Abstract
The prognosis for adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) remains poor, in part from a lack of therapeutic targets. We identified the type I cytokine receptor subunit CRLF2 in a functional screen for B-ALL–derived mRNA transcripts that can substitute for IL3 signaling. We demonstrate that CRLF2 is overexpressed in approximately 15% of adult and high-risk pediatric B-ALL that lack MLL, TCF3, TEL, and BCR/ABL rearrangements, but not in B-ALL with these rearrangements or other lymphoid malignancies. CRLF2 overexpression can result from translocation with the IGH locus or intrachromosomal deletion and is associated with poor outcome. CRLF2 overexpressing B-ALLs share a transcriptional signature that significantly overlaps with a BCR/ABL signature, and is enriched for genes involved in cytokine receptor and JAK-STAT signaling. In a subset of cases, CRLF2 harbors a Phe232Cys gain-of-function mutation that promotes constitutive dimerization and cytokine independent growth. A mutually exclusive subset harbors activating mutations in JAK2. In fact, all 22 B-ALLs with mutant JAK2 that we analyzed overexpress CRLF2, distinguishing CRLF2 as the key scaffold for mutant JAK2 signaling in B-ALL. Expression of WT CRLF2 with mutant JAK2 also promotes cytokine independent growth that, unlike CRLF2 Phe232Cys or ligand-induced signaling by WT CRLF2, is accompanied by JAK2 phosphorylation. Finally, cells dependent on CRLF2 signaling are sensitive to small molecule inhibitors of either JAKs or protein kinase C family kinases. Together, these findings implicate CRLF2 as an important factor in B-ALL with diagnostic, prognostic, and therapeutic implications.
Keywords: JAK2, TSLPR, TSLP
During the past decade, studies using oligonucleotide arrays and high-throughput sequencing have identified several genetic and transcriptional aberrations in B-cell acute lymphoblastic leukemia (B-ALL) (1), leading to three conceptual advances. First, genes involved in normal B-cell development (e.g., PAX5, IKZF1) are frequently mutated in B-ALL (1–3). Second, B-ALL is highly heterogeneous and can exist as multiple, genetically distinct clones within the same individual (1, 4). Third, B-ALL transcriptional profiles cluster based on characteristic chromosomal rearrangements, hereafter defined as rearrangements of TEL, MLL, TCF3, and BCR/ABL (5–8).
However, one third of B-ALL cases lack characteristic rearrangements (9). Transcriptional profiles from a subset of these leukemias cluster with profiles from BCR/ABL–expressing B-ALL (3, 5), suggesting that the former harbor cryptic alterations in tyrosine kinase signaling. Supporting this notion, mutations in JAKs were recently identified in a small percentage of pediatric B-ALL and approximately 20% of ALL in children with Down syndrome (10–14).
Upon ligand binding to a type I cytokine receptor, JAKs phosphorylate substrates including STATs, which in turn affect the transcription of progrowth and antiapoptotic factors (15). JAK enzymatic activity requires interaction with a cytokine receptor, which is believed to serve as a scaffold. As a consequence, JAK gain-of-function mutants do not confer a transformed phenotype in the absence of a compatible cytokine receptor (16).
We performed a retroviral cDNA library screen to identify gain-of-function mutations from primary B-ALL specimens. We made several improvements to older approaches that increase the efficiency of library construction and cDNA representation. In addition, we added selection-based clone recovery, which essentially eliminates false-positive findings. Using this system, we identified CRLF2, a type I cytokine receptor subunit also known as thymic stromal lymphopoietin receptor (TSLPR), as a proto-oncogene in B-ALL. CRLF2 binds its ligand, thymic stromal lymphopoietin (TSLP), as part of a heterodimeric complex with the IL7 receptor subunit (IL7R) (17). TSLP is produced by epithelial cells at sites of inflammation, where it activates myeloid dendritic cells and Th2 immune responses (18, 19). TSLP also promotes early B-cell development (20) and stimulates the growth of some human B-ALLs in vitro (21).
We demonstrate that CRLF2 overexpression is common only in B-ALL cases that lack rearrangements of TEL, MLL, TCF3, and BCR/ABL (5–8) and confers a poor prognosis among children and adults. Similar to recent reports (22, 23), we show that CRLF2 overexpression can result from locus rearrangement, either translocation or intrachromosomal deletion. We identify CRLF2 Phe232Cys and JAK2 Arg683 gain-of-function mutations in mutually exclusive subsets of CRLF2-overexpressing B-ALL that transform growth factor–dependent cells to factor independence. Strikingly, 100% of B-ALL cases with mutant JAK2 overexpress CRLF2, suggesting that CRLF2 is the essential, or at least the overwhelmingly predominate, scaffold for mutant JAK2 activity in B-ALL. Finally, we show that the gene signature associated with CRLF2 overexpression is highly similar in both pediatric and adult cases, and significantly overlaps with a BCR/ABL signature. Together, these studies establish CRLF2 as a key factor in B-ALL, and support its use as a prognostic and therapeutic target.
Results
Mutated CRLF2 Is a Gain-of-Function Oncoprotein in Poor-Prognosis B-ALL.
