Abstract
Fertilization triggers a rise in intracellular Ca2+ concentration ([Ca2+]i) in the egg that initiates a series of events known as egg activation. These events include cortical granule exocytosis that establishes a block to polyspermy, resumption of meiosis, and recruitment of maternal mRNAs into polysomes for translation. Several calcium-dependent proteins, including calcium/calmodulin-dependent protein kinase II (CaMKII), have been implicated in egg activation. However, the precise role of CaMKII in mediating specific events of egg activation and the identity of the isoform(s) present in mouse eggs have not been unequivocally established. Through targeted deletion of the γ isoform of CaMKII, we find that CaMKIIγ is the predominant CaMKII isoform in mouse eggs and that it is essential for egg activation. Although CaMKIIγ−/− eggs exhibit a normal pattern of Ca2+ oscillations after insemination and undergo cortical granule exocytosis, they fail to resume meiosis or to recruit maternal mRNAs. Surprisingly, we find that the recruitment of maternal mRNAs does not directly depend on CaMKII, but requires elevated [Ca2+]i and metaphase II exit. We conclude that CaMKIIγ specifically controls mouse egg activation by regulating cell cycle resumption.
Keywords: CaMKII, fertilization, metaphase II exit
The transition from a fertilization-competent mammalian egg to a developing embryo entails a sequence of events collectively known as egg activation. Early events of egg activation include modifications of the zona pellucida (ZP) that prevent polyspermy, exit from metaphase II arrest, and completion of meiosis, whereas late events include recruitment of maternal mRNAs into polysomes for translation and formation of male and female pronuclei (1). In all animal species studied to date, a rise in intracellular calcium ([Ca2+]i) is the universal trigger of all of the events of egg activation (EEA) (2). Release of a sperm-specific phospholipase C isoform (PLCζ) likely initiates the inositol 1,4,5-trisphosphate (IP3)–mediated increase in [Ca2+]i (3). In mammalian eggs, this increase in [Ca2+]i takes the form of repetitive Ca2+ transients (oscillations) that last several hours (4, 5). Although Ca2+ oscillations can induce all of the EEA, individual events require different numbers of [Ca2+]i transients to be initiated and completed, with early events requiring fewer oscillations than late events (6).
The pathways that connect the rise in [Ca2+]i to the different EEA have only been partially elucidated. Protein kinases, including calcium/calmodulin-dependent protein kinase II (CaMKII), and the phosphatase calcineurin have been postulated as integrators of the Ca2+ signal during egg activation (7–9). However, direct genetic evidence for involvement of these enzymes through loss-of-function studies is lacking, leaving open questions as to whether any single enzyme is essential for all of the EEA or whether these Ca2+-dependent events are mediated by different effectors.
The CaMKII family of serine/threonine kinases mediates many cellular responses to Ca2+ signals. Multiple isoforms of CaMKII are encoded by four genes (α, β, γ, and δ) that have distinct but overlapping expression patterns (10). In mouse eggs, CaMKII activity was originally reported to increase after parthenogenetic activation (11) and to fluctuate in parallel with Ca2+ oscillations (12). Additional investigations using pharmacological inhibitors or constitutively active mutant forms of the enzyme supported a role for CaMKII during egg activation [reviewed in (7)]. However, these approaches failed to reveal the identity of the CaMKII isoforms that might mediate EEA in vivo.
Targeted deletion of CaMKIIα, CaMKIIβ, or CaMKIIδ in mice results in very specific and diverse phenotypes (13–16), but none of these mutant strains display fertility defects. In contrast, we show here that female CaMKIIγ−/− mice are infertile due to egg activation defects. CaMKIIγ−/− eggs display normal sperm-induced [Ca2+]i oscillations and are able to mount a postfertilization ZP block to polyspermy. However, in the absence of CaMKIIγ, cell cycle resumption, decreases in mitogen-activated protein kinase (MAPK) and maturation-promoting factor (MPF) activities, pronuclear formation, and maternal mRNA recruitment do not occur. We also demonstrate that maternal mRNA recruitment does not depend directly on CaMKII but requires increased [Ca2+]i and cell cycle resumption. We conclude that CaMKIIγ controls mouse egg activation by regulating metaphase II exit.
