Abstract
It is well accepted that complex biological processes such as angiogenesis are not controlled by a single family of molecules or individually isolated signaling pathways. In this regard, new insight into the interconnected mechanisms that regulate angiogenesis might be gained by examining this process from a more global network perspective. The coordination of signaling cues from both outside and inside many different cell types is required for the successful completion of angiogenesis. Evidence is accumulating that the multifunctional integrin family of cell adhesion receptors represent an important group of molecules that play active roles in sensing, integrating, and distributing a diverse set of signals that regulate many cellular events required for angiogenesis. Given the ability of integrins to bind numerous extracellular ligands and transmit signals in a bi-directional fashion, we will discuss the multiple ways by which integrins may serve as a functional hub during pathological angiogenesis. In addition, we will highlight potential imaging and therapeutic strategies based on the expanding new insight into integrin function.
Keywords: Integrins, Angiogenesis, Functional Hubs, Extracellular Matrix
Introduction
Angiogenesis can be characterized as an integrated set of cellular, biochemical and molecular processes by which new blood vessels are formed from pre-existing vessels [1]. Central to this process is the concept that networks of “soluble” and “solid state” information must flow in a bidirectional manner between the non-cellular microenvironment and different cell types involved in new vessel development [Figure-1]. The diverse cell types involved in angiogenesis must have the capacity to sense, assimilate, interpret, distribute and respond to complex streams of information in a meaningful way in order for blood vessels to form. A crucial feature in the integration and subsequent distribution of diverse networks such as the World Wide Web, electrical power grids, and cells signaling networks, is the concept of “functional hubs” [2,3]. Functional hubs can act to gather, interpret, integrate and distribute large amounts of complex information to control a given response to changes in bi-directional communication links. Due to the integrated nature of cellular signaling networks disrupting a “function hub”, as opposed to any one individual pathway within an integrated system may have a more pronounced effect [4]. Thus, developing novel approaches to selectively disrupt functional hubs, may lead to more effective strategies for controlling complex biological processes such as angiogenesis.
Figure 1. Bi-direction Flow of Information During Angiogenesis.
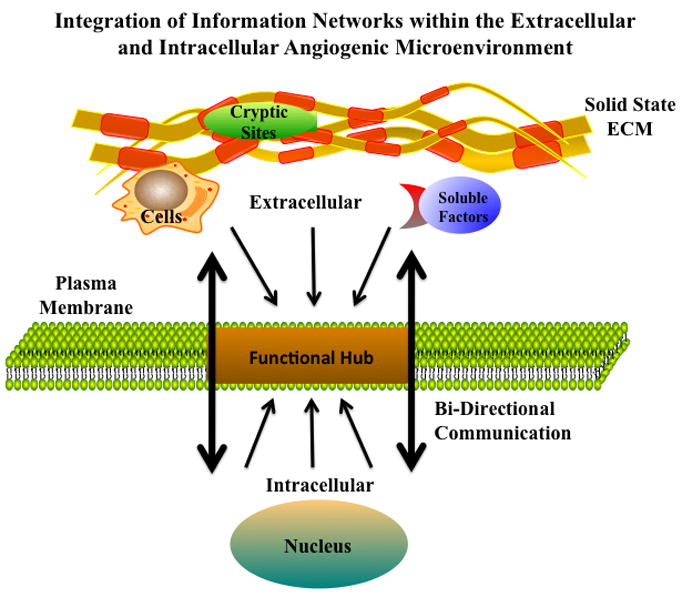
Angiogenesis involve bidirectional integration of numerous extracellular and intracellular angiogenic cues. Schematic representation of the bi-directional flow of signaling cues through a functional hub that gathers, interprets and distributes the regulatory stimuli needed for the formation of functional blood vessels.
Given the number of networks that need to be organized and integrated for new vessels to form, functional hubs may be operationally important during angiogenesis. A major group of cell surface molecules that may serve as functional hubs during angiogenesis is the integrin family and these molecules will be the primary focus of our discussion [5]. Integrins are multifunctional heterodimeric adhesion receptors composed of α and β chains derived from separate gene products [6]. These transmembrane receptors are broadly expressed in all cell types that play roles in angiogenesis. Given their transmembrane structure, ability to form associations with adaptor molecules, and finally, their ability to bind many extracellular ligands, these receptors are well positioned to serve as functional hubs [5,6].
Blood vessel formation regulates physiological processes such as embryonic development, wound healing, and reproduction [7–9]. While angiogenesis plays important roles in normal physiology, it also contributes to pathological processes such as diabetic retinopathy, rheumatoid arthritis and tumor growth [7–11]. Given the broad implications of angiogenesis in these processes, the possibility of modulating angiogenesis for therapy has driven the need for a more complete understanding of this complex cascade. Great strides have been made in understanding the molecular events that control angiogenesis and surprising new mechanistic insight is beginning to emerge. Several new studies have uncovered evidence that while certain angiogenesis inhibitors such as those targeting VEGF signaling may inhibit angiogenesis and slow primary tumor growth, they may also be associated with enhanced tumor cell invasion and metastasis [12,13]. While enthusiasm continues for targeting angiogenesis as a therapeutic modality, these and other opposing experimental results from genetic models and pharmacological inhibition studies illustrates the need for a more detailed understanding of this important process.
Angiogenesis
While several mechanisms can lead to the formation of blood vessels including embryonic vasculogenesis and arteriogenesis, we will focus our discussion on angiogenesis and in particular, the roles of integrins as functional hubs in pathological neovascularization. Angiogenesis can be categorized into at least two types including sprouting and intussuceptive angiogenesis [14,15]. Sprouting angiogenesis involves growth factor stimulation leading to altered cell-cell and cell-extracellular matrix (ECM) interactions, which can enhance proliferation and invasion. These invading cells organize into cords that ultimately form lumens by a process of lumenogenesis [16]. The newly forming blood vessels undergo maturation and stabilization as they recruit supporting cells such as pericytes and smooth muscle cells. In contrast to sprouting angiogenesis, which is associated with extensive proliferation, intussuceptive angiogenesis involves the formation of translumenal tissue bridges that split vessels into higher order branches [4].
In simplistic terms, angiogenesis can be organized into three interconnected stages including an initiation phase, an invasive phase and a maturation phase. During the initiation phase, pro-angiogenic factors can stimulate unique patterns of gene expression leading to altered cell adhesion and proliferation. The invasive phase of angiogenesis can be characterized by enhanced ECM remodeling and invasion. In the maturation phase, endothelial cords undergo lumenogenesis, and recruit cells such as pericytes, fibroblasts, and bone marrow derived cells.
Pathological Angiogenesis
While some of the basic regulatory molecules and cell types that control vessel development may be shared between normal and pathological angiogenesis, distinct differences also exist. These differences may contribute in part to the conflicting results sometimes observed between genetic models focused on normal embryonic vascular development and pharmacological inhibition studies of pathological angiogenesis. Studies have identified a variety of differences between normal and tumor vessels including alterations in the levels and composition of cell surface molecules. Using Serial Analysis of Gene Expression (SAGE), investigators have identified tumor endothelial cell markers that exhibited differential expression [17]. Endothelial cells isolated from certain tumors exhibited enhanced proliferative capacity, reduced ability to undergo senescence and enhanced resistance to serum deprivation as compared to quiescent endothelial cells [18]. Glioblastoma derived tumor endothelial cells were shown to exhibit enhanced resistance to chemotherapeutic drugs as compared to normal brain endothelial cells [19]. This chemoresistance was thought to be at least partly due to elevated levels of survivin [19]. It is interesting to point out that integrin β1-mediated interactions with ECM proteins such as fibronectin have been suggested to regulate survivin [20]. In other studies, enhanced expression of ER chaperone protein GRP78 in brain tumor endothelial cells may contribute to their chemoresistance [21]. De-regulation of growth factor signaling in tumor endothelial cells is also thought to play an important role in chemoresistance [22]. Moreover, some endothelial cells isolated from tumors exhibited aneuploidy [23]. Intriguing new studies also demonstrate differences between tumor and normal endothelial cells in terms of how they respond to mechanical forces [24]. It is interesting to note again, that integrins are a major family molecules known to regulate a cell’s response to growth factors and mechanical forces. Finally, a variety of differences also exist in the physical structures of normal and tumor vessels. The composition and integrity of the ECM including the basement membrane and interstitial matrix can be significantly altered between normal and tumor vessels.
Differences have been documented between the basement membrane components of embryonic vasculature and adult vasculature. During embryonic development the major type of laminin within basement membranes is largely laminin-8 which contains the α4 laminin chain [25]. In contrast, in postnatal mice, increased levels of Laminin-10 are deposited in basement membranes [25]. Interestingly, the α5 chain of laminin-10 has been suggested to be an isotype of laminin that has a freely exposed and integrin accessible RGD site capable of being recognized by αvβ3 integrin [26]. If αvβ3 recognition of this laminin RGD site plays a role in the ability of αvβ3 to regulate new vessel development, it would not be surprising that mice lacking αvβ3 exhibited minimal effects on embryonic vascular development since this potentially important ligand for αvβ3 is not highly expressed in this context. However, during adult pathological angiogenesis when Laminin-10 is strongly expressed along with other RGD containing provisional matrix proteins such as fibrinogen and vitronectin, antagonists of αvβ3 inhibit pathological angiogenesis [27]. Thus, when examining integrin function, the expression of their ligands should also be considered.