We identified CRLF2 in a functional screen for leukemia-derived cDNA that activate tyrosine kinase signaling (Fig. 1A(). In this screen, we infect the murine IL3-dependent cell line BaF3 with retroviral cDNA libraries constructed from bone marrow aspirates involved with more than 80% tumor. Clones that survive IL3 withdrawal invariably harbor tumor-derived cDNAs that obviate the requirement for IL3. After infection with a cDNA library constructed from a B-ALL specimen with 46, XY karyotype, we identified IL3-independent clones that contained a mutated, full-length cDNA transcript of CRLF2.
Fig. 1.
Identification of CRLF2 in B-ALL. (A) Tumor-derived cDNA is packaged into retroviral particles that are used to infect BaF3 cells. Integrants (gray) survive in puromycin selection. Surviving clones (black) are isolated after IL3 withdrawal and their integrated cDNAs are sequenced and repackaged within retrovirus and confirmed. (B) IHC using anti-CRLF2 antibody and interphase FISH. ALL-73 has no detectable CRLF2 expression by IHC, whereas both ALL-74 and ALL-76 have visible expression and surface localization. (Left) FISH demonstrates 2 fusions of the CRLF2 (yellow) and IGH (green) probes in ALL-74, consistent with a reciprocal translocation, but not in ALL-73. (Right) FISH shows one probe centromeric to CRLF2 (yellow) lost in ALL-76, consistent with an intrachromosomal deletion.
We used a combination of quantitative (q) RT-PCR, immunohistochemistry (IHC), and gene expression profiling (GEP) to assay CRLF2 expression in adult B-ALL samples from Dana-Farber Cancer Institute (DFCI; n = 97) and Gruppo Malattie Ematologiche dell’Adulto (GIMEMA; n = 157) cohorts (Fig. 1B and Fig. S1). Cases with CRLF2 overexpression were clearly distinct, and overexpression correlated completely between assays (Fig. 1B and Fig. S1). Overall, CRLF2 was overexpressed in 15 of 120 (12.5%) adult B-ALL cases that lacked characteristic gene rearrangements, compared with 0 of 134 with these rearrangements (P < 10−4). CRLF2 overexpression was not present in 69 cases of T-cell ALL assayed by GEP (P = 0.001 vs. 15 of 120).
We analyzed 90 adult patients with B-ALL that lacked characteristic rearrangements who had available demographic and outcome information, pooled from the DFCI (n = 20) and GIMEMA (n = 70) cohorts (Fig. 2A). CRLF2-overexpressing and nonoverexpressing cohorts had similar median age, sex distribution, and white blood cell counts at presentation. However, disease-free survival (Fig. 2B) was significantly shorter among patients with CRLF2 overexpression (median, 17.8 mo vs. 37.8 mo; P < 0.03). There was also a trend for shorter overall survival among these patients (median, 25.5 mo vs. not reached; P = 0.17). Thus, CRLF2 overexpression is a marker of poor outcome among patients with B-ALL that lack characteristic rearrangements.
Fig. 2.
Demographic and outcome analysis for patients with B-ALL that lack characteristic rearrangements, based on CRLF2 expression. (A) A comparison of 90 adult patients with B-ALL who lack characteristic cytogenetic rearrangements, pooled from GIMEMA (n = 70) and DFCI specimens (n = 20). All values are at the time of diagnosis. Median follow-up for the full cohort is 39.4 months. P values were derived using the Wilcoxon test for quantitative variables and a Fisher exact test for qualitative variables. 1Values were available for only 85 of 90 patients. (B) Disease-free survival and overall survival from the time of diagnosis were estimated using the Kaplan-Meier product-limit method and compared in univariate analysis by the log-rank test.
CRLF2 Overexpression in Pediatric B-ALL and Other Lymphoid Malignancies.
To determine the frequency of CRLF2 overexpression in pediatric B-ALL, we reviewed the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) for B-ALL samples assayed on the Affymetrix U133 platform. Affymetrix HG-U133A and HG-U133Aplus2 arrays contain a single probe set (208303_s_at) that targets the CRLF2 transcript (both complete and partial coding sequence, as well as expression sequence tags).
We identified 9 datasets (Table S1 and Table S2) with a total of 1,253 pediatric ALL cases. Among the 3 datasets that included only high-risk patients, 52 (14.8%) of 351 B-ALL cases that lacked characteristic rearrangements had CRLF2 overexpression compared with 0 of the remaining 130 B-ALL cases (P < 10−4). This may underestimate the frequency of CRLF2 overexpression in the cohort without characteristic rearrangements, as some datasets did not distinguish patients based on karyotype, so patients with characteristic rearrangements were presumably included in the cohort of 351 B-ALL cases. In the six datasets that included both standard-risk and high-risk patients, CRLF2 overexpression was present in only 23 of 559 (4.1%) cases that lacked characteristic rearrangements (P < 10−4 vs. 52 of 351 from the high-risk–only datasets). In addition, 0 of 51 T-ALL cases overexpressed CRLF2 (P = 0.002 vs. 52 of 351 from the high-risk–only datasets).