Results
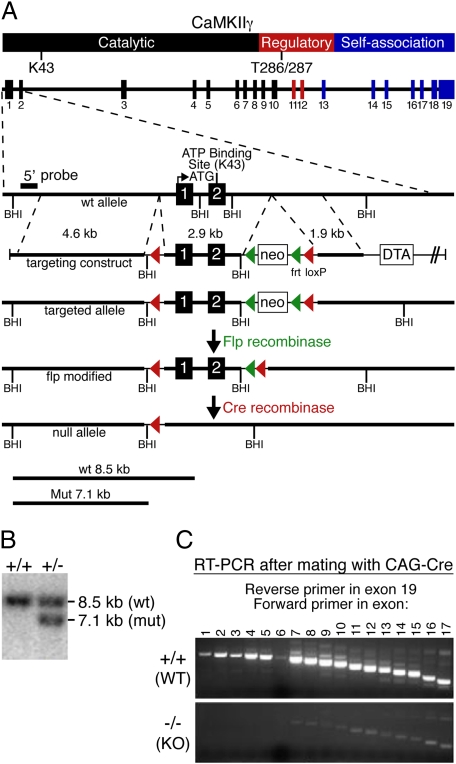
Deletion of CaMKIIγ. We generated a conditional null allele of the mouse CaMKIIγ gene using homologous recombination. LoxP sites were inserted into the CaMKIIγ locus to flank exons 1 and 2, which encode part of the catalytic domain of CaMKIIγ, including the ATP binding motif that is essential for kinase activity (Fig. 1A). Correct targeting was confirmed by Southern blot hybridization (Fig. 1B). We disrupted the gene by crossing mice with the conditional CaMKIIγ allele with a transgenic mouse line carrying the CAG-Cre transgene, which expresses Cre recombinase in the embryo at the zygote stage (17). Using RT-PCR, we confirmed the absence of CaMKIIγ transcripts encoding exons 1 through 5 in homozygous mutant mice (Fig. 1C). An alternative transcript with weak expression that starts at exon 6 cannot generate a functional kinase because the domain required for catalytic activity is missing.
Fig. 1.
Targeting of the mouse CaMKIIγ gene. (A) CaMKIIγ protein structure, intron–exon structure of the CaMKIIγ gene, and gene targeting strategy. (B) Representative Southern blot of genomic DNA from gene-targeted embryonic stem cells digested with BamHI (BHI) using a probe hybridizing to a genomic region upstream of the long arm of the targeted region. Wild-type (WT) and mutant bands are shown. CaMKIIγ genotypes are shown at the top. (C) RT-PCR to detect CaMKIIγ transcripts in WT and CaMKIIγ−/− (KO) mice. The reverse primer lies in exon 19, and the numbers of the forward primers correspond to the exons containing their sequence.
CaMKIIγ−/− (KO) mice were viable, with no obvious morphological or behavioral defects. However, whereas male KO mice and female CaMKIIγ+/− (HET) mice were fertile, we were unable to generate any offspring from female KO mice.
CaMKIIγ Is the Predominant CaMKII Isoform in Mouse Oocytes.
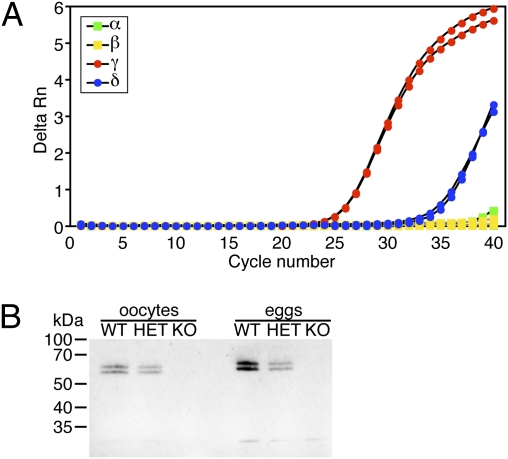
Quantitative real-time RT-PCR on RNA samples from fully grown mouse oocytes showed that the expression of CaMKIIγ was about 500-fold higher than CaMKIIδ and ∼1,700-fold higher than CaMKIIα; CaMKIIβ mRNA was undetectable (Fig. 2A). As a control, all four isoforms were easily detectable in brain tissue. When oocytes from CaMKIIγ WT, HET, and KO mice were analyzed by real-time RT-PCR, we detected a 54% reduction of CaMKIIγ transcript in HET oocytes and no expression in KO oocytes (Fig. S1). Of note, none of the other three isoforms were upregulated in CaMKIIγ−/− oocytes.
Fig. 2.
CaMKIIγ is the predominant CaMKII isoform in mouse oocytes and eggs. (A) Real-time RT-PCR of CaMKIIα, β, γ, and δ transcripts in mouse oocytes. The experiment was performed three times and a representative example is shown. (B) Lysates from 50 oocytes or eggs obtained from mice of the indicated CaMKIIγ genotypes were resolved on a 10% SDS/PAGE gel that was then subjected to immunoblotting with an antibody directed against the C-termini of all four CaMKII isoforms. CaMKIIγ migrates as a doublet. The experiment was performed three times and a representative immunoblot is shown.
CaMKII protein levels were assessed by immunoblots of oocyte and egg lysates from CaMKIIγ WT, HET, and KO mice using an antibody directed against the C terminus of all four CaMKII isoforms (Fig. 2B). Two bands were detected at ∼55 and ∼60 kDa in WT oocytes and eggs. These bands were reduced by ∼50% in HET and not detectable in KO lysates, indicating that two different CaMKIIγ splicing variants are present in oocytes. Consistent with previous reports (18), eggs contained ∼2-fold more CaMKII protein than oocytes. These data demonstrate that the γ isoform is the predominant CaMKII isoform in mouse oocytes. Of note, no additional shorter protein was detectable in KO lysates, indicating that the weak alternative transcript (Fig. 1C) does not result in an abundant stable peptide.