An additional example that illustrates the differential expression of integrin ligands in the vasculature includes the alternatively spliced forms of fibronectin containing EIIIA and EIIIB domains, which are expressed predominately during embryonic development and pathological angiogenesis, but normally lacking in adult vessels [28]. Collectively, these examples help highlight some of the more obvious differences in blood vessels that may contribute to conflicting results sometimes observed between genetic mouse models of integrin function in embryonic vasculogenesis and pharmacological inhibition during pathological angiogenesis.
Critical to the concept of viewing integrins as functional hubs is the appreciation of the diversity of molecules that integrins bind and regulate. Integrins can associate with a wide array of distinct families of molecules including growth factors and their receptors, cytoplasmic adaptors, signaling molecules, other cell surface adhesion receptors, matrix altering proteases, protease inhibitors, cell surface protease receptors, ECM components and anti-angiogenic ECM fragments [Figure-2]. Given that integration of the numerous signaling networks are required for the formation of new blood vessels, integrins appear well suited to serve as functional hubs.
Figure 2. Integrins as Functional Hubs During Angiogenesis.
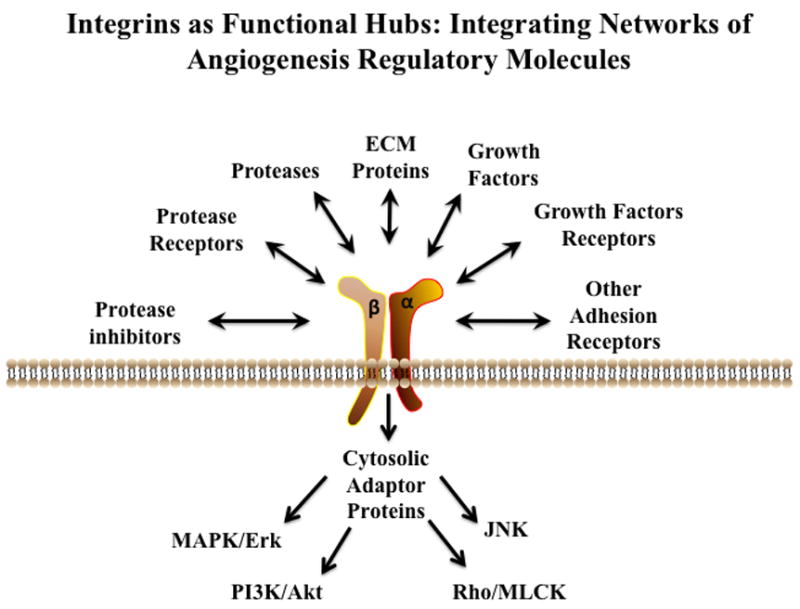
Angiogenesis requires the coordinated activity of a wide array of distinct families of molecules and integrin receptors have the ability to bind many different angiogenesis regulatory factors. Schematic representation illustrating the diversity of angiogenic regulatory proteins and signaling networks that integrins may coordinate during new blood vessel formation.
Integrin Structure, Ligand Binding, and Bi-directional Signaling
Integrins are receptors with at least 24 distinct members generated from 18 α and 8 β subunits [29]. When describing integrin expression as it relates to angiogenesis, we often limit our discussion to endothelial cells and supporting mural cells. Given the evidence implicating many different cell types in angiogenesis, including bone marrow derived cells, fibroblast as well as tumor cells, the regulation of angiogenesis by integrins expressed in these cells may be significantly under estimated. A variety of integrin heterodimers containing β1, β3, β4, β5 and β8 subunits have been shown to play roles in angiogenesis and experimental evidence supporting this notion comes from both genetic models and pharmacological inhibition studies [30].
It would be well beyond the scope of this review to discuss in depth integrin structure, ligand binding and signaling. However, in order to fully appreciate the multiple mechanisms by which integrins may regulate angiogenesis, we will briefly touch on some of the more basic features of integrin binding and signaling. Integrins are composed of an extracellular domain with both α and β chains contributing to a ligand binding surface. The extracellular domains of both the α and β chains are followed by transmembrane domains and short cytoplasmic tails. Structural studies have provided evidence that integrins exist in at least three different configurations, including a low affinity state characterized by a closed headpiece and a bent conformation. An intermediate affinity state characterized by an extended leg region with a closed headpiece and a fully extend conformation with an open headpiece characterizing the high affinity state have also been described [31].
The ability of integrins to bind and associate with various ECM proteins, transmembrane receptors and soluble ligands largely depends on their structural conformation and these distinct conformations are crucial for regulating both inside out and outside in signaling [32]. Studies demonstrate that the cytosolic proteins such as Talin and Kindlins are important control molecules in integrin function [33,34]. Exposure of a cryptic integrin-binding site within talin is thought to promote interactions with the β integrin tails which may facilitate separation of the α and β chains [33]. This physical separation may propagate structural changes through the transmembrane region that ultimately allow extension of the integrin leg-like domains and movement of the head-piece away from the membrane allowing enhanced ligand binding [31]. From outside the cell, extracellular ligand binding may facilitate conformational changes and integrin clustering within the plane of the membrane [5]. Integrin clustering may involve lateral associations between the transmembrane domains thereby facilitating cytoplasmic tail interactions with adapter proteins leading to the formation of large multi-protein signaling complexes [5]. Recent estimates have suggested that well over a 100 different types of cytoplasmic molecules may associate with integrins [5]. This large diversity of protein-protein interactions allows integrins to sense changes in the extracellular environment and integrate and distribute this structural and molecular information into diverse signaling networks [33]. In this regard, a recent study has suggested that integrin-mediated interactions with collagen type-I may result in changes in phosphorylation of more than 350 different proteins [36]. Given this complexity, many questions still remain to be answered as to mechanisms that regulate the molecular switches that define the initiation of a particular integrin-signaling pathway.
Integrin interaction with a variety of ligands has been shown to modulate and integrate signaling networks including MAPK/Erk, PI3k/Akt, Rho/MLCK and the JNk pathways [37,38]. Integrin engagement is thought to enhance phosphorylation of key residues within their cytoplasmic tails, which can potentiate associations with a variety of adaptor molecules and non-receptor kinases such as Fak, Src and ILK [39]. This capacity of integrins to activate, integrate and distribute information from these signaling networks illustrates the potential of these receptors to serve as functional distribution hubs in bi-directional information transfer.
Integrins and Angiogenesis
In addition to the differential expression of integrins within angiogenic and quiescent vessels, both genetic models of integrin function and inhibitor studies have provided compelling evidence that integrins regulate angiogenesis. During our discussion, we will highlight the need for careful interpretation of experimental findings in the context of the particular models being examined. To this end, the potential differences between integrin functions during embryonic vasculogenesis and that of pathological angiogenesis will be addressed. It is important to note that a more meaningful interpretation of the biological functions of integrins might be obtained when considering the expression of their ligands in the context of the models being used. The biological functions of a given receptor is linked to the presence or absence of its ligands and this is of particular significance with integrins, given the expanding number of diverse ligand now ascribed to these multifunctional receptors.
β1 Integrins in Vascular Development and Pathological Angiogenesis
Studies focusing on the functional importance of α5 integrin in embryonic development demonstrated that while α5 null mice are embryonic lethal, blood vessels were formed prior to their death [40]. However, blood vessel formation appeared disrupted as defects in vessel maturation and stability was observed [40]. These studies are consistent with the possibility that α5 integrin may be more important for later steps in vascular maturation and angiogenesis, rather than the early stages of vasculogenesis [40]. The α5 integrin subunit can associate with the β1 chain. β1-integrin knockout mice are also embryonic lethal, thus making it difficult to establish an in-depth molecular understanding of the roles for β1 integrins in angiogenesis. In a series of genetic studies where β1 integrin was specifically knocked out in endothelial and some hematopoietic cells, mutant embryos died before birth, but blood vessels and yolk sac vascular plexus formation did occur, again suggesting limited impact on vasculogenesis (41–43). However distinct defects were observed in sprouting and vascular patterning in these β1 integrin deficient embryos [41–43].
Importantly, a major ECM ligand for α5β1 integrin is fibronectin, and mice deficient in fibronectin also exhibited vascular defects [44]. Interestingly, mice lacking a functional RGD integrin binding site within fibronectin exhibited an embryonic lethal phenotype and showed significant vascular abnormalities [45]. These studies suggest that integrin recognition of this functional RGD site contributes to new vessel formation. In further studies, isoforms of fibronectin such as alternatively spliced forms containing the EIIIA and EIIIB domains were shown to be restricted in expression to embryonic development and angiogenesis [46]. While little information is available on integrin interactions with these forms of fibronectin, evidence suggests that α4β1 and α9β1 may bind to the EIIIA domain and both these β1 integrins have been suggested to impact angiogenesis [47]. Interestingly mice lacking either EIIIA or EIIIB exhibited little obvious defects in embryonic vasculogenesis [46]. However, double EIIIA/EIIIB knockouts were embryonic lethal and exhibited cardiovascular defects, vascular hemorrhage, alterations in vascular yolk sac remodeling, mural cell coverage and angiogenesis [46]. It is interesting to point out that α4β1 integrin has been suggested to play a role in mural cell recruitment [48–50].