We next asked whether CRLF2 is overexpressed in other lymphoid malignancies. We selected a cross-section of chronic lymphocytic leukemia (CLL) specimens, based on karyotype, IGHV somatic hypermutation, ZAP-70 and CD38 expression, CLL family history, and clinical features (Table S3). All 30 specimens had no detectable CRLF2 mRNA. A review of gene expression profiles from GEO dataset GSE6477 (n = 162) failed to identify significant CRLF2 expression in normal plasma cells, monoclonal gammopathy of undetermined significance, smoldering multiple myeloma (MM), newly diagnosed MM, or relapsed MM. Low or undetectable CRLF2 expression was also confirmed in a panel of T-ALL (n = 22) and other (n = 14) cell lines (Table S4).
CRLF2 Locus Rearrangements.
Russell et al. (23) recently demonstrated that the CRLF2 locus, which is located in the pseudoautosomal regions of chromosomes X and Y, can undergo two types of rearrangement: intrachromosomal deletion or translocation with the Ig heavy chain (IGH) locus. Mullighan et al. (22) subsequently mapped the deletions and demonstrated that they juxtapose CRLF2 3′ of the first noncoding exon of P2RY8. In both cases, the chromosome X/Y breakpoints are upstream of CRLF2 and place the full CRLF2 coding sequence under alternate transcriptional control. CRLF2 and IGH are close to the telomeres of chromosome X/Y and 14, respectively. Thus, we designed a FISH strategy using probes against regions flanking CRLF2 and IGH (Fig. S2). Whereas the CRLF2 and IGH loci are clearly separate in cells that lack CRLF2 expression (Fig. 1B), FISH in 3 of 6 B-ALL specimens with high CRLF2 expression demonstrated joining of CRLF2 and IGH probes, consistent with a reciprocal chromosomal translocation (Fig. 1B). Two of the 3 remaining specimens had loss of a centromeric chromosome X/Y probe, consistent with an intrachromosomal deletion (Fig. 1B). The final specimen had neither a deletion nor a translocation, suggesting an additional mechanism for CRLF2 overexpression.
CRLF2/IGH translocations are akin to lymphoma-associated rearrangements involving BCL2 and BCL1 that result from aberrant V(D)J recombination (24). In 6 specimens, we PCR amplified der(14) translocation junctions between IGHJ segments on chromosome 14 and the region centromeric of CRLF2 on chromosome X/Y (Fig. 3A). Junctions involved sequence approximately 8 to 16 kb upstream of the CRLF2 translation start site, with multiple cases clustering near putative V(D)J recombinase recognition signal sequences (Fig. 3B). Thus, CRLF2/IGH translocations appear to result from aberrant V(D)J recombination that can involve cryptic recognition signal sequence in the pseudoautosomal regions.
Fig. 3.
CRLF2/IGH junctions. (A) Junctions were amplified on der(14) using a common IgHJ primer and spaced primers 5′ (centromeric) of CRLF2 coding sequence. N nucleotides are nontemplated insertions. (B) Breakpoints for 4 of the 6 cases (black arrows) clustered near a single putative V(D)J recombinase recognition signal sequence (RSS, blue). The expected sites of cleavage by the V(D)J recombinase are in red. Cases MUTZ-5 and 7243 were reported by Russell et al. (23).
CRLF2 Phe232Cys Is a Gain-of-Function Mutation.
Sequencing of CRLF2 in 35 B-ALL specimens, including 14 overexpressing cases, identified multiple single nucleotide variants. We assayed the function of the 4 nonsynonymous variants (711T > G, 746G > A, 789A > G, 984C > T) by retroviral expression in BaF3 cells. Of these, only CRLF2 711T > G (Phe232Cys; Fig. 4A) conferred cytokine independence in murine BaF3 and human UT7 megakaryoblastic leukemia cells (Fig. 4B). The 711T > G mutation was present in 3 of 14 overexpressing cases (21.4%) and was a somatic mutation. Sequencing of cDNA from all three cases demonstrated expression of the mutant allele (Fig. 4A). The other three nonsynonymous variants were polymorphisms, as they were present in germline specimens and were recovered from healthy donor peripheral blood lymphocytes (PBLs).
Fig. 4.
CRLF2 and JAK2 mutations in B-ALL. (A) The CRLF2 Phe232Cys mutation that results from 711T > G is present in genomic and cDNA, as shown for ALL-40. (B) UT7 cells and BaF3 cells that stably express CRLF2 and/or JAK2 alleles were grown in the absence of GM-CSF (UT7) or IL3 (BaF3). (Bottom Right) TSLP 1 ng/mL was added at d 0 as indicated. Growth is the number of viable cells relative to the number of cells initially seeded on d 0. Error bars represent 1 SD. (C) Unmodified BaF3 cells (-) and BaF3 cells that stably express WT CRLF2, CRLF2 Phe232Cys (FC), and/or IL7R were grown in the presence of IL3 except for the lane marked (-IL3). Protein lysates were separated by gel electrophoresis in the presence of reducing or nonreducing conditions and immunoblotted with an anti-CRLF2 antibody. (D) The 14 sequenced cases with CRLF2 overexpression fall into 3 categories based on CRLF2 and JAK2. Sequence traces for 3 different JAK2 alleles amplified from genomic DNA are shown.