Female Infertility in CaMKIIγ−/− Mice Is Due to an Egg Activation Defect. To rule out that the absence of CaMKIIγ caused abnormalities of ovary development, we performed histological analyses. Ovaries of KO females displayed no morphological or histological abnormalities and contained normal numbers of follicles with readily identifiable growing and fully grown oocytes, as well as corpora lutea, which signifies that ovulation occurred (Fig. S2).
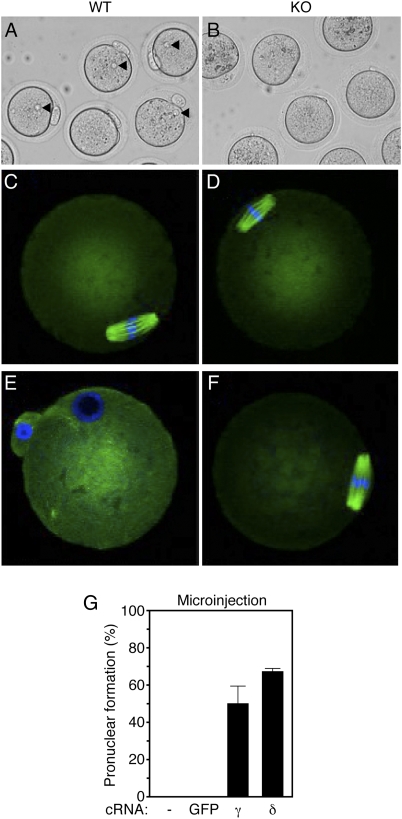
Based on the observation that eggs express predominantly CaMKIIγ, we next tested whether eggs lacking CaMKIIγ were able to undergo egg activation. Thus, ovulated eggs were collected from the oviducts of WT and KO mice. There was no difference in the number or appearance of the eggs in the two groups. We then parthenogenetically activated eggs with 10 mM SrCl2 and examined them for the presence of a female pronucleus (PN). SrCl2 activates mouse eggs by eliciting Ca2+ oscillations very similar to those seen at fertilization (19). Whereas 85% of WT eggs had a PN (Fig. 3A), indicative of effective activation, no pronuclei were detected in any KO eggs (Fig. 3B).
Fig. 3.
CaMKIIγ KO female mice are infertile because of a failure to resume meiosis II. (A and B) WT and KO eggs were subjected to SrCl2 activation, and pronuclei (PN) formation was assessed 6–7 h postactivation. Arrowheads indicate the maternal PN observed in WT, but not KO, eggs. (C–F) Spindle morphology in WT and KO eggs before (C and D) and after (E and F) SrCl2 activation. Green, β-tubulin; blue, DNA. Representative images are shown. (G) Microinjection of CaMKIIγ or CaMKIIδ cRNA into KO oocytes. As controls, uninjected oocytes (-) and oocytes injected with GFP cRNA were used. Oocytes were matured for 16 h and then SrCl2 activated, and PN formation was determined 6–7 h after activation. The experiment was performed twice. Results are expressed as mean ± range.
To determine whether failure to form a PN in KO eggs was caused by failure to resume meiosis, WT and KO eggs were fixed and stained for DNA and β-tubulin to visualize the chromosomes and the meiotic spindle before and after activation (Fig. 3 C–F). Both WT and KO ovulated eggs possessed a spindle, with the chromosomes tightly aligned at the metaphase plate (Fig. 3 C–D). After SrCl2 activation, WT eggs completed meiosis and formed a PN, but eggs from KO mice remained arrested at metaphase II (Fig. 3 E and F). Similar results were obtained when in vitro fertilization was performed. Whereas 68–84% of ZP-intact WT and HET eggs were fertilized, there was no fertilization of KO eggs (Fig. S3). Removal of the ZP had no effect on the fertilization failure of null eggs.
Although KO eggs appeared normal, successfully matured, and arrested at metaphase II, failure of these eggs to undergo egg activation could still be attributed to abnormal oocyte development. To rule out this possibility, we injected KO oocytes with a cRNA encoding wild-type and full-length CaMKIIγ. As a result, 53% of injected KO oocytes formed a PN after SrCl2 activation, whereas no PN were observed in KO oocytes that were not injected or that expressed GFP (Fig. 3G). Remarkably, this rescue was not isoform specific because 71% of oocytes injected with the same concentration of CaMKIIδ cRNA also formed a PN after activation. Moreover, both CaMKIIγ and CaMKIIδ cRNA-injected KO eggs cleaved to the two-cell stage when cultured further. These results demonstrate that the observed infertility phenotype of KO females results from the failure of eggs to undergo egg activation but not from abnormal oocyte development or endocrine dysfunction. Moreover, these results indicate that the loss of CaMKII protein rather than the loss of specific features of the γ isoform causes infertility.