While many studies are consistent with a limited role for α5 and β1 integrins in the early stages of vasculogenesis, pharmacological inhibition of α5β1 can disrupt pathological angiogenesis [51,52]. α4β1 may play a functional role in the mobilization and recruitment of monocytes to the vasculature and pharmacological blockade of α4β1 was shown to reduce the extent of monocyte-stimulated angiogenesis [49]. Blocking α4β1 interactions with VCAM-1 resulted in reduced pericyte coverage of blood vessels, increased apoptosis and decreased angiogenesis [48]. Moreover, while vasculogenesis did occur in α4 integrin deficient mice, some vessels exhibited a reduced pericyte and smooth muscle cell coverage [50]. These studies taken together with observation in EIIIA/B fibronectin domain mutant mice provide support for a role for this integrin-ligand pair in angiogenesis.
Collagen and several collagen-binding integrins including α1β1, α2β1 and α3β1 have been implicated in regulating blood vessel formation. For example, mice harboring defects in the expression of collagen as well as mice with defects in collagen metabolism have been shown to exhibit vascular abnormalities [53,54]. While, vascular defects were observed in these collagen mutant mice, early embryonic vasculogenesis still occurred (53,54). Previous studies have demonstrated that distinct cryptic collagen epitopes including the HUIV26 and the HU177 sites play roles in postnatal pathological angiogenesis [55–61]. In addition, soluble fragments containing the non-collagenous (NC) domains of collagen were shown to inhibit pathological angiogenesis [62]. Finally, a number of studies have demonstrated that specific antagonists of collagen binding integrins inhibit pathological angiogenesis [63–65].
As mentioned previously, it is important to distinguish between the roles that integrins and their ligands play in normal embryonic vascular development and their roles in pathological angiogenesis, which may differ. For instance, while NC1 domains of collagen-IV chains are known to bind integrins and inhibit pathological angiogenesis, mice deficient in individual collagen chains, and thus their soluble NC domains, exhibited minimal abnormalities in early embryonic vasculogenesis [62,66]. Interestingly, mice lacking the collagen binding α1 integrin, exhibited few if any defects in embryonic vasculogenesis, yet pharmacological targeting of this integrin inhibited pathological angiogenesis [63,64,67]. In other studies, while mice lacking the α2 integrin failed to demonstrate an obvious alteration in embryonic vasculogenesis, pharmacological inhibition of α2β1 inhibited pathological angiogenesis [62,68]. Finally, other β1 integrins including α6β1, α7β1 and α9β1 have recently been reported to regulating blood vessel development and angiogenesis [69–71]. Taken together, these studies and many others, demonstrate the need to distinguish between embryonic vasculogenesis and postnatal pathological angiogenesis in regards to integrin function in new vessel formation.
αv Integrins in Vascular Development and Pathological Angiogenesis
Among the most extensively studied set of integrins in angiogenesis are the αv containing heterodimers and in particular αvβ3. Numerous studies have provided evidence that pharmacological antagonists of αvβ3 inhibit pathological angiogenesis in many in vivo models [27,72–74]. As with the majority of the integrins examined to date, mice lacking αv exhibited few defects in early vasculogenesis [75]. However, some abnormal leaky vessels were detected in the brain and gut [75]. Given these findings, it would be interesting to examine whether alterations in expression or composition of particular integrin ligands occur within these distinct tissues. In further studies, pathological angiogenesis in these αvβ3 deficient mice was shown to be elevated, which may be due in part to increased VEGFR signaling [76]. In contrast, signaling deficient β3 knockin mice harboring mutations in key phosphorylation sites exhibited few alterations in embryonic vasculogenesis, but cytokine induced and tumor angiogenesis in these mice was inhibited [77].
In more recent studies, β3 signaling deficient mice where shown to exhibit defects in recruitment of bone marrow derived cells to sites of neovascularization [78]. Bone marrow transplants from β3 wild type mice appeared to rescue the defects observed in angiogenesis [78]. Interestingly, in previous studies, αvβ3 expressing glioblastoma tumors implanted in β3 wild type mice exhibited enhanced tumor angiogenesis and elevated tumor infiltration of bone marrow derived macrophage as compared to those tumors transplanted in β3 null mice [79]. However, while increased angiogenesis was observed, the primary tumors size was essentially the same [79]. Bone marrow transplantation or macrophage depletion reversed this glioblastoma phenotype, suggesting that host-derived αvβ3 expressing cells may differentially regulate angiogenesis and tumor growth. These important studies provide new evidence that β3 integrin expressed in bone marrow derived cells may play a critical role in angiogenesis as well as tumor growth. In further studies, abnormal coronary vessel maturation was detected in male, but not female β3 deficient mice [80]. It would be interesting to speculate that the altered tissue projections within lumens of these coronary vessels may be associated with alterations in intusscuptive angiogenesis given the nature of the morphological changes observed. Finally, while not an αv containing integrin, α6β4 has also been implicated in angiogenesis [81]. Deletion of α6 results in loss of α6β4 and α6β1, which resulted in an embryonic lethal phenotype [82]. However, no obvious defects in embryonic vasculogenesis were reported [82]. In contrast, mice harboring a mutant signaling deficient β4 chain exhibited significant reductions in pathological angiogenesis [81]. Collectively, these examples help illustrate the careful interpretation that is needed in examining the functional impact of integrins in embryonic vasculogenesis and that of pathological angiogenesis. Furthermore, these examples emphasize the need for a more an in-depth understanding of the molecular contributions of integrins to angiogenesis from cell types other than endothelial cells.
Regulation of the Initiation Stage of Angiogenesis by Integrins
Integrins bind and regulate numerous factors known to influence angiogenesis and thereby may act as functional hubs in facilitating the coordination and distribution of information from diverse signaling networks. Integrins may regulate the expression and activity of pro-angiogenic molecules such as growth factors and growth factor receptors as well as anti-angiogenic factors such as ECM fragments [62, 82]. Integrin-mediated regulation of growth factor signaling can modulate crucial events during the initiation phase of angiogenesis including proliferation, cell adhesion and expression of matrix altering enzymes. In addition to binding growth factor and growth factor receptors, integrin interaction with ECM components may also impact the expression and activity of receptors that control cell-cell interactions such as cadherins [83].
Integrin-Growth Factor and Growth Factor Receptor Associations
Growth factors such as VEGF, FGF, IGF-1, and EGF can be secreted as well as immobilized within the ECM [84,85]. These growths factors may be recognized in a matrix bound state and stimulate proliferation and motility [84,85]. Evidence suggests that integrins may associate with a number of these immobilized pro-angiogenic molecules. Integrins such as αvβ3, α3β1 and α9β1 may interact with distinct isoforms of VEGF-A and these interactions may enhance endothelial cell adhesion, migration and survival [71,85]. In addition to VEGF, integrins bind FGF1, Angiopoietins (Ang1), Connective Tissue Growth Factor (CTGF) and Cysteine-rich angiogenic protein 61 (Cyr61) [86]. Studies demonstrate that αvβ3 may bind immobilized FGF1 and enhanced cell adhesion, migration and proliferation [86]. Moreover, α5β1 may associate with Ang -1 and this interaction may promote signaling leading to migration that is independent from the Ang-1 receptor Tie-2 [87].
In addition to interactions with secreted and matrix bound growth factors, integrins also associate with growth factor receptors. VEGF-A- mediated activation of VEGFR2 recruits Src and facilitates phosphorylation of the β3 cytoplasmic tail [88]. The phosphorylated β3 tail may promote interactions between αvβ3 and VEGFR2, thereby enhancing endothelial proliferation and survival [88]. Further studies suggest that αvβ3 may associate with other growth factor receptors including FGF, IGF-1, PDGF and HGF receptors [89–92]. The molecular mechanisms governing these associations and their functional impact on angiogenesis are not fully understood, but these complexes likely enhance and integrate numerous signaling networks important for new vessel formation. Interactions between β1-integrins and ECM can also influence growth factor signaling. For example, β1-integrin collagen interaction was shown to inhibit VEGFR2 signaling by recruitment of the tyrosine phosphatase Shp-2 leading to de-phosphorylating VEGFR2 [93].
In addition to pro-angiogenic factors, integrins also regulate the response of vascular cells to anti-angiogenic factors. The anti-angiogenic chemokine CXCL4 may bind αvβ3 in endothelial cells and inhibit adhesion and migration [94]. Moreover, endogenous angiogenesis inhibitors such as such as Canstatin and Tumstatin bind β1 and β3 integrins leading to inhibition of endothelial cell proliferation and survival [95,96]. Finally, the Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) may inhibit angiogenesis by an MMP independent mechanisms involving TIMP-2 binding to α3β1 integrin [97]. This novel association leads to Shp-1 dependent inactivation of FGF and VEGF receptors, thereby inhibiting endothelial cell proliferation [97].
Integrin-Mediated Alteration of Cell Adhesion During Angiogenesis
Alterations in cell-cell junctions are thought to play vital roles in the initiation of angiogenesis and embryonic vascular development, as mice deficient in VE-cadherin exhibit vascular abnormalities [98]. Experimental support for integrins in regulating cadherin-dependent endothelial cell behavior comes from studies examining VE-cadherin. αvβ3 interactions with fibronectin disrupts VE-cadherin distribution in endothelial cell junctions and this disruption was associated with Src activation [83]. This αvβ3-mediated disruption of VE-cadherin decreased cell-cell interaction and may promote migration. Previous studies have also suggested that FGF signaling may play an important role in regulating cadherin expression in endothelial cells. FGF2 stimulation of endothelial cells in vitro was also shown to decrease the surface expression of several cadherins including E, N, P and VE-cadherins and this inhibition appeared to depend at least in part, on JNK signaling [99]. In this regard, integrin are known to modulate the JNK pathway and integrins such as αvβ3 and α5β1 have been shown to associate with FGF and FGF receptors. Interestingly, Syndecan-1, a cell surface ECM receptor was also shown to bind and subsequently activate αvβ3 [100]. This novel syndecan-integrin association appears to play a role in angiogenesis as a specific peptide antagonist (Synstatin) of this interaction inhibited angiogenesis and tumor growth in vivo [100]. Thus, integrin signaling and cross talk with other cell adhesion receptors may play an important role in regulating cell adhesion and integrin activation thereby providing endothelial cells with an enhanced capacity to migrate during the initiation phase of angiogenesis.