The CRLF2 Phe232 residue is near the junction of the extracellular and transmembrane domains. Mutations that introduce cysteine residues in this region of other receptor tyrosine kinases, such as the erythropoietin receptor, can activate signal transduction through intermolecular disulfide-bonded dimers (25, 26). To confirm that CRLF2 Phe232Cys promotes constitutive dimerization, we performed immunoblots in BaF3 cells expressing WT CRLF2 or CRLF2 Phe232Cys under both reducing and nonreducing conditions (Fig. 4C). Under nonreducing conditions, the molecular weight of the CRLF2 Phe232Cys band, but not the WT band, was doubled, consistent with constitutive dimerization through the cysteine residues.
JAK2 Mutations Are Highly Associated with CRLF2 Overexpression.
The absence of gain-of-function CRLF2 mutations in most cases with CRLF2 overexpression raised the possibility that other factors within the same signaling cascade may harbor mutations. JAK mutations were recently reported in children with B-ALL (11, 13, 14). Thus, we sequenced for previously identified mutations in JAK1 and JAK2 and identified JAK2 Arg683Gly (n = 4), Arg683Ser (n = 1), and Arg683Thr (n = 1) substitutions (Fig. 4D) in 6 of 14 adult B-ALL cases that overexpress WT CRLF2. Of note, expression of CRLF2 Phe232Cys and JAK2 mutant alleles was mutually exclusive (Fig. 4D), suggesting that they function within the same pathway.
Mutant JAK proteins can transform growth factor–dependent cells only when expressed in combination with a type I cytokine receptor (11). The specific receptor that the JAK associates with, along with the particular JAK mutation, can affect the transformed phenotype (27). Thus, we asked whether overexpression of CRLF2 is essential for B-ALL associated with mutant JAK2. We linked gene expression (GEO GSE11877) and JAK mutation status from a cohort of 207 patients with high-risk pediatric B-ALL (Fig. S3A) (3, 14). CRLF2 expression among the 207 patients was clearly bimodal, with overexpression in 29 cases (14.0%; Fig. S3A). Twenty of the 207 patients also had mutations in JAK1 (n = 3), JAK2 (n = 16), or JAK3 (n = 1) (14). Strikingly, all 16 patients (100%) with JAK2 exon 16 mutations overexpressed CRLF2, as did 2 of the 3 patients with JAK1 mutations.
CRLF2 and JAK2 Contribute to Cytokine-Independent Growth.
The finding that all cases of B-ALL with JAK2 mutations overexpressed CRLF2 raised the possibility that JAK2 associates directly with CRLF2, either in the presence or the absence of IL7R. The stoichiometry of a WT CRLF2/IL7R complex is believed to be 1:1 (17). RT-PCR and gene expression profiling demonstrated that IL7R expression did not differ between the CRLF2 overexpressing and nonoverexpressing cases (Fig. S3B). This argues that mutated JAK2 signals with CRLF2 in the absence of IL7R. To test this possibility, we coexpressed WT and mutant versions of CRLF2 and JAK2 in the presence or absence of IL7R in BaF3 cells (Fig. 4B). Although WT CRLF2 or mutant JAK2 alone were insufficient to confer IL3 independence in BaF3 cells, the combination readily transformed the cells to IL3-independent growth in the absence of IL7R. Of note, the combination of WT CRLF2 with WT JAK2 provided a growth advantage versus JAK2 alone (Fig. 4B).
We next asked whether CRLF2 Phe232Cys renders cells hypersensitive to TSLP. Unlike in cells that express CRLF2/IL7R, the addition of TSLP had no effect on the growth of BaF3 cells expressing CRLF2 Phe232Cys (Fig. 4B). However, TSLP promoted the growth of cells that express CRLF2 Phe232Cys and IL7R (Fig. 4B), suggesting that CRLF2 Phe232Cys retains the ability to signal within a heterodimer. In contrast, TSLP did not promote the growth of cells that express CRLF2/mutant JAK2 (Fig. S3C).
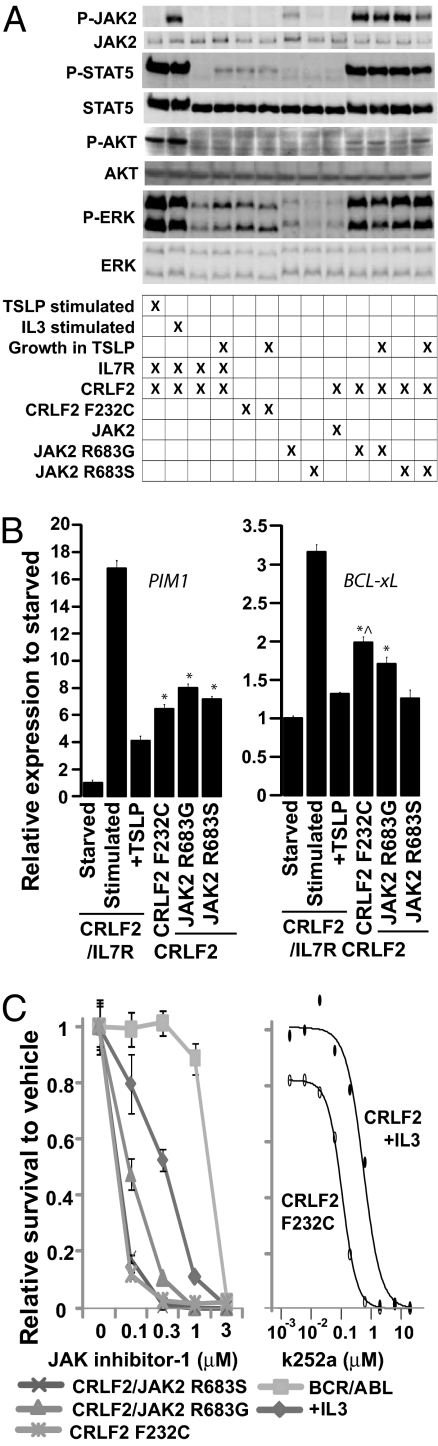
Both CRLF2 Phe232Cys and CRLF2/mutant JAK2 promoted the phosphorylation of the JAK targets STAT5 and ERK (Fig. 5A). Phosphorylation of STAT5 by CRLF2 Phe232Cys was less robust than by CRLF2/mutant JAK2 but comparable to CRLF2/IL7R/TSLP. CRLF2 Phe232Cys also promoted the up-regulation of transcriptional targets downstream of JAKs (BCL-xL and PIM1) to a comparable or greater extent than WT CRLF2/mutant JAK2 (Fig. 5B).