CaMKIIγ−/− Eggs Display a Normal Pattern of Ca2+ Oscillations. To determine whether the failure of KO eggs to undergo egg activation was due to an abnormal [Ca2+]i response, we measured Ca2+ oscillations in response to sperm in WT, HET, and KO eggs (Fig. S4). KO eggs displayed a normal pattern of Ca2+ oscillations, and there was no difference compared with WT eggs in the baseline 340/380 fluorescence ratio, the duration or amplitude of the first Ca2+ transient, the time to the first Ca2+ transient, or the persistence of [Ca2+]i oscillations. However, KO eggs had a significantly greater number of Ca2+ rises in the time period monitored (P < 0.05).
CaMKIIγ−/− Eggs Undergo Cortical Granule Exocytosis. To ascertain whether KO eggs can undergo any EEA, WT and KO female mice were mated with WT males, and at different times after fertilization we assessed different EEA. We allowed fertilization to occur in vivo to eliminate the possibility of spontaneous parthenogenetic activation of eggs after extended culture.
One of the earliest EEA after sperm–egg fusion is the ZP block to polyspermy, which creates a physical barrier that prevents additional sperm entry (20). The release of cortical granule (CG) contents upon fertilization results in modification of two of the glycoproteins of the ZP (ZP2 and ZP3), such that sperm binding is impaired (20). ZP3 undergoes changes in its carbohydrate moiety and ZP2 is proteolytically processed. Proteolytic cleavage of ZP2 was assayed as a measure of the block to polyspermy.
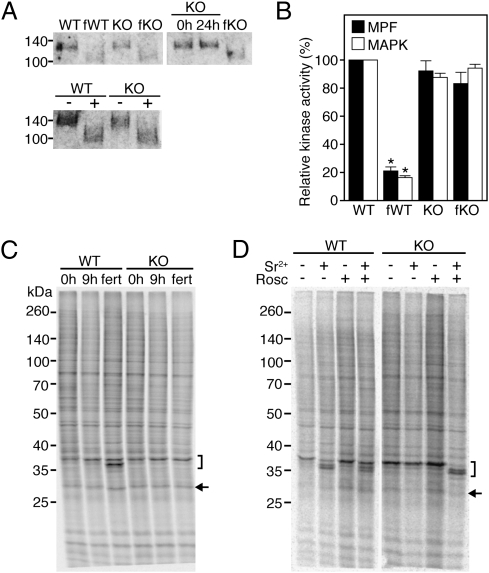
Freshly ovulated eggs as well as eggs/embryos collected 36 h after hCG treatment (∼24 h after fertilization) from the oviducts of WT and KO female mice were processed for Western Blot analysis. In unfertilized WT and KO eggs, a 120-kDa band corresponding to full-length ZP2 was detectable (Fig. 4A, Upper-Left Panel). After fertilization, both WT (fWT) and KO (fKO) eggs possessed only the ∼90 kDa cleaved polypeptide, demonstrating that KO eggs can mount a block to polyspermy. Of note, cleavage of ZP2 did not occur in KO eggs that were collected from unmated females 36 h after hCG treatment (Fig. 4A, Upper-Right Panel), demonstrating that the ZP2 conversion was triggered by sperm entry and was not a consequence of egg aging. These results indicate that the postfertilization block to polyspermy is independent of CaMKII. The formal possibility that sperm-borne CaMKII mediated the block to polyspermy was excluded by parthenogenetically activating WT and KO eggs and observing that activated KO eggs cleaved ZP2 to the same extent as eggs exposed to sperm (Fig. 4A, Lower Panel).
Fig. 4.
Events of egg activation (EEA) in CaMKIIγ KO eggs. (A) Proteolytic cleavage of ZP2 as a proxy for the postfertilization block to polyspermy. (Upper Left Panel) Protein extracts from WT or KO eggs were prepared before (WT, KO) or 24 h after (fWT, fKO) fertilization and subjected to SDS/PAGE followed by immunoblot with an antibody against ZP2. (Upper Right Panel) Protein extracts from KO eggs were prepared and treated as described for the Left Panel. 0 h, unfertilized metaphase II eggs; fKO, KO female mice were mated to males and eggs were collected at ∼24 h after fertilization (36 h after hCG); 24h, eggs collected at the same time point from unmated females. (Lower Panel) WT and KO eggs were parthenogenetically activated using 10 mM SrCl2. −, eggs cultured without SrCl2; +, eggs cultured with SrCl2. The experiment was performed three times and a representative example is shown. (B) MPF and MAPK assays of metaphase II eggs before (WT, KO) and 12 h after (fWT, fKO) fertilization. Kinase activities were measured in single eggs and are expressed relative to unfertilized WT eggs. Solid bars, MPF activity; open bars, MAPK activity. Data are expressed as the mean ± SEM of five experiments. *P < 0.001, one-way ANOVA. (C) Changes in protein synthesis in metaphase II eggs after fertilization as assessed by [35S]-methionine metabolic radiolabeling and SDS/PAGE. Eggs were isolated from WT and KO mice before (0 h) or 9 h after (fert) fertilization. As a control, eggs were isolated at the same time from unmated females (9h). The experiment was conducted three times and a representative autoradiogram is shown. (D) Changes in protein synthesis in metaphase II eggs after parthenogenetic activation. Metaphase II eggs were isolated from WT and KO mice and treated with SrCl2 (Sr2+), roscovitine (Rosc), or SrCl2 + roscovitine. The experiment was conducted twice and a representative autoradiogram is shown. Bracket depicts 35-kDa complex; arrow indicates position of spindlin (C and D).