Integrin Mediated Regulation of Protease Expression
The ability of vascular cells to remodel the local extracellular microenvironment is a critical step in angiogenesis and the expression of proteolytic enzymes such as MMPs and serine proteases are thought to regulate angiogenesis in both a positive and negative manner [101]. The coordinated expression of proteases and their respective inhibitors likely facilitate the transition from the initiation phase to the invasive stage of angiogenesis. Endothelial cells, tumor cells, fibroblasts, and bone marrow derived cells all contribute to the enhanced levels of proteases within the angiogenic microenvironment. Thus, it is important to consider integrins expressed in these diverse cell types as to their ability to regulate protease expression. Previous studies demonstrate that α5β1 binding to the 120kd cell-binding domain of fibronectin enhances expression of MMP-3 and MMP-9 while interaction with the CS-1 domain suppressed MMPs [102,103]. In endothelial cells, α2β1 interactions with collagen, enhanced expression of MMP-1, while α5β1 expressed in macrophages increased expression of MMP-9 [104,105]. Over expression of αvβ6 in squamous carcinoma cells enhanced expression of MMP-3 and MMP-9 [106,107]. These studies highlight some of the many examples by which integrins may regulate the complex mechanisms that contribute to the early initiation phase of angiogenesis.
Regulation of the Invasive Stage of Angiogenesis by Integrins
Following initiation, the invasive phase of angiogenesis can be characterized by remodeling of the local microenvironment. While integrins can regulate the expression of proteolytic enzymes, many of these proteases are secreted as zymogens and thus need to be activated and localized to discrete sites to facilitate directed cellular invasion. An expanding body of evidence has demonstrated that integrins play functional roles in the activation and localization of matrix degrading proteases. This enhanced activity leads to structural alterations in the local ECM, providing less restrictive pathways for vascular cell invasion and migration. In addition, structural changes of the ECM can expose Matrix Immobilized Cryptic ECM Epitopes (MICEE) that regulates cell adhesion, proliferation and migration [55–61,108].
Involvement of Integrins in Protease Activation and Cell Surface Localization
Previous studies have demonstrated that β1 interactions with collagen enhanced the activation of MMPs including MMP-2, MMP-9, and MT1-MMP in a variety of cell types [109–111]. Interestingly, MMP-2 activation following collagen binding appeared to depend on the structural conformation of collagen as the activation of MMP-2 was observed in cell attached to native but not denatured collagen type-I [112]. Moreover, this MMP-2 activation was associated with increased levels of MT1-MMP. Further evidence supporting a role for integrins in regulating protease activity comes from studies examining αvβ3 dependent activation of the serine protease tPA in vascular smooth muscle cells [113].
In addition to playing roles in expression and activation of proteolytic enzymes, evidence now indicates that integrins can bind proteolytic enzymes at the cell surface thereby localizing activity to sites of migration and invasion. In this regard, we previously made the novel observation that αvβ3 co-localized with MMP-2 on angiogenic blood vessels and invasive tumor cells and this interaction was associated with enhanced collagen degradation and invasion [114]. Inhibiting αvβ3-MMP-2 interactions reduced angiogenesis and tumor growth, suggesting a functional role for this protease-integrin association [115,116]. Interestingly, since our early observations, many new studies have now documented further examples of integrin-protease associations and their functional implications [Table-I]. Integrin αvβ3 has also been shown to associate with the serine protease thrombin, the serine protease receptor uPAR as well as the secreted lysosomal protease procathepsin-X [117–119]. In other studies, MT1-MMP was found associated with αvβ3 in endothelial tips cells, while β1 integrin was found associated with MT1-MMP in non-migrating endothelial cells, suggesting that αvβ3- MT1-MMP interactions may promote endothelial cell invasion and migration [120]. In addition to these studies, MMP-9 has also been shown to associate with a number of integrins including αMβ2, α5β1 and αvβ5 in diverse cell types including tumor cells, epithelial cells and neutrophils [110,121–123]. Given the numerous studies demonstrating integrin-protease interactions it would not be surprising that many of these associations contribute to the regulation of angiogenesis. Finally, proteolytic exposure of cryptic regulatory elements (MICEE) buried in the three-dimensional structure of ECM proteins has been shown to regulate endothelial and tumor cell adhesion and migration in vitro and angiogenesis and tumor growth in vivo [55–61].
Table I.
Examples of Integrin-Protease Associations
| Integrin | Proteolytic Enzyme | Reference |
|---|---|---|
| α2β1 | MMP-1 | 152 |
| α4β1 | ADAM-7 | 153 |
| α4β1 | ADAM-28 | 153 |
| α5β1 | MMP-9 | 119 |
| α5β1 | ADAM-15 | 154 |
| α5β1 | ADAM-17 | 155 |
| α6β1 | ADAM-2 | 156 |
| α6β1 | ADAM-9 | 157 |
| α9β1 | ADAM-9 | 158 |
| α9β1 | ADAM-7 | 158 |
| α9β1 | ADAM-12 | 159 |
| α9β1 | ADAM-28 | 158 |
| α9β1 | ADAM-33 | 158 |
| α4β7 | ADAM-28 | 160 |
| αvβ3 | MMP-2 | 114 |
| αvβ3 | MT1-MMP | 120 |
| αvβ3 | Thrombin | 117 |
| αvβ3 | ProCathepsin-X | 119 |
| αvβ3 | ADAM-15 | 160 |
| αvβ3 | ADAM-23 | 161 |
| αvβ5 | MMP-9 | 162 |
| αvβ5 | ADAM-9 | 163 |
| αL/β2 | MMP-9 | 164 |
| αM/β2 | MMP-9 | 165 |
| αM/β2 | Elastase | 164 |
| αM/β2 | Protease-3 | 167 |
Integrins in the Regulation of Maturation Phase of Angiogenesis
During the maturation phase of angiogenesis, endothelial cells begin to re-establish new cell-cell and cell-ECM connections. In addition, bone morrow derived progenitor cells may also be recruited to the sites of neovascularization [124]. Following endothelial cord formation, the process of lumenogenesis begins. The molecular mechanisms regulating lumenogenesis is not completely understood, but new insight is beginning to emerge and integrins may play a role [16]. Lumenogenesis is thought to involve several steps and studies have implicated molecules such as CDC42, Rac-1, and MT-1 MMP in this process [16]. Importantly, α2β1 may also contribute to lumen formation as antibodies directed to α2β1 inhibit this process [16].
Blood vessels continue to mature by assembling new vascular basement membranes. Recruitment of mural cells such as pericytes and smooth muscle cells contribute to vascular basement membrane assembly. For example, fibroblast, pericytes and vascular smooth muscle cells are all thought to contribute to the formation of basement membranes and their synthesis and proper assembly may be integrin dependent. Genetic evidence supporting this idea comes from studies demonstrating basement membranes abnormalities in α3 integrin deficient mice [125]. Interestingly, mice deficient in Akt exhibited defects in the assembly of blood vessel associated fibronectin and targeting fibroblast derived from these mice with a stimulating anti-β1 antibody reversed this defect [126]. The assembly of other basement membrane components such as laminin and collagen type-IV has also been suggested to depend in part on β1 integrins [127,128]. Blood vessel stability involves pericyte and smooth muscle cell coverage and evidence suggests that recruitment of mural cells to blood vessels may depend on β1 integrins. For instance, vascular maturation was impaired in mice harboring deletions in mural cell β1 integrins and blocking α4β1 interactions with VCAM-1 resulted in reduced pericyte coverage of blood vessels, leading to increased apoptosis and decreased angiogenesis [48,129].
Integrins and their Ligands as Selective Targets in Angiogenic Imaging
Concerted efforts are now underway to exploit the new molecular understanding of integrins for imaging and therapeutics. In addition to detecting areas pathological neovascularization, and assessing the efficacy of anti-angiogenic drugs, early detection of tumors that are transitioning from a benign to a malignant phenotype is also of great interest. Detection of the early stages of angiogenesis may provide a means for selective targeting of sites of malignant tumor progression at a time when therapeutic intervention may result in more beneficial effects.
Numerous studies have focused on detecting changes in expression of growth factor receptors and their circulating or matrix bound ligands as potential markers of angiogenesis [130]. In other studies, work has focused on quantifying the relative levels of circulating bone marrow-derived endothelial progenitor cells as surrogate markers of angiogenesis [131]. Importantly, just as investigators have exploited the altered expression of growth factor receptors and their liagnds, the differential expression of integrins and their ligands, may also represent clinically useful targets for imaging. One of the better-studied integrins in this context is αvβ3. A variety of studies have documented up-regulation of αvβ3 in angiogenic blood vessels as compared to normal vessels, and imaging agents linked to antagonists targeting αvβ3 have demonstrated great promise in selectively homing to sites of neovascularization [132]. Given the expanding body of evidence for the functional contribution of αvβ3 expressing bone marrow derived cells in angiogenesis, αvβ3-directed imaging may provide a clinically useful approach in the near future.
