Abstract
Epithelial cancer cells often over express mucins that are aberrantly glycosylated. Although it has been realized that these compounds offer exciting opportunities for the development of immunotherapy for cancer, their use is hampered by the low antigenicity of classical immunogens composed of a glycopeptide derived from a mucin conjugated to a foreign carrier protein. We have designed, chemically synthesized, and immunologically evaluated a number of fully synthetic vaccine candidates to establish a strategy to overcome the poor immunogenicity of tumor-associated carbohydrates and glycopeptides. The compounds were also designed to study in detail the importance of TLR engagement for these antigenic responses. We have found that covalent attachment of a TLR2 agonist, a promiscuous peptide T-helper epitope, and a tumor-associated glycopeptide, gives a compound (1) that elicit in mice exceptionally high titers of IgG antibodies which recognize MCF7 cancer cells expressing the tumor-associated carbohydrate. Immunizations with glycolipopeptide (2), which contains lipidated amino acids instead of a TLR2 ligand, gave significantly lower titers of IgG antibodies demonstrating that TLR engagement is critical for optimum antigenic responses. Although mixtures of compound 2 with Pam3CysSK4 (3) or monophosphoryl lipid A (4) elicited similar titers of IgG antibodies compared to 1, the resulting antisera had an impaired ability to recognize cancer cells. It was also found that it is essential to covalently link the helper T-epitope to B-epitope probably because internalization of the helper T-epitope by B-cells requires assistance of the B-epitope. The results presented here show that synthetic vaccine development is amenable to structure activity relationship studies for successful optimization of carbohydrate-based cancer vaccines.
Keywords: Antibodies, Antigens, Peptides, Epitopes, Cytokines, Tumor immunity
Introduction
The over-expression of oligosaccharides, such as Globo-H, LewisY, and Tn antigens is a common feature of oncogenically transformed cells.[1–3] Numerous studies have shown that this abnormal glycosylation can promote metastasis[4] and hence the expression of these compounds is strongly correlated with poor survival rates of cancer patients. A broad and expanding body of preclinical and clinical studies demonstrates that naturally acquired, passively administered or actively induced antibodies against carbohydrate-associated tumor antigens are able to eliminate circulating tumor cells and micro-metastases in cancer patients.[5–8]
Traditional cancer vaccine candidates composed of a tumor-associated carbohydrate (Globo-H, LewisY, and Tn) conjugated to a foreign carrier protein (e.g. Keyhole limpet hemocyanin; KLH and Bovine serum albumin; BSA) have failed to elicit sufficiently high titers of IgG antibodies in most patients. It appears that the induction of IgG antibodies against tumor-associated carbohydrates is much more difficult than eliciting similar antibodies against viral and bacterial carbohydrates. This observation is not surprising because tumor associated saccharides are self-antigens and consequently tolerated by the immune system. The shedding of antigens by the growing tumor reinforces this tolerance. In addition, a foreign carrier protein such as KLH can elicit a strong B-cell response, which may lead to the suppression of an antibody response against the carbohydrate epitope. The latter is a greater problem when self-antigens such as tumor-associated carbohydrates are employed. Also, linkers that are utilized for the conjugation of carbohydrates to proteins can be immunogenic leading to epitope suppression.[9, 10] It is clear that the successful development of a carbohydrate-based cancer vaccine requires novel strategies for the more efficient presentation of tumor-associated carbohydrate epitopes to the immune system, resulting in a more efficient class switch to IgG antibodies.[11–20]
Advances in the knowledge of the cooperation of innate and adaptive immune responses[21–26] is offering new avenues for vaccine design for diseases such as cancer, for which traditional vaccine approaches have failed. The innate immune system responds rapidly to families of highly conserved compounds, which are integral parts of pathogens and perceived as danger signals by the host. Recognition of these molecular patterns is mediated by sets of highly conserved receptors, such as Toll-like receptors (TLR), whose activation results in acute inflammatory responses such as direct local attack against invading pathogens and the production of a diverse set of cytokines. Apart from antimicrobial properties, the cytokines and chemokines also activate and regulate the adaptive component of the immune system.[27] In this respect, cytokines stimulate the expression of a number of co-stimulatory proteins for optimum interaction between T-helper cells and B-cells and antigen presenting cells (APC). In addition, some cytokines and chemokines are responsible for overcoming suppression mediated by regulatory T-cells. Other cytokines are important for directing the effector T-cell response towards a Th-1 or Th-2 phenotype.[28]
Self-adjuvanting synthetic vaccines provide an attractive remedy for protein conjugate vaccine candidates and have been pursued in the field of peptide-based vaccines. [29, 30] Recently, we described a fully synthetic three-component vaccine candidate (compound 1, Figure 1) composed of a tumor-associated MUC-1 glycopeptide B-epitope, a promiscuous helper T-cell epitope and a TLR2 ligand.[31, 32] The exceptional antigenic properties of the three-component vaccine were attributed to the absence of any unnecessary features that are antigenic and may induce immune suppression. It contains, however, all the mediators required for eliciting relevant IgG immune responses. Furthermore, attachment of the TLR2 agonist Pam3CysSK4 (3)[30, 33, 34] to the B- and T-epitopes ensures that cytokines are produced at the site where the vaccine interacts with immune cells. This leads to a high local concentration of cytokines facilitating maturation of relevant immune cells. Apart from providing danger signals, the lipopeptide 3 facilitates the incorporation of the antigen into liposomes and promotes selective targeting and uptake by antigen presenting cells and B-lymphocytes.