MAPK and MPF Activities Remain High in Inseminated CaMKIIγ−/− Eggs. The activities of both MPF and MAPK are high in metaphase II eggs and decrease after metaphase II exit and entry into interphase. MPF inactivation, which precedes the decline in MAPK, is required for meiosis resumption and release of the second polar body, whereas a decrease in MAPK activity is likely required for pronuclear formation (7). MAPK and MPF activities were measured simultaneously in single eggs from WT and KO female mice before and after fertilization. Both activities decreased ∼80% after fertilization in WT eggs, but remained virtually unchanged in KO eggs (Fig. 4B). This finding is consistent with the arrest of KO eggs at metaphase II (Fig. 3F).
CaMKIIγ−/− Eggs Are Unable to Recruit Maternal mRNAs. Recruitment of maternal mRNAs into polysomes for translation is another EEA that occurs several hours after fertilization. Among the handful of polypeptides that exhibit changes after fertilization, a few can be readily detected in one-dimensional (1D) SDS/PAGE gels and are well-accepted markers of maternal mRNAs recruited after fertilization or activation. The 35-kDa complex is a set of polypeptides that can be resolved into three bands on SDS/PAGE gels. Whereas the upper band is predominant in unfertilized eggs, the middle and lower bands appear at different time points after fertilization (21). Similarly, a 30-kDa complex that has been identified as spindlin (22) is composed of two bands, with the upper band being predominant before fertilization and the lower band appearing after fertilization (21). To ascertain whether inseminated KO eggs recruit maternal mRNAs, freshly ovulated eggs, as well as eggs recovered 21 h after hCG treatment (∼9 h after fertilization) from the oviducts of mated females, were metabolically radiolabeled with [35S]-methionine and newly synthesized polypeptides were visualized by autoradiography after SDS/PAGE (Fig. 4C). The pattern of newly synthesized polypeptides was virtually identical in WT and KO eggs that were freshly ovulated (0 h) or collected 21 h after hCG from unmated females (9 h). After fertilization, a faster migrating band within the 35-kDa complex and a faster electrophoretic mobility of spindlin were detectable only in WT but not KO eggs. Thus, KO eggs seem to be unable to recruit maternal mRNAs in response to sperm.
These findings could be interpreted as evidence that this EEA is directly CaMKII dependent. Because recruitment of maternal transcripts is a late EEA, an alternative explanation could be that cell cycle resumption is required for maternal mRNA recruitment. To test the latter possibility, unfertilized WT and KO eggs were induced to resume meiosis by treatment with roscovitine, an inhibitor of CDC2 kinase (the catalytic component of MPF). Under these conditions, WT and KO eggs were able to exit metaphase II, complete meiosis, and form pronuclei, but they were unable to recruit maternal transcripts (Fig. 4D). Thus, cell cycle resumption per se is not sufficient to trigger maternal mRNA recruitment.
Roscovitine triggers parthenogenetic activation in the absence of a Ca2+ increase in the egg; activation with SrCl2, on the other hand, results in several Ca2+ oscillations (23). We reasoned that an increase in Ca2+ could be needed to trigger maternal mRNA recruitment. As expected, parthenogenetic activation using SrCl2 alone resulted in maternal mRNA recruitment in WT, but not KO, eggs. However, the addition of both SrCl2 and roscovitine resulted in maternal mRNA recruitment in both WT and KO eggs (Fig. 4D). This result suggests that the recruitment of maternal mRNAs requires cell cycle resumption and Ca2+, but it can take place in the absence of CaMKII.
Discussion
CaMKII has been implicated in egg activation in frogs and mice (11, 24). CaMKII inhibitors inhibit cell cycle progression in both parthenogenetically activated and fertilized mouse eggs (12, 25, 26). Conversely, expression of a truncated, constitutively active form of CaMKIIα triggers cell cycle resumption (27), reduction in MAPK and MPF activities, PN formation, and maternal mRNA recruitment, all in the absence of Ca2+ oscillations (28). Interestingly, CG exocytosis is abnormal in these eggs (28). Also, CaMKII activity oscillates in synchrony with each Ca2+ transient (12). These and other reports led to the idea that CaMKII is a master integrator of several EEA (29). In contrast to this proposal, the results described here establish that CaMKIIγ is responsible only for meiotic resumption.