As we have emphasized throughout our discussion, it is important to consider the corresponding ligands that bind a given receptor, and this concept is also relevant in terms of their potential use in angiogenesis imaging. The alternatively spliced form of fibronectin containing the EIIIA domain, which has been suggested to be a ligand for α4β1 and α9β1, is highly expressed in angiogenic blood vessels with little if any expression in normal quiescent adult blood vessels. This differential expression may be useful as a selective target for angiogenesis imaging. Importantly, while ECM proteins are well-known integrin ligands, an early hallmark of angiogenesis involves proteolytic remodeling of the local ECM surround blood vessels. Thus, structurally altered forms of ECM proteins within the vascular basement membrane may also provide a highly selective target to distinguish early angiogenic vessels from quiescent vessels. Studies have suggested that some of the earliest events during angiogenesis are associated with secretion of proteolytic enzymes from resident cells or from cells that have been recruited to the sites of neovascularization [133]. In this respect, we identified the HUIV26 cryptic collagen-IV epitope within the vascular basement membranes of angiogenic tissues. This cryptic epitope was shown to be a ligand for integrin αvβ3 [55]. Importantly, the proteolytic exposure of this cryptic ECM site was shown to be an early event in angiogenesis as exposure of the HUIV26 epitope was observed between 24 and 48 hours following FGF2 stimulated angiogenesis in the chick CAM and could also be detected within 24 hours in the mouse retina following induction of neovascularization [55,134]. Exposure of the HUIV26 cryptic sites was largely dependant on MMP-9, as a specific reduction in the exposure of the HUIV26 cryptic epitope was observed in MMP-9 null mice with only minimal reductions observed in MMP-2 knockout mice [56]. In a separate study, exposure of the HUIV26 site within vascular basement membranes was also detected within 18 to 24 hrs after induction of angiogenesis in the thymus of mice and again this was shown to be dependent in large part on MMP-9 [57].
In further studies, we identified a second cryptic collagen epitope termed HU177, and in contrast to the HUIV26 site, the HU177 cryptic collagen site could be selectively exposed within a variety distinct types of collagen [59]. Importantly, the HU177 cryptic sites were shown to be exposed within angiogenic but not quiescent blood vessels in several models [59–61,135–137]. Interestingly, little if any exposure of the HU177 epitope was detected within vessels from the pupillary membranes of mice, however 24 hours after VEGF stimulation the HU177 cryptic epitope could be detected in these tissues [136]. HU177 cryptic epitope was shown to be specifically exposed in a time dependent manner in basement membranes following ischemia induced angiogenesis in the hindlimb model and again its expression correlated with the ischemia induced expression of MMPs. The expression of HU177 epitope was markedly reduced in MMP-9 null mice [137]. Moreover, exciting new studies have recently demonstrated a statistically significant increase in the levels of soluble form of the HU177 cryptic epitope in serum from primary melanoma patients as compared controls [138]. In addition, to these collagen epitopes, we have also identified a novel cryptic epitope within laminin that may also be selectively exposed within the basement membrane of tumor associated angiogenic blood vessels [108]. Collectively, these studies demonstrate that potential of exploiting these novel cryptic ECM sites within well-known integrin ligands as targets for highly selective imaging of angiogenesis and tissue invasion.
Inhibition of the Integrin Functional Hub During Angiogenesis
As mention above, the notion that integrins serve as functional hubs integrating and distributing networks of angiogenic signaling cues from a variety of cell types, provides a strong rational for targeting integrins to control aberrant neovascularization. Given the known consequences of disrupting functional hubs associated with other types of network such as transportation systems and electrical power grids, disrupting integrins, might result in important therapeutic benefits alone or in combination with chemotherapy or radiation. Significant efforts have been focused on developing anti-angiogenic agents targeting integrins. αv containing integrins such as αvβ3 and αvβ5 have been a major focus in the development of antagonists of angiogenesis and numerous studies have provided convincing data that blocking αv integrins can inhibit pathological angiogenesis [74]. As discussed previously, blocking αv integrins may disrupt mechanisms within several phases of angiogenesis including cellular proliferation, migration, survival signaling and proteolytic activity. Several antibodies targeting αv integrins have been developed, some of which are being currently tested in clinical trials including, Abegrin/Etarcizumab (anti-αvβ3), CNT095 (anti-αv) and Abciximab (anti-αIIbβ3/αvβ3) [139–141]. In addition, peptide and non-peptidic mimetic are also being developed that target αv integrins such Cilengitide [142]. Interestingly, new strategies aimed at disrupting functional associations of αvβ3 with MMP2 (TSRI265) and Syndecan-1 (SSTN) have also been developed and demonstrate anti-angiogenic activity [100,116].
In addition to αv integrins several antagonists directed to different β1 integrins have also been generated, including Volociximab (anti-α5β1), ATN-161 (anti-α5β1) and E7820 (anti-α2) [51,52,143]. Integrin antagonists such as Efalizumab, a humanized antibody targeting αLβ2 and Natalizumab directed to α4 have been developed to treat inflammatory disease such as psoriasis and Chron’s [144,145]. Given the emerging findings that bone marrow derived myeloid cells may contribute to angiogenesis, and β2 integrins can be expressed in a variety of bone marrow derived cells, it would be interesting to determine whether these antagonists may also impact the angiogenic cascade.
Selective Targeting of Cryptic Integrin Ligands
Given the broad expression of integrins in essentially all tissues as well as their multiple roles in normal as well as pathological conditions, it is critical to carefully determine the most appropriate use of integrin antagonists alone and in combination with existing therapies to fully realize their clinical potential. Complicating the effective use of integrin antagonists in the clinical setting is the emerging realization that certain integrins may regulate angiogenesis and tumor progression in a positive manner while others may negatively impact these processes. Moreover, blocking one specific integrin may alter the functional activity of a second different integrin, a phenomenon known as trans-dominant integrin regulation [146]. Thus, developing novel approaches that selectively inhibit the pro-angiogenic activity without enhancing vessel growth or tumor invasion will be critical to the successful use of integrin inhibitors for the treatment of tumor progression. As mentioned previously, an important aspect in regulating any receptor mediated signaling cascade is to consider both the receptor and its ligands. Just as investigators have targeted the VEGFR and its ligand VEGF, the possibility exists that targeting integrin ligands such as defined ECM proteins may also be of therapeutic utility. However, given the ubiquitous expression of integrin ligands, a highly selective approach is needed.
One potential strategy makes use of the observations that the ECM surrounding angiogenic blood vessels as well as the invasive fronts of tumors are often structurally altered. These structural alterations in existing ECM components may serve as solid-state conformational switches that facilitate an invasive phenotype (Figure 3). A rapidly growing body of evidence is now documenting examples of structurally altered or alternatively spliced forms of ECM components accumulating at sites of angiogenesis and tumor invasion [147–151]. For example, cryptic sites and alternatively spliced forms of many ECM molecules have been detected including cryptic sites in Vitronectin, Fibrinogen, Fibronectin, Laminin and Collagen (Table-II). The localized exposure of these cryptic sites may not simply represent ECM destruction, but represent solid-state conformational switches, thereby providing temporally controlled and highly selective exposure of novel integrin ligands. The temporally regulated exposure of cryptic integrin ligands may allow the initiation of novel integrin signaling events restricted to sites of tissue invasion.
Figure 3. Solid-State Conformational Switches within the ECM.
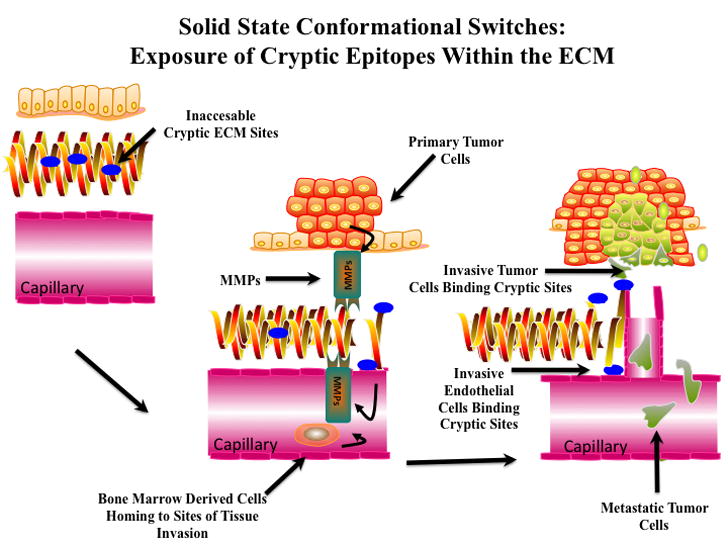
A crucial event during angiogenesis involves structural modification of the local ECM. Schematic representation of proteolytic exposure of solid-state conformational switches (Cryptic Epitopes). Proteolytic activity from both resident cells and bone marrow derived cells homing to sites of angiogenesis may contribute to the localized exposure of these cryptic regulatory switches. Many solid-state cryptic sites represent integrin ligand that regulates invasive cellular behavior restricted to sites of tissue invasion. These cryptic sites may allow highly selective targeting for imaging and therapeutic intervention.
Table 2.