FIGURE 1.
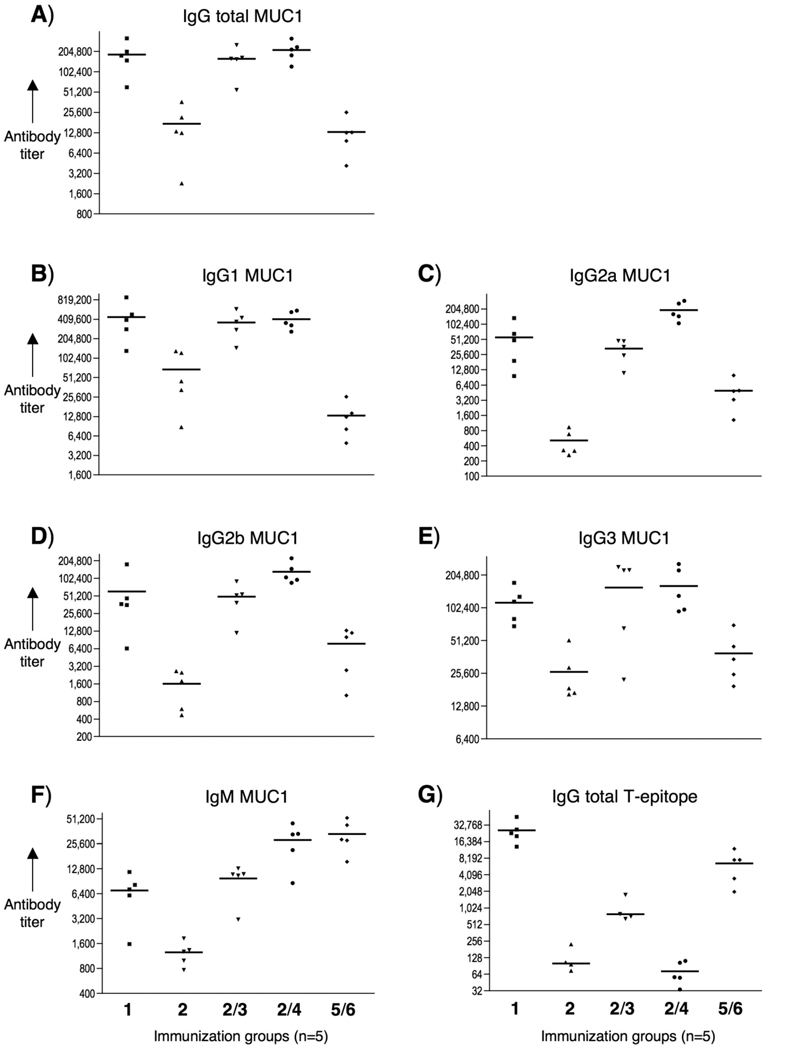
ELISA anti-MUC1 and anti-T-epitope antibody titers after 4 immunizations with 1, 2, 2/3, 2/4 and 5/6. ELISA plates were coated with A-F) BSA-MI-MUC1 conjugate or G) neutravidin-biotin-T-epitope and titers were determined by linear regression analysis, plotting dilution vs. absorbance. Titers were defined as the highest dilution yielding an optical density of 0.1 or greater relative to normal control mouse sera. Each data point represents the titer for an individual mouse after 4 immunizations and the horizontal lines indicate the mean for the group of five mice.