Fig. 5.
Signal activation and dependence on JAK signaling. (A) BaF3 cells expressing CRLF2, IL7R, and/or JAK2 proteins were starved of cytokine for 6 h, starved then stimulated with cytokine (TSLP 20 ng/mL or IL3 500 pg/mL) or grown in the presence of TSLP 1 ng/mL. Protein lysates were subjected to immunoblotting with antibodies against total and phosphorylated (P-) JAK2, STAT5, AKT, and ERK. (B) Quantitative RT-PCR of transcriptional targets in BaF3 cells that stably express CRLF2, IL7R, and/or JAK2 proteins grown in the absence of cytokine, starved of cytokine for 6 h, or stimulated with TSLP 20 ng/mL after 6 h cytokine starvation. *P < 0.05 versus cells that express CRLF2/IL7R grown in the presence of TSLP. ^P < 0.05 versus CRLF2/JAK2R683G and CRLF2/JAK2R683S. (C) BaF3 cells that stably express BCR/ABL, CRLF2, and/or JAK2 were grown in the absence of cytokines except where +IL3 indicates 500 pg/mL IL3. Data represents the ratio of viable cells exposed to JAK inhibitor-1 (Calbiochem) or k252a at varying concentrations for 72 h, compared with the same cell line exposed to vehicle. Error bars indicate 1 SD.
Whereas cells expressing WT CRLF2/mutant JAK2 had constitutively phosphorylated JAK2, cells expressing CRLF2 Phe232Cys and CRLF2/IL7R cells treated with TSLP had no detectable phospho-JAK2 (Fig. 5A). Yet, cells expressing either the CRLF2 Phe232Cys or CRLF2/mutant JAK2 were highly sensitive to a small-molecule JAK inhibitor (Fig. 5C), suggesting that CRLF2 signaling involves a JAK other than JAK2. Cells expressing CRLF2 Phe232Cys had little or no AKT phosphorylation, in contrast with cells expressing CRLF2/mutant JAK2 (Fig. 5A). However, cells expressing CRLF2/IL7R grown in the presence of TSLP also had minimal or no AKT phosphorylation despite the fact that stimulation of these cells with TSLP after a brief cytokine starvation clearly promoted AKT phosphorylation (Fig. 5A). Thus, the absence of AKT phosphorylation in CRLF2 F232C-expressing cells may reflect a limitation in the sensitivity of immunoblotting. As expected, TSLP had no effect on STAT5, ERK, AKT, or JAK2 phosphorylation in the absence of IL7R (Fig. 5A).
CRLF2 Transcriptional Signature in Adult and Pediatric B-ALL.
To characterize the dysregulated genes associated with CRLF2 overexpression, we performed a supervised analysis of gene expression profiles from the 22 B-ALL cases (8 CRLF2 overexpressing, 14 nonoverexpressing) that were validated by qRT-PCR (Fig. S1B). CRLF2 overexpression defined a 130–probe set (105-gene) “CRLF2 adult signature” (Fig. S4A and Table S5). Up-regulated genes in the CRLF2 adult signature included CD10, PKC iota, and the STAT5-induced negative regulator SOCS2, whereas genes showing decreased expression included the class I and class II HLAs. Higher SOCS2 expression among CRLF2 overexpressing cases was confirmed by qRT-PCR (123.1 fold higher than donor PBLs vs. 53.5 fold for CRLF2 nonoverexpressing cases; P < 0.05). Interestingly, several agents with activity against PKC family kinases had selective toxicity in BaF3-CRLF2 Phe232Cys cells, compared with BaF3 cells expressing WT CRLF2 (Fig. 5C and Fig. S5).
We applied a similar supervised approach to identify differentially expressed genes based on CRLF2 expression in the pediatric B-ALL GEO datasets GSE11877 and GSE12995, and compared these with the CRLF2 adult signature of 105 genes using Gene Set Enrichment Analysis (GSEA). Both pediatric signatures showed significant enrichment of the adult set (P < 0.001; false discovery rate (FDR) of 0; Fig. S4B).