Examples of cryptic ECM sites and alternatively spliced forms of ECM
| Cryptic ECM Site/Alternatively Spliced | Receptors | References |
|---|---|---|
| Cryptic HUIV26 Site in Collagen-IV | αvβ3 | 55 |
| Cryptic HU177 Site in Collagen | β1 | 59, Unpublished |
| Cryptic STQ Site in Laminin | Unknown | 108 |
| Cryptic Site in Laminin | Unknown | 151 |
| Cryptic Site (RGD) in Vitronectin | αvβ3 | 147 |
| Cryptic Site in Vitronectin-2 | Unknown | 148 |
| Cryptic site in Fibrinogen | αvβ6 | 149 |
| Cryptic Site in Fibronectin | αvβ3, α5β1 | 150 |
| Alternatively Spliced EIIIA Domain | α4β1/α9β1 | 28 |
| Alternatively Spliced EIIIB Domain | Unknown | 28 |
Cryptic integrin ligands may represent highly selective therapeutic targets that can be exploited to inhibit integrin signaling that is restricted in large part, to sites of tissue invasion. For example, the HUIV26 cryptic site within collagen type-IV was shown to be recognized by integrin αvβ3 [55]. Thus blocking αvβ3 dependent binding to the HUIV26 cryptic site by targeting the HUIV26 site rather that the integrin receptor, results in highly selective inhibition of αvβ3-mediated signaling at sites of tissue invasion. Several studies have provided evidence that targeting the HUIV26 cryptic epitope inhibits angiogenesis, tumor growth and metastasis in numerous models [55–58]. In similar studies, a second highly conserved cryptic collagen epitope (HU177) that can be exposed in many genetically distinct type of collagen was also identified. Studies have recently shown that specific targeting of this highly restricted cryptic collagen epitope can also potently inhibit angiogenesis and tumor growth in vivo [59–61]. Importantly, this unique cryptic collagen epitope (HU177) also represents a cryptic integrin ligand (unpublished observations). A murine antibody directed to this site has been humanized and clinical trials are currently underway to examine its effects in patients with malignant tumors [60,61]. Collectively, these studies and others are beginning to provide evidence for the utility of targeting unique cryptic integrin ligands to selectively control pathological angiogenesis.
Conclusions
With the growing appreciation of the importance of many integrated signaling networks in controlling both physiological and pathological blood vessel formation, additional molecular insight into angiogenesis, and perhaps the development of novel anti-angiogenic approaches, might be achieved by studying this process from a more global rather than a reductionist perspective. In this regard, an interesting concept in systems biology and network theory suggests that targeting certain hubs that integrate circuits within a system may result in a more pronounced disruption than targeting a single pathway. Intriguing evidence suggests that integrins function in sensing changes in both the extracellular and intracellular microenvironments and in turn, integrates and distributes these complex streams of information into diverse signaling networks to ultimately regulate cellular behavior. Thus, in some respects integrins may represent functional hubs that operate during the various stages of angiogenesis. Given the emerging evidence that particular integrins may regulate distinct processes during angiogenesis in either a positive or negative manner, a more global understanding how these receptors coordinate the diversity of pro- and anti-angiogenic stimuli may lead to the identification of highly selective new strategies to regulate pathological angiogenesis.
Acknowledgments
This work was supported in part by grants 2ROICA91645 to PCB and a grant from Cancer Innovations Inc to PCB. Additional support was provided from grant P20RR15555 to Robert Friesel and subproject to PCB. We would like to apologize to all those investigators whose important work was not discussed do to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Barabasi AL, Oltvar ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004;5:101–13. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 3.Almaas E. Biological impacts and context of network theory. J Exp Biol. 2007;210:1548–58. doi: 10.1242/jeb.003731. [DOI] [PubMed] [Google Scholar]
- 4.Albert R, Jeong H, Barabasi A-L. Error and attack tolerance of complex networks. Nature. 2000;27406:378–82. doi: 10.1038/35019019. [DOI] [PubMed] [Google Scholar]
- 5.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314:131–44. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 7.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Hand Exp Pharmacol. 2006;176:157–212. doi: 10.1007/3-540-36028-x_6. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson L, Kreuger J, Claesson-Welsh L. Building blood vessels--stem cell models in vascular biology. J Cell Biol. 2007;177:751–5. doi: 10.1083/jcb.200701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akalu A, Cretu A, Brooks PC. Targeting integrins for the control of tumor angiogenesis. Expert Opin Investig Drugs. 2005;14:1475–86. doi: 10.1517/13543784.14.12.1475. [DOI] [PubMed] [Google Scholar]
- 11.Gagne P, Akalu A, Brooks PC. Challenges facing antiangiogenic therapy for cancer: impact of the tumor extracellular environment. Expert Rev Anticancer Ther. 2004;4:129–40. doi: 10.1586/14737140.4.1.129. [DOI] [PubMed] [Google Scholar]
- 12.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Anti-angiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burri PH, Hlushchuk PR, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–88. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 15.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–12. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 16.Davis DE, Koh AW, Stratman N. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res Embryo Today. 2007;81:270–85. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- 17.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Gene expressed in human endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 18.deVos FYFL, Willemse PHB, deVries EGE, Gietema JA. Endothelial cell effects of cytotoxics:balance between desired and unwanted effects. Cancer Treatment Rev. 2004;30:495–513. doi: 10.1016/j.ctrv.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Virrey JJ, Guan S, Li W, Schonthal AH, Chen TC, Hofman FM. Increased survivin expression confers chemoresistance to tumor-associated endothelial cells. Am J Path. 2008;173:575–85. doi: 10.2353/ajpath.2008.071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu Y-M, King M, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-α-induced apoptosis via the AKT/Survivin pathway. J Biol Chem. 2003;278:50402–11. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 21.Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M, et al. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–75. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–72. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 23.Hida K, Klagsbrun M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res. 2005;65:2507–10. doi: 10.1158/0008-5472.CAN-05-0002. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Nat’l Acad Sci U S A. 2008;105:11305–10. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physio Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, Timpl R. Domain IVa of laminin α5 chain is cell-adhesive and binds β1 and αvβ3 integrins through Arg-Gly-Asp. FEBS Lett. 2001;509:181–5. doi: 10.1016/s0014-5793(01)03167-2. [DOI] [PubMed] [Google Scholar]
- 27.Brooks PC, Clark RA, Cheeresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 28.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009 doi: 10.1007/s10456-009-9136-6. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 32.Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev. 2002;186:141–63. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- 33.Wegener KL, Campbell ID. Transmembrane and cytoplasmic domains in integrin activation and protein-protein interactions. Mol Membr Biol. 2008;25:76–87. doi: 10.1080/09687680802269886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma Y-Q, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15:313–8. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, Nagasami C, et al. Activation of integrin αIIbβ3 by modulation of transmembrane helix association. Science. 2003;300:795–8. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Lu B, Yang Q, Fearns C, Yates JR, 3rd, Lee JD. Combined integrin phosphoproteomic analyses and small interfering RNA--based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res. 2009;69:3713–20. doi: 10.1158/0008-5472.CAN-08-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somanatn PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. 2009;53:53–64. doi: 10.1007/s12013-008-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serini G, Napione L, Bussolino F. Integrins team up with tyrosine kinase receptors and plexins to control angiogenesis. Curr Opin Hematol. 2008;15:235–42. doi: 10.1097/MOH.0b013e3282fa745b. [DOI] [PubMed] [Google Scholar]
- 39.Sepulveda JL, Gkretsv Wu C. Assembly and signaling of adhesion complexes. Curr Top Dev Biol. 2005;68:183–225. doi: 10.1016/S0070-2153(05)68007-6. [DOI] [PubMed] [Google Scholar]
- 40.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 41.Lei L, Liu D, Huang Y, Jovin I, Shai S-Y, Kyriakides T, et al. Endothelial expression of β1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol. 2008;28:794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlson TR, Hu H, Braren R, Kim YH, Wang RA. Cell-autonomous requirement for β1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008;135:2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. β1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237:75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- 44.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, et al. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–78. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–74. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 48.Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin α4β1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–51. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J, Jin H, et al. Integrin α4β1promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–52. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 50.Grazioli A, Alves CS, Konstantopoulos K, Yang JT. Defective blood vessel development and pericyte/pvSMC distribution in α4 integrin-deficient mouse embryos. Dev Biol. 2006;293:165–77. doi: 10.1016/j.ydbio.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 51.Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, et al. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14:7924–9. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doñate F, Parry GC, Shaked Y, Hensley H, Guan X, Beck I, et al. Pharmacology of the novel antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2): observation of a U-shaped dose-response curve in several preclinical models of angiogenesis and tumor growth. Clin Cancer Res. 2008;14:2137–44. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- 53.Löhler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38:597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- 54.Deguchi J-O, Huang H, Libby P, Aikawa E, Whittaker P, Sylvan J, et al. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest. 2009;89:315–26. doi: 10.1038/labinvest.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, et al. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–37. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odaka C, Tanioka M, Itoh T. Matrix metalloproteinase-9 in macrophages induces thymic neovascularization following thymocyte apoptosis. J Immunol. 2005;174:846–53. doi: 10.4049/jimmunol.174.2.846. [DOI] [PubMed] [Google Scholar]