To establish the optimal architecture of a fully synthetic three-component cancer vaccine, we have chemically synthesized, and immunologically evaluated vaccine candidates 1–6. The compounds were also designed to establish the importance of TLR signaling for optimal immune responses. In this respect, recent studies employing mice deficient in TLR signaling have cast doubt about the importance of these innate immune receptors for adaptive immune responses.[35–38]
Results
Chemical synthesis
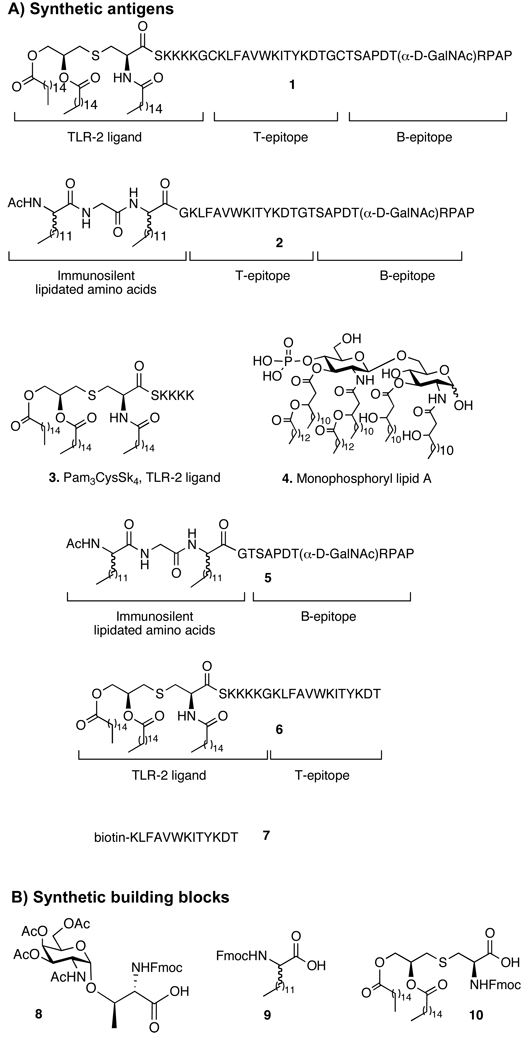
Compound 1 (Figure 1), which contains as B-epitope a tumor-associated glycopeptide derived from MUC-1,[39–45] the well-documented murine MHC class II restricted helper T-cell epitope KLFAVWKITYKDT derived from the Polio virus,[46] and the TLR2 agonist 3,[34] was previously shown to elicit exceptional high titers of IgG antibodies in mice.[31] Compound 2 has a similar architecture as 1, however, the TLR2 ligand has been replaced by lipidated amino acids.[47] The lipidated amino acids do not induce production of cytokines, however, they enable incorporation of the compound into liposomes. Thus, glycolipopeptide 2 is ideally suited to establish the importance of TLR engagement for antigenic responses against tumor-associated glycopeptides. To determine the importance of covalent attachment of the TLR ligand, liposomal preparations of compound 2 and Pam3CysSK4 (3) or monophosphoryl lipid A (MPL-A) (4), which are TLR2 and TLR4 agonists, respectively were employed.[34, 48] Finally, compounds 5 and 6, which are composed of a MUC-1 glycopeptide B-epitope linked to lipidated amino acids and the helper T-epitope attached to Pam3CysSK4, were employed to establish the importance of covalent linkage of the B- and helper T-epitope. Compound 1 was prepared as described previously.[31, 32] Compound 2 was synthesized by solid phase peptide synthesis using a Rink amide resin, 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids, Fmoc-Thr-(AcO3-α-d-GalNAc) (8),[49–51] and Fmoc protected lipidated amino acid (9).[52, 53] The standard amino acids were introduced using O-(benzotriazol-1-yl)-N,N,N’,N'-tetramethyl-uronium hexafluorophosphate (HBTU)/1-hydroxy-benzotriazole (HOBt) as activating reagent, the glycosylated amino acid was installed with O-(7-azabenzotriazol-1-yl)-N,N,N’,N'-tetramethyl-uronium hexafluorophosphate (HATU)/ 1-hydroxy-7-azabenzotriazole (HOAt) and the lipidated amino acids with benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBOP)/HOBt. After completion of the assembly of the glycolipopeptide, the N-terminal Fmoc protecting group was removed with 20% piperidine in N,N-dimethylformamide (DMF), and the resulting amine capped by acetylation with acetic anhydride and diisopropylethyl amine (DIPEA) in N-methylpyrrolidone (NMP). Next, the acetyl esters of the saccharide moiety were cleaved with 60% hydrazine in methanol and treatment with reagent B (trifluoroacetic acid (TFA), H2O, phenol, triethylsilane (TES), 88/5/5/2, v/v/v/v) resulted in removal of the side chain protecting groups and release of the glycopeptide from the solid support.