We find that CaMKIIγ is the predominant CaMKII isoform expressed in mouse eggs and that CaMKIIγ-deficient female but not male mice are sterile because of egg activation defects. Even though KO eggs elicit an apparently normal pattern of Ca2+ oscillations, they remain arrested at metaphase II and fail to recruit maternal mRNAs, but do mount a ZP block to polyspermy. Expressing not only CaMKIIγ but also CaMKIIδ in CaMKIIγ KO eggs rescues the egg activation defect. This result strongly suggests that isoform-independent CaMKII activity per se is required for egg activation, rather than CaMKIIγ isoform-specific features. Our results also demonstrate that the sole role of CaMKII in the oocyte is to trigger cell cycle resumption, which in turn results in recruitment of maternal mRNAs in a CaMKII-independent manner.
CG exocytosis, and the subsequent modification of the ZP to prevent polyspermy, is one of the earliest EEA. Although it is well established that a rise in [Ca2+]i is required for CG release, the downstream Ca2+-dependent mediators of this signal are still poorly defined. Pharmacological inhibitors of CaMKII block CG exocytosis in both fertilized and ethanol-activated mouse eggs (26). Nevertheless, expressing an artificially truncated, constitutively active form of CaMKIIα in mouse eggs results in abnormal CG exocytosis, even though other EEA are normal (28). Both myosin light-chain kinase and protein kinase C have been implicated in CG exocytosis (30, 31). In these studies, inhibition of CG release appeared incomplete, raising the possibility that more than one effector is involved in CG exocytosis. We assayed the proteolytic cleavage of ZP2 as a proxy for the ZP block to polyspermy rather than staining for CGs because we found that the poor CG staining observed for this mouse strain precluded quantifying CG release. We found a comparable extent of proteolytic ZP2 cleavage in CaMKIIγ-deficient eggs and WT eggs, indicating that CaMKII is not essential for CG exocytosis or the establishment of the postfertilization ZP block to polyspermy.
By analogy to the events of synaptic vesicle exocytosis in hippocampal neurons, a two-step mechanism of CG exocytosis has been proposed (32). The first step involves undocking of secretory granules associated with the cortical cytoskeleton, and the second step involves active translocation of undocked granules toward the plasma membrane. In the hippocampus, dissociation is caused by CaMKII phosphorylation of synapsin, whereas translocation is mediated by MLCK phosphorylation of myosin II (32). One explanation for the ability of CaMKIIγ-deficient eggs to undergo CG exocytosis is that CaMKII is not involved in this process, indicating that its function in secretion is not universal. Alternatively, although impaired, undocking of CGs could still occur such that some CGs are released and trigger ZP modifications. Finally, some CGs may not be trapped in the actin cytoskeleton and thus could translocate to the plasma membrane even in the absence of CaMKII.
Failure of inseminated or parthenogenetically activated CaMKIIγ−/− eggs to exit metaphase II is consistent with a role for CaMKII in cell cycle resumption after fertilization. To exit meiosis, cyclin B (the regulatory component of MPF) must be destroyed by the ubiquitin ligase activity of the anaphase-promoting complex or cyclosome (APC/C). In frog eggs, EMI2, a protein that binds to and inhibits the APC/C activator CDC20, has been described as the missing link between [Ca2+]i rises and cyclin B degradation (33). The Ca2+ rise induced by sperm entry activates CaMKII in the egg. CaMKII phosphorylates EMI2 creating a docking site for Xenopus polo-like kinase 1 (Plx1), which in turn phosphorylates EMI2 and causes its destruction by the ubiquitin/proteasome system. Thus, the APC/C is liberated from repression and triggers meiosis II exit. Although the mechanism of EMI2 degradation has not been demonstrated in mammals, current evidence suggests that it is similar, if not identical, to the mechanism in frogs. For example, mammalian EMI2 is required for both establishment and maintenance of metaphase II arrest (34, 35) and mouse EMI2 contains motifs for phosphorylation by both CaMKII and the mammalian ortholog of Plx1 (Plk1) (36).
Fertilization induces recruitment of maternal mRNAs via polyadenylation (37). mRNA recruitment into polysomes for translation is essential for further development, as inhibiting mRNA polyadenylation shortly after fertilization results in a marked decrease in transcriptional activity in zygotes (38). Microarray analysis of polysomal mRNAs in metaphase II eggs and late zygotes demonstrates that thousands of transcripts are differentially translated in the zygote as compared to the unfertilized egg (39). Nevertheless, only a few maternal transcripts that are mobilized during egg activation have been identified, including spindlin (22), and cyclin A2 (40). Whereas the presence of a cytoplasmic polyadenylation element (CPE) in the 3′UTR of mRNAs is widely recognized as a determinant for translation during meiotic maturation (41), the sequence responsible for marking transcripts for recruitment after fertilization is poorly defined. The mechanistic connection between elevation in [Ca2+]i and translation is also poorly defined, but CaMKII has been proposed as the mediator of the Ca2+ signal (29).
