation of a subset of M2-associated genes in macrophages isolated from obese adipose tissue. TNF-α, IL-1β, CXCL10, and IL-6 are up-regulated 8- to 41-fold in ATMs from ob/ob mice, whereas the expression of M1-associated Nos2 and IL-12α are down-regulated. In contrast all six of these M1-associated genes are up-regulated in Tg ATMs but only by a marginal two- to threefold. M2-associated genes are disparately regulated as well. IL-1Rβ and Ym1/Chi313 are substantially down-regulated in HFD and ob/ob models of obesity but up-regulated in lipodystrophy. The opposite holds for IL-10 and TGFβ, which are up-regulated in obesity but down-regulated in lipodystrophy. Triply positive F4/80+CD11b+CD11c+ macrophages, described to be found in obese WAT (15), were present as well in the adipose tissue of the lipodystrophic mice. In fact the percentages of CD11c+ cells within the F4/80+CD11b+ gate were increased for epididymal and s.c. WAT as well as interscapular BAT (Fig. 5C). Therefore, the ATMs from neither obesity nor lipodystrophy have purely M1 or M2 phenotypes, but are mixtures of expression corresponding to the two categories. Most importantly, ATMs from obese and lipodystrophic mice differ significantly from one another and both are different from ATMs from WT mice.
Distinctions between ATMs in obesity and lipodystrophy were further investigated using surface markers for macrophage activation. The proportion of CD11b+F4/80+ macrophages that also express CD40 is increased 4.1-fold in HFD mice (P < 0.04) and 3.6-fold in ob/ob mice (P < 0.003) compared to relevant controls but is unchanged in ATMs from Tg mice (Fig. 5D). Inasmuch as ATMs contribute to insulin resistance in obesity, which appears to be the case, ATMs in lipodystrophy have a distinct phenotype and activation state that does not contribute to insulin resistance.
Challenges with lipopolysaccharide (LPS), a prototypic endotoxin and TLR4 ligand, create a systemic inflammatory response thought to mimic some features of insulin resistance (30). We challenged WT and Tg mice with low dose LPS to further assess ATM responses. Epididymal adipose tissue was harvested 2 h after the i.p. injection of LPS, and mRNA expression was determined for proinflammatory cytokines. The responses in WT mice were dramatic, with large increases in the 2 h expression of mRNAs encoding TNF-α (8.7-fold, P < 10−8), IL-6 (26-fold, P < 10−5), and IL-1β (90-fold, P < 10−6) (Fig. 5 E–G). This is in sharp contrast to the situation with Tg mice, where the identical i.p. dose of LPS had no effect on the 2 h TNF-α or IL-6 mRNA expression and a markedly blunted effect on IL-1β mRNA expression (10-fold compared to the 90-fold seen in WT mice). This suggested an inability to respond to challenge, akin to anergy or LPS tolerance, despite the fact that the Tg mice had never before been challenged with LPS.
Given our interest in using the antiinflammatory drug salicylate to treat insulin resistant states related to inflammation (8, 22, 24, 25), we also analyzed the ability of this drug to suppress LPS-induced cytokine gene expression. Mice were chronically treated with salicylate in their diet (4 g/kg), as we typically do to treat insulin resistance in obese C57BL/6J mice. Salicylate suppressed LPS-induced cytokine gene expression by 50–70% in WT mice: TNF-α mRNA decreased from 8.7- to 3.5-fold over basal (P < 0.0001), IL-6 mRNA expression decreased from 26-fold to 10-fold over basal (P < 0.01), and IL-1β expression decreased from 90- to 40-fold over basal (P < 0.03) (Fig. 5 E–G). In contrast, salicylate treatment of Tg mice had no effect on the expression of the cytokine mRNAs in adipose tissue. The inability of LPS to induce the expression of TNF-α and IL-6 and the inability of salicylate to suppress expression of all three cytokines underscores the significant differences in the activities of ATMs from Tg vs. WT or obese epididymal fat. It further identifies a population of ATMs in lipodystrophy different from obese ATMs in terms of gene expression, CD40 activation, LPS response, and involvement in insulin resistance.