Pure compound 2 was obtained after purification by RP-HPLC on a C-4 semi-preparative column. A similar protocol was used for the synthesis of compound 5. Derivative 6 was synthesized by solid phase peptide synthesis on a Rink amide resin and after assembly of the peptide, the resulting product was coupled manually with N-Fmoc-(R)-(2,3-bis(palmitoyloxy)-(2R-propyl)-(R)-cysteine (10).[54] The N-Fmoc group of the product was removed with 20% piperidine in DMF and the resulting amine was coupled with palmitic acid using PyBOP, HOBt, and DIPEA in DMF. The lipopeptide was treated with reagent B to release it from the resin and to remove side chain protecting groups.
Compound 7, which is composed of the helper T-epitope linked to biotin, was required for ELISA studies and conveniently synthesized by automated solid-phase peptide synthesis on Rink amide resin using standard Fmoc-chemistry. The biotin was coupled manually to the N-terminal amino-group by reaction with succinimidyl-6-(biotinamido)hexanoate (EZ-link® NHS-Biotin reagent; Pierce Endogen, Inc.) in the presence of DIPEA. The biotinylated peptide was subsequently released from the resin with concomitant removal of the side-chain protecting groups by treatment with reagent B.
Immunizations and Immunology
Compounds 1 and 2 were incorporated into phospholipid-based small uni-lamellar vesicles by hydration of a thin film of egg phosphatidylcholine (PC), phosphatidylglycerol (PG), cholesterol (Chol), and compound 1 or 2 (molar ratios: 65/25/50/10) in a HEPES buffer (10 mm, pH 6.5) containing NaCl (145 mm) followed by extrusion through 100 nm Nuclepore® polycarbonate membrane. Incorporation of compounds 1 and 2 into the liposomes were determined by quantitative carbohydrate analysis using high pH anion exchange chromatography (HPAEC-PAD). Only small batch-to-batch variations were observed in antigen concentration in the liposomes. Groups of five female BALB/c mice were immunized subcutaneously four times at weekly intervals with liposomes containing 3 µg of saccharide. Furthermore, similar liposomes were prepared of a mixture of glycolipopeptide 2 with Pam3CysSK4 (3) or MPL-A (4) (molar ratios: PC/PG/Chol/2/3 or 4, 65/25/5/5/5) in HEPES buffer and administered four times at weekly intervals prior to sera harvesting. Finally, mice were immunized with a liposomal preparation of compound 5 and 6 (molar ratios: PC/PG/Chol/5/6, 65/25/5/5/5) employing standard procedures.