In hippocampal neurons, mRNAs are sequestered in an inhibitory multiprotein complex that suppresses translation. Phosphorylation of CPE binding protein by CaMKII results in polyadenylation of these mRNAs, which disrupts the complex and allows translation (42). The finding that expressing constitutively active CaMKII in mouse eggs triggers maternal mRNA recruitment (28) is consistent with CaMKII being the effector of this EEA and has led to speculation that a mechanism similar to the mechanism in neurons leads to mRNA recruitment after fertilization. Our results, however, demonstrate that this is not the case. Although CaMKII is necessary for mRNA recruitment, the sole function of the enzyme appears to be to trigger cell cycle resumption. Cell cycle resumption results, through an as-yet-unidentified CaMKII-independent pathway, in maternal mRNA recruitment only in the presence of elevated [Ca2+]i. We speculate that the inconsistency between our results and the aforementioned work (28) is due to overexpression of CaMKII resulting in downstream effects that do not normally occur under physiological conditions.
Using a genetic loss of function approach, we were able to dissect the pathways involved in triggering the different EEA. The results presented here suggest a shift in our view of this process (Fig. 5). We provide evidence that there is not a sole integrator of the Ca2+ signal responsible for all of the EEA. Instead, CaMKIIγ triggers cell cycle resumption, which in turn results in the recruitment of maternal mRNAs in a Ca2+-dependent manner. On the other hand, the postfertilization ZP block to polyspermy is entirely CaMKIIγ independent. In the future, it will be of interest to identify the direct targets of CaMKII responsible for cell cycle resumption, the downstream effectors that trigger the recruitment of maternal mRNAs, and the molecular effectors of the CaMKII-independent ZP block to polyspermy. Moreover, the realization that CaMKIIγ is specifically required for fertilization should stimulate efforts to search for mutations in the CaMKIIγ gene in infertile women.
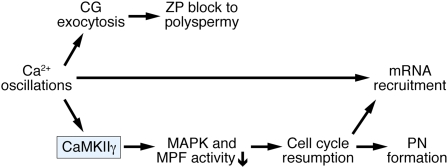
Fig. 5.
Working model of mouse egg activation. A rise in [Ca2+]i activates the γ isoform of CaMKII that in turn triggers meiotic resumption by decreasing MAPK and MPF activity. Cortical granule (CG) exocytosis and the subsequent ZP block to polyspermy are triggered by [Ca2+]i in a CaMKII-independent manner. Maternal mRNA recruitment is elicited by [Ca2+]i and requires cell cycle resumption but does not directly depend on CaMKII.
Materials and Methods
Generation of CaMKIIγ−/− Mice. Details of gene targeting and generation of mutant mice are described in SI Materials and Methods.
Oocyte, Egg, and Embryo Collection and Microinjection.
Details of protocols are described in SI Materials and Methods.
Parthenogenetic Activation, in Vitro Fertilization, and Ca2+ Imaging.
Details are described in SI Materials and Methods.
Immunoblotting.
Complete protocol is described in SI Materials and Methods.
Histochemistry and Immunofluorescence.
Full protocols are described in SI Materials and Methods.
In Vitro Synthesis of cRNA.
Protocol is described in SI Materials and Methods.
MPF and MAPK Assays.
MPF and MAPK activities were measured in single eggs, as previously described (43).
[35S]-Methionine Metabolic Radiolabeling and SDS/PAGE.
Comprehensive protocols are described in SI Materials and Methods.
RNA Isolation and Quantitative Real-Time RT-PCR.
Comprehensive protocols are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Robert E. Hammer, Eric Small, Mariano G. Buffone, and Karen Schindler for helpful discussions, and Jose Cabrera for assistance with graphics. We are grateful to Laurinda Jaffe and Gary Wessel for critical comments on the manuscript. This work was supported by the Emmy Noether Program Grant BA-2258/2-1 of the Deutsche Forschungsgemeinschaft (J.B.) and by a training grant from the NIH (T32 HD 007305 22) (F.E.D.). Work in the laboratory of E.N.O. was supported by the National Institutes of Health, the Donald W. Reynolds Center for Clinical Cardiovascular Research, the Robert A. Welch Foundation, and the Leducq Foundation. Work in the laboratory of R.M.S. was supported by a grant from the National Institutes of Health (HD22732, R.M.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912658106/DCSupplemental.