Discussion
Despite being opposites in terms of increased vs. decreased adipose tissue mass, obesity and lipodystrophy share common metabolic consequences, thus providing an opportunity to compare and contrast their underlying pathophysiological mechanisms. Obesity is accompanied by altered numbers and activities of immune cells in adipose tissue (13, 14, 15, 28), where infiltrating macrophages, in particular, are suspected to be potential mediators of insulin resistance and diabetes (14, 31), although numerous questions about mechanism remain (32). In contrast, inflammation has not been considered to be a significant contributor to the metabolic derangements seen in lipodystrophy (1). We have therefore evaluated biochemical, cellular, and pharmacological parameters of inflammation in the circulations and tissues of aP2-nSREBP transgenic mice, a favored model for studies of lipodystrophy.
Significantly elevated levels of proinflammatory cytokines and chemokines (e.g., TNF-α, IL-6, MCP-1) and decreased levels of antiinflammatory cytokines (e.g., IL-10) in the circulations of lipodystrophic mice provided the first clues about systemic inflammation. A whole-body histological scan found that the histopathologic picture in Tg mice is one of generalized macrophage infiltration into all adipose tissue depots, with significant numbers of T and B lymphocytes and mast cells also present (Fig. S7 ). This is not the case in other tissues, including the severely steatotic livers of the lipodystrophic mice. Inflammatory infiltrates in the adipose tissue were confirmed using RT-PCR to quantify relative mRNA expression levels corresponding to proteins previously shown to be up-regulated in obese adipose tissue. We therefore concluded that the circulating evidence for inflammation in the lipodystrophic mice originated in the dystrophic fat. Importantly and consistent with our results, increased levels of expression of proinflammatory cytokines and macrophages (17, 18) have been found in s.c. adipose tissue from humans with HIV-associated lipodystrophy, the most common form of lipodystrophy seen today. This data reinforces the relevance of the lipodystrophic mouse model used here to clinical practice.
We next investigated whether antiinflammatory strategies for treating insulin resistance in obesity might also be helpful for treating insulin resistance in lipodystrophy. Salicylates are antiinflammatory drugs known to decrease inflammation, glucose, and insulin levels in rodents and humans with obesity and diabetes (8, 22, 24, 25); we are currently conducting multicenter clinical trials to determine whether salicylate might be used as a treatment for obesity-induced insulin resistance (www.tinsal-t2d.org) (24, 25). High-dose salicylates do not promote the redistribution of ectopic fat, as is seen with thiazolidinediones, but work at least in part by inhibiting NF-κB signaling (8, 33). We therefore looked at the ability of salicylates to improve insulin resistance in the lipodystrophic mice, but there was no improvement despite positive effects in control obese mice. Similarly ineffective was the myeloid deletion of Ikkβ in lipodystrophic mice (Tg/IkkβΔmye), despite the fact that this maneuver improves insulin resistance in obese mice through a blockade of NF-κB signaling in adipose tissue macrophages (26). We therefore believe that despite there being large numbers of adipose tissue macrophages and additional evidence of inflammation in both adipose tissue and the circulations of lipodystrophic mice, these apparently do not participate in the pathogenesis of insulin resistance and therefore cannot be used as pharmacological targets for reversal. Inasmuch as mouse models predicts human responses, and this model has (20, 21), the results presented here predict that antiinflammatory strategies including salicylates will be of little use in lipodystrophic patients.
So what distinguishes adipose tissue inflammation in lipodystrophy from adipose tissue inflammation in obesity and allows antiinflammatory maneuvers to improve insulin resistance in the latter but not the former? There is evidence of accelerated adipocyte apoptosis in the Tg mice, suggesting that the infiltrating macrophages were recruited in response to dead or dying adipocytes. Although adipocyte apoptosis is not known to be a major feature of obesity, infiltrating macrophages in response to dead or dying adipocytes is (28). This suggests that macrophage recruitment and activation might occur in both conditions, but the responses to apoptotic and necrotic cells may be different.
ATMs from lean mice have been described as having M2 or alternatively activated characteristics, whereas those in the expanding adipose tissue of obesity have more of an M1 polarity (15). M1 and M2 categories were originally used to describe the in vitro responses of elicited macrophages. LPS and/or IFN-γ challenge yield an M1 phenotype and the production of proinflammatory cytokines and ROS having antimicrobial activity. IL-4 and/or IL-13 stimulation elicit an M2 phenotype more related to tissue repair and the suppression of inflammation (34). That the ATMs in lipodystrophy have neither a purely M1 nor purely M2 polarization is not surprising, after all these macrophages are not responding to simple in vitro stimuli but to the much more complex in vivo environment of lipodystrophic fat. Like the ATMs in obesity, ATMs in lipodystrophy appear to have more of a mixed, intermediate type of polarity. We found it more surprising and interesting that the macrophages isolated from obese adipose tissue and those isolated from lipodystrophic adipose tissue are so different from each other in terms of surface marker expression, gene expression profiling, and responses—or the lack of response—to potent stimuli.