Anti-MUC-1 antibody titers of anti-sera were determined by coating microtiter plates with the MUC-1 derived glycopeptide TSAPDT(α-d-GalNAc)RPAP conjugated to BSA and detection was accomplished with anti-mouse IgM or IgG antibodies labeled with alkaline phosphatase. Mice immunized with 1 elicited exceptional high titers of anti-MUC-1 IgG antibodies (Table 1 and Figure 2). Sub-typing of the IgG antibodies (IgG1, IgG2a, IgG2b, and IgG3) indicated a bias towards a Th2 immune response. Furthermore, the observed high IgG3 titer is typical of an anti-carbohydrate response. Immunizations with glycolipopeptide 2, which contains lipidated amino acids instead of TLR2 ligand, resulted in significantly lower titers of IgG antibodies demonstrating that TLR engagement is critical for optimum antigenic responses. However, liposomal preparations of compound 2 with the adjuvant 3 or 4 elicited IgG (total) titers similar to 1. In the case of the mixture of 2 with Pam3CysSK4, the immune response was biased towards a Th2 response as evident by high IgG1 and low IgG2a,b titers. On the other hand, the use of monophosphoryl lipid A lead to significant IgG1 and IG2a,b responses, and thus this preparation elicited a mixed Th1/Th2 response. Finally, liposomes containing compound 5 and 6 did not induce measurable titers of anti MUC-1 antibodies, indicating that the B- and T epitope need to be covalent linked for antigenic responses. Next, possible antigenic responses against the helper T-epitope were investigated. Streptavidine-coated microtiter plates were treated with the helper T-epitope modified with biotin (7). After the addition of serial dilutions of sera, detection was accomplished with anti-mouse IgM or IgG antibodies labeled with alkaline phosphatase. Interestingly, compound 1 elicited low, whereas mixtures of 2 with 3 or 4 elicited no, antibodies against the helper T-epitope.
TABLE 1.
ELISA anti-MUC1 and anti-T-epitope antibody titers[a] after 4 immunizations with various preparations.
| IgG total | IgG1 | IgG2a | IgG2b | IgG3 | IgM | IgG total | |
|---|---|---|---|---|---|---|---|
| Immunization[b] | MUC1 | MUC1 | MUC1 | MUC1 | MUC1 | MUC1 | T-epit. |
| 1 | 177,700 | 398,200 | 49,200 | 37,300 | 116,200 | 7,200 | 23,300 |
| 2 | 13,300 | 44,700 | 300 | 1,800 | 18,600 | 1,300 | 100 |
| 2/3 | 160,500 | 279,800 | 36,200 | 52,500 | 225,600 | 11,000 | 700 |
| 2/4 | 217,400 | 359,700 | 161,900 | 106,000 | 131,700 | 33,400 | 100 |
| 5/6 | 12,800 | 12,700 | 4,800 | 10,100 | 34,400 | 29,000 | 7,600 |
Anti-MUC1 and anti-T-epitope antibody titers are presented as the median for groups of five mice. ELISA plates were coated with BSA-MI-MUC1 conjugate for anti-MUC1 antibody titers or neutravidin-biotin-T-epitope for anti-T-epitope antibody titers. Titers were determined by linear regression analysis, plotting dilution vs. absorbance. Titers are defined as the highest dilution yielding an optical density of 0.1 or greater relative to normal control mouse sera.