- 58.Jo N, Ju M, Nishijima K, Robinson GS, Adamis AP, Shima DT, et al. Inhibitory effect of an antibody to cryptic collagen type IV epitopes on choroidal neovascularization. Mol Vis. 2006;12:1243–9. [PubMed] [Google Scholar]
- 59.Cretu A, Roth JM, Caunt M, Akalu A, Policarpio D, Formenti S, et al. Disruption of endothelial cell interactions with the novel HU177 cryptic collagen epitope inhibits angiogenesis. Clin Cancer Res. 2007;13:3068–68. doi: 10.1158/1078-0432.CCR-06-2342. [DOI] [PubMed] [Google Scholar]
- 60.Pernasetti F, Nickel J, Clark D, Baeuerle PA, Van Epps D, Freimark B. Novel anti-denatured collagen humanized antibody D93 inhibits angiogenesis and tumor growth: An extracellular matrix-based therapeutic approach. Int J Oncol. 2006;29:1371–9. [PubMed] [Google Scholar]
- 61.Freimark B, Clark D, Pernasetti F, Nickel J, Myszka D, Baeuerle PA, et al. Targeting of humanized antibody D93 to sites of angiogenesis and tumor growth by binding to multiple epitopes on denatured collagens. Mol Immunol. 2007;44:3741–50. doi: 10.1016/j.molimm.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 62.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, et al. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–61. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 63.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through α1β1 and α2β1 integrins. Proc Natl Acad Sci USA. 1997;94:13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcinkiewicz C, Weinreb PH, Calvete JJ, Kisiel DG, Mousa SA, Tuszynski GP, et al. Obtustatin: a potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003;63:2020–3. [PubMed] [Google Scholar]
- 65.Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T, et al. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J Biol Chem. 2008;283:2335–43. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 66.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2003;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 67.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–13. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 68.Holtkötter O, Nieswandt B, Smyth N, Müller W, Hafner M, Schulte V, et al. Integrin α2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–94. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 69.Lee T-H, Seng S, Li H, Kennel SJ, Avraham HK, Avraham S. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of α6β1 integrin in angiogenesis. J Biol Chem. 2006;281:40450–60. doi: 10.1074/jbc.M607525200. [DOI] [PubMed] [Google Scholar]
- 70.Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, et al. Role for the α7β1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- 71.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin α9β1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–96. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 72.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 73.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–22. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nemeth JA, Nakada MT, Trikha M, Lang Z, Gordon MS, Jayson GC, et al. Alpha-v integrin as therapeutic targets in oncology. Cancer Invest. 2007;25:632–46. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 75.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–19. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 76.Renolds AR, Renolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in β3-integrin-deficient mice. Cancer Res. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 77.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–07. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng W, McCabe NP, Mahabeleshwar GH, Somantha PR, Philips DR, Byzova TV. The angiogenic response is dictated by β3 integrin on bone marrow-derived cells. J Cell Biol. 2008;183:1145–57. doi: 10.1083/jcb.200802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanamori M, Kawaguchi T, Berger MS, Pieper RO. Intracranial microenvironment reveals independent opposing functions of host αvβ3 expression on glioma growth and angiogenesis. J Biol Chem. 2006;281:37256–64. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- 80.Weis SM, Lindquist JN, Barnes LA, Lutu-Fuga KM, Cui J, Wood MR. Cooperation between VEGF and β3 integrin during cardiac vascular development. Blood. 2007;109:1962–70. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 82.Georges-Labouesse E, Mark M, Messaddeq N, Gansumuller A. Essential role of α6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–6. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Jin G, Miao H, Li JY, Usam S, Chien S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci U S A. 2006;3:1774–9. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark RA. Synergistic signaling from extracellular matrix-growth factor complexes. J Invest Dermatol. 2008;128:1354–5. doi: 10.1038/jid.2008.75. [DOI] [PubMed] [Google Scholar]
- 85.Hutchings H, Ortega N, Plouët J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;11:1520–2. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 86.Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, et al. Direct binding of integrin αvβ3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008;283:18066–75. doi: 10.1074/jbc.M801213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 2007;5:655–65. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- 88.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;8:882–92. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toledo MS, Suzuki E, Handa K, Hakomori S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J Biol Chem. 2005;280:16227–34. doi: 10.1074/jbc.M413713200. [DOI] [PubMed] [Google Scholar]
- 90.Clemmons DR, Maile LA. Interaction between insulin-like growth factor-I receptor and αvβ3integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol Endocrinol. 2005;19:1–11. doi: 10.1210/me.2004-0376. [DOI] [PubMed] [Google Scholar]
- 91.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–7. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, et al. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signaling pathway in endothelial cells. BMC Cell Biol. 2005;6:8–14. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitola S, Brenchio B, Piccinini M, Tertoolen L, Zammataro L, Breier G, et al. Type I collagen limits VEGFR-2 signaling by a SHP2 protein-tyrosine phosphatase-dependent mechanism. Circ Res. 2006;98:45–54. doi: 10.1161/01.RES.0000199355.32422.7b. [DOI] [PubMed] [Google Scholar]
- 94.Aidoudi S, Bujakowska K, Kieffer N, Bikfalvi A. The CXC-chemokine CXCL4 interacts with integrins implicated in angiogenesis. PLoS ONE. 2008;3:e2657. doi: 10.1371/journal.pone.0002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with αvβ3and αvβ5 integrins. Cancer Res. 2005;65:4353–61. doi: 10.1158/0008-5472.CAN-04-3536. [DOI] [PubMed] [Google Scholar]
- 96.Sudhakar A, Boosani CS. Inhibition of tumor angiogenesis by tumstatin: insights into signaling mechanisms and implications in cancer regression. Pharm Res. 2008;25:2731–9. doi: 10.1007/s11095-008-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–80. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 98.Gory-Fauré S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, et al. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- 99.Wu JC, Yan HC, Chen WT, Chen WH, Wang CJ, Chi YC, et al. JNK signaling pathway is required for bFGF-mediated surface cadherin downregulation on HUVEC. Exp Cell Res. 2008;314:421–429. doi: 10.1016/j.yexcr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.López-Otín C, Matrisian LM. Emerging roles of proteases in tumor suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 102.Tremble P, Damsky CH, Werb Z. Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol. 1995;29:1707–20. doi: 10.1083/jcb.129.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by α5β1 and α4β1integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–79. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Langholz O, Röckel D, Mauch C, Kozlowska E, Bank I, Krieg T, et al. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1integrins. J Cell Biol. 1995;131:1903–15. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie B, Laouar A, Huberman E. Fibronectin-mediated cell adhesion is required for induction of 92-kDa type IV collagenase/gelatinase (MMP-9) gene expression during macrophage differentiation. The signaling role of protein kinase C-beta. J Biol Chem. 1998;273:11576–82. doi: 10.1074/jbc.273.19.11576. [DOI] [PubMed] [Google Scholar]
- 106.Ylipalosaari M, Thomas GJ, Nystrom M, Salhimi S, Marshall JF, Huotari V, et al. αvβ6 integrin down-regulates the MMP-13 expression in oral squamous cell carcinoma cells. Exp Cell Res. 2005;309:273–83. doi: 10.1016/j.yexcr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 107.Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF. αvβ6 integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol. 2001;116:898–904. doi: 10.1046/j.1523-1747.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- 108.Akalu A, Roth JM, Caunt M, Policarpio D, Liebes L, Brooks PC. Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 2007;67:4353–63. doi: 10.1158/0008-5472.CAN-06-0482. [DOI] [PubMed] [Google Scholar]
- 109.Borrirukwanit K, Lafleur MA, Mercuri FA, Blick T, Price JT, Fridman R, et al. The type I collagen induction of MT1-MMP-mediated MMP-2 activation is repressed by αvβ3 integrin in human breast cancer cells. Matrix Biol. 2007;26:291–305. doi: 10.1016/j.matbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 110.Wang XQ, Su P, Paller AS. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J Biol Chem. 2003;278:48770–8. doi: 10.1074/jbc.M308818200. [DOI] [PubMed] [Google Scholar]
- 111.Knoblauch A, Will C, Goncharenko G, Ludwig S, Wixler V. The binding of Mss4 to alpha-integrin subunits regulates matrix metalloproteinase activation and fibronectin remodeling. FASEB J. 2007;21:497–510. doi: 10.1096/fj.06-7022com. [DOI] [PubMed] [Google Scholar]
- 112.Wang DR, Sato M, Li LN, Miura M, Kojima N, Senoo H. Stimulation of pro-MMP-2 production and activation by native forms of extracellular type I collagen in cultured hepatic stellate cells. Cell Struct Funct. 2003;28:505–13. doi: 10.1247/csf.28.505. [DOI] [PubMed] [Google Scholar]