From a clinical perspective, the most important result of this study, however, is the finding that insulin resistance and hyperglycemia are not improved in the Tg mice by antiinflammatory drug or genetic manipulations. The fact that these mice predicted leptin responsiveness in human lipodystrophy and that obese mice predicted salicylate responsiveness in subjects with type 2 diabetes and the metabolic syndrome together argue that our current findings predict that lipodystrophic patients will not respond to antiinflammatory strategies, including salicylates. This can and most likely will be tested in human subjects, but the likelihood of positive clinical results is substantially reduced by these findings.
Materials and Methods
Mice.
Study mice were aP2-nSREBP-1c and ob/ob (Jackson Laboratories), C57BL/6J and IkkβΔmye (26). In selected experiments mice were fed for 16 weeks with either standard chow (10% kcal fat) or HFD (60% kcal fat) (Research Diets). Sodium salicylate (4 g/kg) was incorporated into Purina 5008 chow by Harlan Teklad. Experiments were conducted in accordance with the National Institutes of Health guidelines under protocols approved by the Joslin Institutional Animal Care and Use Committee.
Methods.
Serum insulin, TNFα, IL-6, and IL-10 were measured in the Joslin Diabetes Endocrinology Research Center Assay Core. Serum MCP-1 was measured by Luminex (Linco). For quantitative Real-time RT-PCR, total RNA was extracted (RNeasy, QIAGEN) and cDNA synthesized (Advantage RT-for-PCR kit, BD Biosciences). PCR amplifications (Applied Biosystems) were normalized against 18S and TATA box binding protein (Tbp). Immunohistochemistry was performed by the Joslin Histology Core and the Longwood Specialized Histopathology Core at the Brigham and Women's Hospital. For flow cytometry, stromal-vascular fractions from adipose tissue and spleen were stained with specific fluorescent antibodies and analyzed by FACS (LSRII, BD Biosciences). RNA was extracted (TRIzol, Invitrogen) from sorted macrophages (CD11b+F4/80+) and analyzed by FACS (FACSAria, BD Biosciences). WAT transplantation experiments were performed as previously described (27).
Statistics.
Data are presented as mean ± SEM. Student t tests were used for statistical analysis. A probability level of P < 0.05 was considered to be statistically significant.
For additional information, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank O. Gavrilova for helpful discussions, M. Karin for providing the IkkβΔmye mice, C. Bertochi and D. Jamieson for expert research assistance, and J. LaVecchio and G. Buruzula at Joslin Diabetes Endocrinology Research Center/Harvard Stem Cell Institute Flow Cytometry Core. These studies were supported by National Institutes of Health Grants R01 DK45943, R37 DK51729, and R01 DK73547 to S.E.S.; P30 DK36836 (Joslin Diabetes Endocrinology Research Center); postdoctoral fellowships from the Ministry of Education and Science of Spain (L.H.) and American Diabetes Association (7-04-MN-47), and the Helen and Morton Adler Chair (S.E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905310107/DCSupplemental.
References
- 1.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 2.Villarroya F, Domingo P, Giralt M. Lipodystrophy in HIV 1-infected patients: Lessons for obesity research. Int J Obes (Lond) 2007;31:1763–1776. doi: 10.1038/sj.ijo.0803698. [DOI] [PubMed] [Google Scholar]
- 3.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 5.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 8.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JA, et al. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2004;286:E261–E271. doi: 10.1152/ajpendo.00056.2003. [DOI] [PubMed] [Google Scholar]
- 18.Lagathu C, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 19.Shimomura I, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 21.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 22.Hundal RS, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldfine AB, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and Type 2 Diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilova O, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: Signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 30.Anderson PD, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 31.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Neels JG, Olefsky JM. Inflamed fat: What starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 34.Lacy-Hulbert A, Moore KJ. Designer macrophages: Oxidative metabolism fuels inflammation repair. Cell Metab. 2006;4:7–8. doi: 10.1016/j.cmet.2006.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.