To identify pathways up-regulated in CRLF2-overexpressing B-ALL, we applied GSEA to the CRLF2 differential expression signatures (from both GSE11877 and GSE12995) using gene sets from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (28). Four pathways, all involved in cytokine receptor signaling, were identified as significant (P < 0.01 and FDR < 0.10) in both datasets: “cytokine–cytokine receptor interaction,” “JAK-STAT signaling,” “neuroactive ligand receptor interaction,” and “ECM receptor interaction.” The enrichment of the JAK-STAT signaling pathway (Fig. S4C) further suggests that CRLF2 overexpression in B-ALL supports aberrant JAK-STAT activation. In agreement with the BaF3 data (Fig. 5B), BCL-xL and PIM1 were among the up-regulated genes enriched in the JAK-STAT signaling pathway.
Finally, we hypothesized that CRLF2 overexpression promotes a similar gene expression pattern to BCR/ABL. To address this, a BCR/ABL signature was obtained from the Oncomine Concepts Map (http://www.oncomine.org), which identified the top up-regulated and down-regulated genes (29). Using GSEA, the pediatric CRLF2 overexpression signatures from both datasets showed striking enrichment of the BCR/ABL-associated genes (P < 0.001, FDR = 0; Fig. S4D). Together, these findings establish common expression patterns between BCR/ABL–positive and CRLF2-overexpressing B-ALL in both children and adults.
Discussion
Using a selection-based cDNA library screen, we identified the cytokine receptor subunit CRLF2 as a factor in poor-risk B-ALL. The screen, which uses tumor-derived mRNA to assay for transcripts that can substitute for IL3 signaling, is broadly applicable to other tumor types, as many gain-of-function alterations in epithelial and mesenchymal malignancies (e.g., mutant EGFR, KIT, ERBB2, ALK, and EWS) confer IL3 independence in BaF3 cells (29–34). The alterations identified by the screen are functionally relevant and therefore attractive therapeutic targets.
Our data suggests that routine screening of B-ALL for CRLF2 expression, either by IHC or flow cytometry, offers prognostic value. CRLF2 overexpression was associated with high-risk disease in children and a dismal prognosis in adults. Marked enrichment of the adult CRLF2 overexpression signature among pediatric B-ALL suggests that CRLF2 overexpression drives a similar disease in children and adults. Like FLT3 mutations in acute myelogenous leukemia, the presence of CRLF2 overexpression could guide the decision whether to pursue allogeneic stem cell transplantation or conventional chemotherapy for patients with B-ALL (35, 36). Of note, screening for CRLF2 identifies a larger cohort of high-risk cases than JAK2 sequencing, as only some cases that overexpress CRLF2 harbor JAK2 mutations but all cases with JAK2 mutations overexpress CRLF2.
CRLF2/IGH translocations appeared to involve cryptic recognition signal sequences for the V(D)J recombinase centromeric of CRLF2 (Fig. S3). Mullighan et al. recently reported that both P2RY8-CRLF2 (22) and IKZF1 (encodes the lymphoid differentiation factor IKAROS) (2) rearrangements can also involve aberrant V(D)J recombination. Translocations resulting from aberrant V(D)J recombination are common in more mature B-cell neoplasms, leading to the suggestion that V(D)J recombination during receptor revision is more error-prone than V(D)J recombination earlier in B-cell ontogeny (37). The discovery of CRLF2 rearrangements, as well as less frequent IGH translocations in B-ALL (38–40), challenges this possibility.
The association of transcriptional signatures between CRLF2 overexpressing B-ALL and BCR/ABL–expressing B-ALL suggests that activation of JAK/STAT signaling by CRLF2 Phe232Cys, CRLF2 with mutant JAK2, or CRLF2 with another partner can mimic BCR/ABL signaling. In agreement, IKZF1 mutations are common in BCR/ABL–expressing B-ALL and lymphoid blast crisis of chronic myelogenous leukemia (2, 3), and were present in 14 of the 16 cases of B-ALL with JAK2 mutations reported by Mullighan et al. (14). Importantly, the use of imatinib has drastically improved outcomes for patients with BCR/ABL–expressing B-ALL (41). Similar improvements may be possible by targeting CRLF2/JAK signaling, either with small-molecule kinase inhibitors or monoclonal antibodies against CRLF2.
The finding that all 22 B-ALLs with JAK2 mutations (6 from our cohort, 16 from GEO GSE11877) overexpressed CRLF2 suggests that CRLF2 is the essential, or at least overwhelmingly predominate, scaffold for mutant JAK2 signaling in B-ALL. Interestingly, the JAK2 V617F mutation associated with myeloid proliferations has not been observed in B-ALL. This raises the question whether JAK2 V617F is capable of signaling in combination with CRLF2. Alternatively, JAK2 V617F could confer a different substrate selectivity from the JAK mutants observed in B-ALL (14).