- 113.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA. LRP and αvβ3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:1351–9. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- 114.Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson GW, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–93. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 115.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 116.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin αvβ3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98:119–224. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bar-Shavit R, Eskohjido Y, Fenton JW, Esko JD, Vlodavsky I. Thrombin adhesive properties: induction by plasmin and heparan sulfate. J Cell Biol. 1993;123:1279–87. doi: 10.1083/jcb.123.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xue W, Mizukami I, Todd RF, Petty HR. Urokinase-type plasminogen activator receptors associate with β1 and β3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57:1682–9. [PubMed] [Google Scholar]
- 119.Lechner AM, Assfalg-Machleidt I, Zahler S, Stoeckelhuber M, Machleidt W, Jochum M, et al. RGD-dependent binding of procathepsin X to integrin αvβ3 mediates cell-adhesive properties. J Biol Chem. 2006;281:39588–97. doi: 10.1074/jbc.M513439200. [DOI] [PubMed] [Google Scholar]
- 120.Gálvez BG, Matías-Román S, Yáñez-Mó M, Sánchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–21. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stefanidakis M, Ruohtula T, Borregaard N, Gahmberg CG, Koivunen E, Stefanidakis M, et al. Intracellular and cell surface localization of a complex between αMβ2 integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J Immunol. 2004;172:7060–8. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 122.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The α3β1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–42. [PubMed] [Google Scholar]
- 123.Björklund M, Heikkilä P, Koivunen E. Peptide inhibition of catalytic and noncatalytic activities of matrix metalloproteinase-9 blocks tumor cell migration and invasion. J Biol Chem. 2004;279:29589–97. doi: 10.1074/jbc.M401601200. [DOI] [PubMed] [Google Scholar]
- 124.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 125.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–88. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 126.Somanath PR, Kandel ES, Ha N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem. 2007;82:22964–76. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lohikangas L, Gullberg D, Johansson S. Assembly of laminin polymers is dependent on β1-integrins. Exp Cell Res. 2001;265:135–44. doi: 10.1006/excr.2001.5170. [DOI] [PubMed] [Google Scholar]
- 128.Fleischmajer RS, Perlish RS, MacDonald ED, Schechter A, Murdoch AD, Iozzo RV, et al. There is binding of collagen IV to β1 integrin during early skin basement membrane assembly. Ann N Y Acad Sci. 1998;857:212–27. doi: 10.1111/j.1749-6632.1998.tb10118.x. [DOI] [PubMed] [Google Scholar]
- 129.Abraham S, Kogata N, Fässler R, Adams RH. Integrin β1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–70. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 130.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–33. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 131.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 132.Chen X. Multimodality imaging of tumor integrin αvβ3 expression. Mini-Rev Med Chem. 2006;6:227–34. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- 133.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu J, Rodrigue ZD, Kim JJ, Brooks PC. Generation of monoclonal antibody to cryptic collagen sites by using subtractive immunization. Hybridoma. 2000;19:375–85. doi: 10.1089/02724570050198893. [DOI] [PubMed] [Google Scholar]
- 135.Petitclerc E, Strömblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, et al. Integrinαvβ3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–30. [PubMed] [Google Scholar]
- 136.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–10. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gagne PJ, Tihonov N, Li X, Glaser J, Qiao J, Silberstein M, et al. Temporal exposure of cryptic collagen epitopes within ischemic muscle during hindlimb reperfusion. Am J Pathol. 2005;167:1349–59. doi: 10.1016/S0002-9440(10)61222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ng B, Zakrzewski J, Warycha M, Christos PJ, Bajorin DF, Shapiro RL, et al. Shedding of distinct cryptic collagen epitope (HU177) in sera of melanoma patients. Clin Cancer Res. 2008;14:6253–8. doi: 10.1158/1078-0432.CCR-07-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, et al. Phase I and pharmacokinetic study of etaracizumab (Abegrin™), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- 140.Mullamitha SA, Ton NC, Parker GJM, Jackson A, Julyan PJ, Roberts C, et al. Phase I evaluation of a fully human anti-αv integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 141.Pathak A, Zhao R, Huang J, Stouffer GA. Eptifibatide and abciximab inhibit insulin-induced focal adhesion formation and proliferative responses in human aortic smooth muscle cells. Cardiovasc Diabetol. 2008;7:36–47. doi: 10.1186/1475-2840-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Philips P, et al. Phase I clinical trial in children with refractory brain tumors: pediatric brain tumor consortium study PBTC-012. J Clin Oncol. 2008:26919–24. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 143.Keizer RJ, Zamacona MK, Jansen M, Critchley D, Wanders J, Beijnen JH, et al. Application of population pharmacokinetic modeling in early clinical development of the anticancer agent E7820. Invest New Drugs. 2009;27:140–52. doi: 10.1007/s10637-008-9164-x. [DOI] [PubMed] [Google Scholar]
- 144.Costanzo A, Talamonti M, Botti E, Spalone G, Papoutsaki M, Chimenti S. Efficacy of efalizumab in psoriasis patients previously treated with tumor necrosis factor blockers. Dermatology. 2009 doi: 10.1159/000213758. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 145.MacDonald JK, Mcdonald JW. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007:1. doi: 10.1002/14651858.CD006097.pub2. [DOI] [PubMed] [Google Scholar]
- 146.Díaz-González F, Forsyth J, Steiner B, Ginsberg MH. Trans-dominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–51. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seiffert D, Smith JW. The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem. 1997;272:13705–10. doi: 10.1074/jbc.272.21.13705. [DOI] [PubMed] [Google Scholar]
- 148.Bloemendal HJ, de Boer HC, Koop EA, van Dongen AJ, Goldschmeding R, Landman WJ, et al. Activated vitronectin as a target for anticancer therapy with human antibody. Cancer Immunol Immunother. 2004;53:799–808. doi: 10.1007/s00262-004-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fouchier F, Penel C, Pierre Montero M, Bremond P, Champion S. Integrin αvβ6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn2+ Eur J Cell Biol. 2007;86:143–60. doi: 10.1016/j.ejcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 150.Hocking DC, Kowallski K. A cryptic fragment from fibronectin’s III1 module localizes to lipid rafts and stimulates cell growth and contractility. J Cell Biol. 2002;158:175–84. doi: 10.1083/jcb.200112031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 152.Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, et al. Pro-collagenase-1 (matrix metalloproteinase-1) binds the α2β1 integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem. 2001;276:29368–74. doi: 10.1074/jbc.M104179200. [DOI] [PubMed] [Google Scholar]
- 153.Bridges LC, Sheppard D, Bowditch RD. ADAM disintegrin-like domain recognition by the lymphocyte integrins α4β1 and α4β7. Biochem J. 2005;387:101–8. doi: 10.1042/BJ20041444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nath D, Slocombe PM, Stephens PE, Warn A, Hutchingson GR, Yamada KM, et al. Interaction of metargidin (ADAM-15) with αvβ3 and α5β1 integrins on different haemopoietic cells. J Cell Sci. 1999;112:579–87. doi: 10.1242/jcs.112.4.579. [DOI] [PubMed] [Google Scholar]
- 155.Bax DV, Messent AJ, Tart J, van Hoang M, Kott J, Maciewicz RA, et al. Integrin α5β1 and ADAM-17 interact in vitro and co-localize in migrating HeLa cells. J Biol Chem. 2004;279:22377–86. doi: 10.1074/jbc.M400180200. [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Sampson NS. Mediation of sperm-egg fusion: evidence that mouse egg α6β1 integrin is the receptor for sperm fertilinbeta. Chem Biol. 1999;6:1–10. doi: 10.1016/S1074-5521(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 157.Nath D, Slocombe PM, Webster A, Stephens PE, Dochertt AJ, Murphy G. Meltrin gamma (ADAM-9) mediates cellular adhesion through α6β1 integrin, leading to a marked induction of fibroblast cell motility. J Cell Sci. 2000;113:2319–28. doi: 10.1242/jcs.113.12.2319. [DOI] [PubMed] [Google Scholar]
- 158.Bridges LC, Bowditch RD. ADAM-Integrin interactions: potential integrin regulated ectodomain-shedding activity. Curr Pharm Des. 2005;11:837–47. doi: 10.2174/1381612053381747. [DOI] [PubMed] [Google Scholar]
- 159.Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–30. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- 160.Zhang XP, Kamata T, Yokoyama K, Puzon-McLaughlin W, Takada Y. Specific interaction of the recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15) with integrin αvβ3. J Biol Chem. 1998;273:7345–50. doi: 10.1074/jbc.273.13.7345. [DOI] [PubMed] [Google Scholar]
- 161.Cal S, Freije JM, López JM, Takada Y, López-Otín ADAM 23/MDC3, a human disintegrin that promotes cell adhesion via interaction with the αvβ3 integrin through an RGD-independent mechanism. Mol Biol Cell. 2000;11:1457–69. doi: 10.1091/mbc.11.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Björklund M, Heikkilä P, Koivunen E. Peptide inhibition of catalytic and noncatalytic activities of matrix metalloproteinase-9 blocks tumor cell migration and invasion. J Biol Chem. 2004;279:29589–97. doi: 10.1074/jbc.M401601200. [DOI] [PubMed] [Google Scholar]
- 163.Zhou M, Graham R, Russell G, Croucher PI. MDC-9 (ADAM-9/Meltrin gamma) functions as an adhesion molecule by binding the αvβ5 integrin. Biochem Biophys Res Commun. 2001;280:574–80. doi: 10.1006/bbrc.2000.4155. [DOI] [PubMed] [Google Scholar]
- 164.Stefanidakis M, Bjorklund M, Ihanus E, Gahmberg CG, Koivunen E. Identification of a negatively charged peptide motif within the catalytic domain of progelatinases that mediates binding to leukocyte β2 integrins. J Biol Chem. 2003;278:34674–84. doi: 10.1074/jbc.M302288200. [DOI] [PubMed] [Google Scholar]
- 165.Stefanidakis M, Ruohtula T, Borregaard N, Gahmberg CG, Koivunen E. Intracellular and cell surface localization of a complex between αMβ2 integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J Immunol. 2004;172:7060–8. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 166.Cai TQ, Wright SD. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J Exp Med. 1996;184:1213–23. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.David A, Kacher Y, Specks U, Aviram I. Interaction of proteinase 3 with CD11b/CD18 (beta2 integrin) on the cell membrane of human neutrophils. J Leukoc Biol. 2003;74:551–7. doi: 10.1189/jlb.1202624. [DOI] [PubMed] [Google Scholar]