References
- 1.Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- 2.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 3.Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbertson KS, Whittingham DG, Cobbold PH. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature. 1981;294:754–757. doi: 10.1038/294754a0. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki S, Igusa Y. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature. 1981;290:702–704. doi: 10.1038/290702a0. [DOI] [PubMed] [Google Scholar]
- 6.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, et al. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 10.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Winston NJ, Maro B. Calmodulin-dependent protein kinase II is activated transiently in ethanol-stimulated mouse oocytes. Dev Biol. 1995;170:350–352. doi: 10.1006/dbio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 12.Markoulaki S, Matson S, Ducibella T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev Biol. 2004;272:15–25. doi: 10.1016/j.ydbio.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 14.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 15.van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, et al. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–825. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- 16.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 18.Abbott AL, Fissore RA, Ducibella T. Identification of a translocation deficiency in cortical granule secretion in preovulatory mouse oocytes. Biol Reprod. 2001;65:1640–1647. doi: 10.1095/biolreprod65.6.1640. [DOI] [PubMed] [Google Scholar]
- 19.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- 20.Wong JL, Wessel GM. Defending the zygote: Search for the ancestral animal block to polyspermy. Curr Top Dev Biol. 2006;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. [DOI] [PubMed] [Google Scholar]
- 21.Howlett SK, Bolton VN. Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J Embryol Exp Morphol. 1985;87:175–206. [PubMed] [Google Scholar]
- 22.Oh B, Hwang SY, Solter D, Knowles BB. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development. 1997;124:493–503. doi: 10.1242/dev.124.2.493. [DOI] [PubMed] [Google Scholar]
- 23.Rogers NT, Halet G, Piao Y, Carroll J, Ko MS, et al. The absence of a Ca(2+) signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction. 2006;132:45–57. doi: 10.1530/rep.1.01059. [DOI] [PubMed] [Google Scholar]
- 24.Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, et al. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J, Bierle BM, Gallicano GI, Capco DG. Calcium/calmodulin-dependent protein kinase II and calmodulin: Regulators of the meiotic spindle in mouse eggs. Dev Biol. 1998;204:464–477. doi: 10.1006/dbio.1998.9038. [DOI] [PubMed] [Google Scholar]
- 26.Tatone C, Delle Monache S, Iorio R, Caserta D, Di Cola M, et al. Possible role for Ca(2+) calmodulin-dependent protein kinase II as an effector of the fertilization Ca(2+) signal in mouse oocyte activation. Mol Hum Reprod. 2002;8:750–757. doi: 10.1093/molehr/8.8.750. [DOI] [PubMed] [Google Scholar]
- 27.Madgwick S, Levasseur M, Jones KT. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- 28.Knott JG, Gardner AJ, Madgwick S, Jones KT, Williams CJ, et al. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol. 2006;296:388–395. doi: 10.1016/j.ydbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Matson S, Markoulaki S, Ducibella T. Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol Reprod. 2006;74:169–176. doi: 10.1095/biolreprod.105.046409. [DOI] [PubMed] [Google Scholar]
- 31.Tsaadon L, Kaplan-Kraicer R, Shalgi R. Myristoylated alanine-rich C kinase substrate, but not Ca2+/calmodulin-dependent protein kinase II, is the mediator in cortical granules exocytosis. Reproduction. 2008;135:613–624. doi: 10.1530/REP-07-0554. [DOI] [PubMed] [Google Scholar]
- 32.Ducibella T, Matson S. Secretory mechanisms and Ca2+ signaling in gametes: Similarities to regulated neuroendocrine secretion in somatic cells and involvement in emerging pathologies. Endocr Pathol. 2007;18:191–203. doi: 10.1007/s12022-007-0015-7. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt A, Rauh NR, Nigg EA, Mayer TU. Cytostatic factor: An activity that puts the cell cycle on hold. J Cell Sci. 2006;119:1213–1218. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]
- 34.Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, et al. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;2:4–10. doi: 10.1186/1747-1028-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. doi: 10.1242/dev.127.17.3795. [DOI] [PubMed] [Google Scholar]
- 38.Aoki F, Hara KT, Schultz RM. Acquisition of transcriptional competence in the 1-cell mouse embryo: Requirement for recruitment of maternal mRNAs. Mol Reprod Dev. 2003;64:270–274. doi: 10.1002/mrd.10227. [DOI] [PubMed] [Google Scholar]
- 39.Potireddy S, Vassena R, Patel BG, Latham KE. Analysis of polysomal mRNA populations of mouse oocytes and zygotes: Dynamic changes in maternal mRNA utilization and function. Dev Biol. 2006;298:155–166. doi: 10.1016/j.ydbio.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Fuchimoto D, Mizukoshi A, Schultz RM, Sakai S, Aoki F. Posttranscriptional regulation of cyclin A1 and cyclin A2 during mouse oocyte meiotic maturation and preimplantation development. Biol Reprod. 2001;65:986–993. doi: 10.1095/biolreprod65.4.986. [DOI] [PubMed] [Google Scholar]
- 41.Mendez R, Richter JD. Translational control by CPEB: A means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- 42.Atkins CM, Nozaki N, Shigeri Y, Soderling TR. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2004;24:5193–5201. doi: 10.1523/JNEUROSCI.0854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 2000;127:4147–4156. doi: 10.1242/dev.127.19.4147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.