Unlike most proto-oncogenes, which are activated by a single genetic event that results in either overexpression or somatic mutation, CRLF2 appears to require 2 genetic events. First, CRLF2 is overexpressed through intrachromosomal deletion, translocation to IGH, or possibly a third mechanism (22, 23). Second, somatic mutation activates either CRLF2, JAK1, JAK2, or possibly other factors in JAK/STAT signaling (14, 22). Of note, the combined overexpression of WT CRLF2 and JAK2 in BaF3 cells conferred a growth advantage in the absence of cytokines (Fig. 4B), suggesting that the first of the 2 events is likely to be the CRLF2 locus rearrangement, which provides some advantage to a preleukemic clone. In addition, overexpression of WT CRLF2 in mouse fetal liver cells supports the proliferation of precursor B cells and STAT5 phosphorylation (23).
Similar to CRLF2 F232C, mutations that promote the dimerization of other cytokine receptors (e.g., RET, erythropoietin receptor) occur in both hematologic and nonhematologic tumors (25, 26). However, these other receptors canonically signal in response to ligand as homodimers. In contrast, CRLF2 responds to TSLP as a heterodimer with IL7R and may signal independently of TSLP as a homodimer when harboring the F232C mutation or as a monomer through mutant JAK2 (Fig. S6).
Many aspects of CRLF2 signaling remain unclear. First, what kinase or kinases associate with CRLF2, either in its WT or mutant forms? In a previous study, TSLP signaling was inhibited by a dominant-negative TEC kinase, but not by kinase-deficient JAK1 or JAK2. Thus, activation of STAT5 and other targets induced by TSLP or mutation of CRLF2 may not involve JAKs. Conversely, the sensitivity of cells expressing CRLF2 Phe232Cys to an enzymatic JAK inhibitor (Fig. 5C) argues that JAKs (or a kinase sensitive to this inhibitor) are involved. A final possibility is that JAK activation in this setting does not involve canonical JAK autophosphorylation.
The second question is whether mutant JAK2 signaling involves CRLF2 alone or a dimeric complex that includes CRLF2. The low levels of IL7R expression in all cases of B-ALL (Fig. S3B) suggest that overexpressed CRLF2 signals in the absence of IL7R, and thus independently of TSLP. Finally, what is the role of TSLP in signaling by overexpressed CRLF2 and is it dependent on which mutation is present? Cells that expressed CRLF2 Phe232Cys and IL7R proliferated more rapidly in response to TSLP (Fig. 4B), but those that expressed CRLF2/mutant JAK2 did not (Fig. S3C). Experiments are under way to determine whether this difference applies to B-ALL in vivo.
Levels of TSLP within the bone marrow microenvironment have not been described, but could be adequate to stimulate B-ALL proliferation. Importantly, TSLP liberated from keratinocytes in a murine model of atopic dermatitis stimulated polyclonal B-cell lymphoproliferation (42). Although no association has been identified between B-ALL and atopy, it will be important to reexamine this issue with a focus specifically on CRLF2-overexpressing B-ALL.
Materials and Methods
The studies were approved by the DFCI institutional review board.
Cell Lines.
BaF3 cells were maintained in RPMI medium with 10% FCS and 500 pg/mL IL3. UT7 cells were maintained in RPMI medium with 10% FCS and 500 pg/mL GM-CSF (Bayer).
Survival and Inhibitor Assays.
Survival of BaF3 and UT7 cells transduced with CRLF2 and/or JAK2 constructs was determined by growing all cell lines in the presence of growth factors until d 0. On d 0, cells were seeded at 104 to 105/mL in media in the presence or absence of growth factors (IL3 500 pg/mL or TSLP 1 ng/mL) or JAK inhibitor-1 (Calbiochem) and the number of cells was then counted daily. All assays were performed in triplicate or more.
Immunoblotting.
Antibodies were used against CRLF2 (R&D Systems), STAT5 (Santa Cruz Biotechnology), and phospho-STAT5, JAK2, phospho-JAK2, AKT, phospho-AKT, ERK, and phospho-ERK (Cell Signaling). For nonreducing immunoblot, we used protein dissolved in SDS sample buffer without 2-mercaptoethanol.
FISH.
FISH was performed as described (6). For each sample, 100 cells were scored. For all cases with rearrangements, the frequency of cells with the rearrangement was ≥80% of the fraction of leukemia involvement within that bone marrow specimen, as measured by histologic examination.
Additional methods are outlined in SI Methods.
Supplementary Material
Acknowledgments
The authors thank Dr. Bob Distel and Yanan Kuang from the Dana-Farber Cancer Institute Translational Research Laboratory for assistance with HPLC; Marc Dibona for assistance with FISH; Drs. Shai Izraeli and A. Thomas Look for helpful discussion; and Dr. Roberto Bellucci, Gorka Murga, Ilene Galinsky, RNP, Dr. Ann Mullally, and Dr. Takaomi Sanda for assistance with obtaining primary samples and cell lines. This work was supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (D.M.W.), the Compagnia di San Paolo, Turin, the Dunkin Donuts Rising Star Program, the Kristen Amico Fund, and the Claudia Adams Barr Program in Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911726107/DCSupplemental.
References
- 1.Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 2.Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 3.Mullighan CG, et al. Children’s Oncology Group. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullighan CG, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaretti S, et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res. 2005;11:7209–7219. doi: 10.1158/1078-0432.CCR-04-2165. [DOI] [PubMed] [Google Scholar]
- 6.Fine BM, et al. Gene expression patterns associated with recurrent chromosomal translocations in acute lymphoblastic leukemia. Blood. 2004;103:1043–1049. doi: 10.1182/blood-2003-05-1518. [DOI] [PubMed] [Google Scholar]
- 7.Juric D, et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25:1341–1349. doi: 10.1200/JCO.2006.09.3534. [DOI] [PubMed] [Google Scholar]
- 8.Moos PJ, et al. Identification of gene expression profiles that segregate patients with childhood leukemia. Clin Cancer Res. 2002;8:3118–3130. [PubMed] [Google Scholar]
- 9.Faderl S, Kantarjian HM, Talpaz M, Estrov Z. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91:3995–4019. [PubMed] [Google Scholar]
- 10.Malinge S, et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood. 2007;109:2202–2204. doi: 10.1182/blood-2006-09-045963. [DOI] [PubMed] [Google Scholar]
- 11.Bercovich D, et al. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down’s syndrome. Lancet. 2008;372:1484–1492. doi: 10.1016/S0140-6736(08)61341-0. [DOI] [PubMed] [Google Scholar]
- 12.Gaikwad A, et al. Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol. 2009;144:930–932. doi: 10.1111/j.1365-2141.2008.07552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney L, et al. Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood. 2009;113:646–648. doi: 10.1182/blood-2008-08-170928. [DOI] [PubMed] [Google Scholar]
- 14.Mullighan CG, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene. 2000;19:2496–2504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci USA. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25. doi: 10.1016/S0065-2776(08)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reche PA, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 20.Levin SD, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 21.Brown VI, et al. Thymic stromal-derived lymphopoietin induces proliferation of pre-B leukemia and antagonizes mTOR inhibitors, suggesting a role for interleukin-7Ralpha signaling. Cancer Res. 2007;67:9963–9970. doi: 10.1158/0008-5472.CAN-06-4704. [DOI] [PubMed] [Google Scholar]
- 22.Mullighan CG, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell LJ, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 24.Küppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Gross AW, Lodish HF. Active conformation of the erythropoietin receptor: random and cysteine-scanning mutagenesis of the extracellular juxtamembrane and transmembrane domains. J Biol Chem. 2006;281:7002–7011. doi: 10.1074/jbc.M512638200. [DOI] [PubMed] [Google Scholar]
- 26.Kjaer S, Kurokawa K, Perrinjaquet M, Abrescia C, Ibáñez CF. Self-association of the transmembrane domain of RET underlies oncogenic activation by MEN2A mutations. Oncogene. 2006;25:7086–7095. doi: 10.1038/sj.onc.1209698. [DOI] [PubMed] [Google Scholar]
- 27.Mullighan CG. JAK2—a new player in acute lymphoblastic leukaemia. Lancet. 2008;372:1448–1450. doi: 10.1016/S0140-6736(08)61342-2. [DOI] [PubMed] [Google Scholar]
- 28.Kanehisa M. The KEGG database. Novartis Found Symp. 2002;247:91–101. [PubMed] [Google Scholar]
- 29.Ross ME, et al. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood. 2003;102:2951–2959. doi: 10.1182/blood-2003-01-0338. [DOI] [PubMed] [Google Scholar]
- 30.Shimamura T, et al. Non-small-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res. 2006;66:6487–6491. doi: 10.1158/0008-5472.CAN-06-0971. [DOI] [PubMed] [Google Scholar]
- 31.George RE, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slupianek A, et al. Fusion tyrosine kinases induce drug resistance by stimulation of homology-dependent recombination repair, prolongation of G(2)/M phase, and protection from apoptosis. Mol Cell Biol. 2002;22:4189–4201. doi: 10.1128/MCB.22.12.4189-4201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malinge S, et al. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008;112:4220–4226. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- 34.Jiang J, et al. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65:8968–8974. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- 35.Gaidzik V, Döhner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin Oncol. 2008;35:346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman T, Rowe JM. Hematopoietic stem cell transplantation for adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2009;16:453–459. doi: 10.1097/MOH.0b013e3283309a40. [DOI] [PubMed] [Google Scholar]
- 37.Wang JH, et al. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature. 2009;460:231–236. doi: 10.1038/nature08159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akasaka T, et al. Five members of the CEBP transcription factor family are targeted by recurrent IGH translocations in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) Blood. 2007;109:3451–3461. doi: 10.1182/blood-2006-08-041012. [DOI] [PubMed] [Google Scholar]
- 39.Russell LJ, et al. A novel translocation, t(14;19)(q32;p13), involving IGH@ and the cytokine receptor for erythropoietin. Leukemia. 2009;23:614–617. doi: 10.1038/leu.2008.250. [DOI] [PubMed] [Google Scholar]
- 40.Russell LJ, et al. t(6;14)(p22;q32): a new recurrent IGH@ translocation involving ID4 in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) Blood. 2008;111:387–391. doi: 10.1182/blood-2007-07-092015. [DOI] [PubMed] [Google Scholar]
- 41.Ottmann OG, Pfeifer H. First-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia in adults. Curr Opin Oncol. 2009;21(Suppl 1):S43–S46. doi: 10.1097/01.cco.0000357476.43164.6b. [DOI] [PubMed] [Google Scholar]
- 42.Demehri S, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.