Liposomal preparations were employed. Individual anti-MUC1 titers for IgG total, IgG1, IgG2a, IgG2b, IgG3 and IgM, and anti-T-epitope for IgG total are reported in Fig. 2.
FIGURE 2.
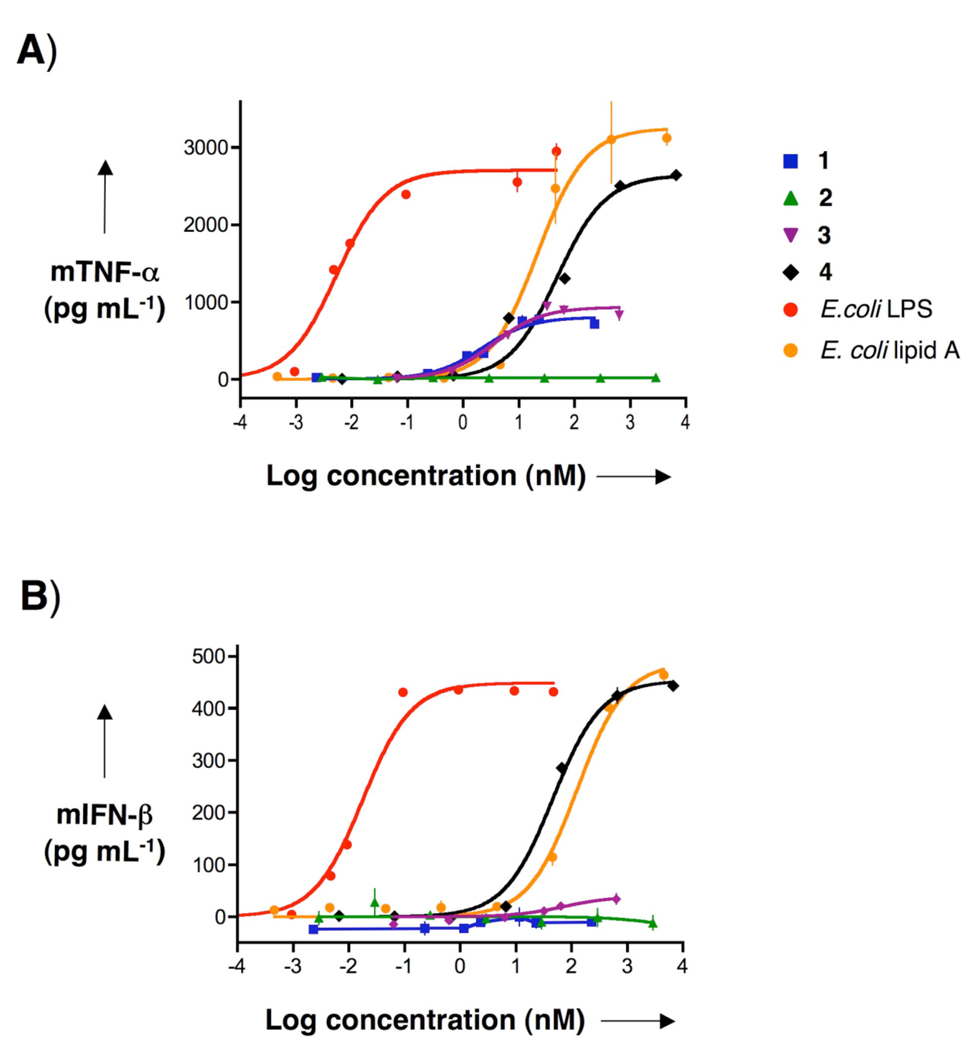
TNF-α and IFN-β production by murine macrophages after stimulation with synthetic compounds 1–4, E. coli LPS, and E. coli lipid A. Murine 264.7 RAW γNO(−) cells were incubated for 5.5 h with increasing concentrations of 1–4, E. coli LPS, or E. coli lipid A as indicated. A) TNF-α and B) IFN-β in cell supernatants were measured using ELISAs. Data represent mean values ± SD (n=3).
Compounds 3 or 4 have been employed for initiating the production of cytokines by interacting with TLR2 or TLR4, respectively, on the surface of mononuclear phagocytes.[55] After activation with 3, the intracellular domain of TLR2 recruits the adaptor protein MyD88 resulting in the activation of a cascade of kinases leading to the production of a number of cytokines and chemokines. On the other hand, LPS and lipid As induce cellular responses by interacting with the TLR4/MD2 complex, which results in the recruitment of the adaptor proteins MyD88 and TRIF leading to the induction of a more complex pattern of cytokine. TNF-α secretion is the prototypical measure for activation of the MyD88-dependent pathway, whereas secretion of IFN-β is commonly used as an indicator of TRIF-dependent cellular activation.
To examine cytokine production, mouse macrophages (RAW γNO(−) cells) were exposed over a wide range of concentrations to compounds 1–4, E. coli 055:B5 LPS and prototypic E. coli bisphosphoryl lipid A.[56] After 5.5 h, the supernatants were harvested and examined for mouse TNF-α and IFN-β using commercial or in-house developed capture ELISAs, respectively (Figure 3). Potencies (EC50, concentration producing 50% activity) and efficacies (maximal level of production) were determined by fitting the dose-response curves to a logistic equation using PRISM software. Compounds 1 and 3 induced secretion of TNF-α with similar efficacies and potencies, indicating that attachment of the B- and T-epitopes had no effect on cytokine responses. As expected, none of the compounds induced the production of INF-β. Furthermore, compound 2 did not induce TNF-α and IFN-β secretion, indicating that its lipid moiety is immunosilent. Compound 4 stimulated the cells to produce TNF-α and INF-β but it was less potent than E. coli 055:B5 LPS. It displayed a much larger efficacy of TNF-α production compared to compounds 1 and 3. The reduced efficacy of compounds 1 and 3 is probably a beneficial property, because LPS can over-activate the innate immune system which leads to symptoms of septic shock.
FIGURE 3.
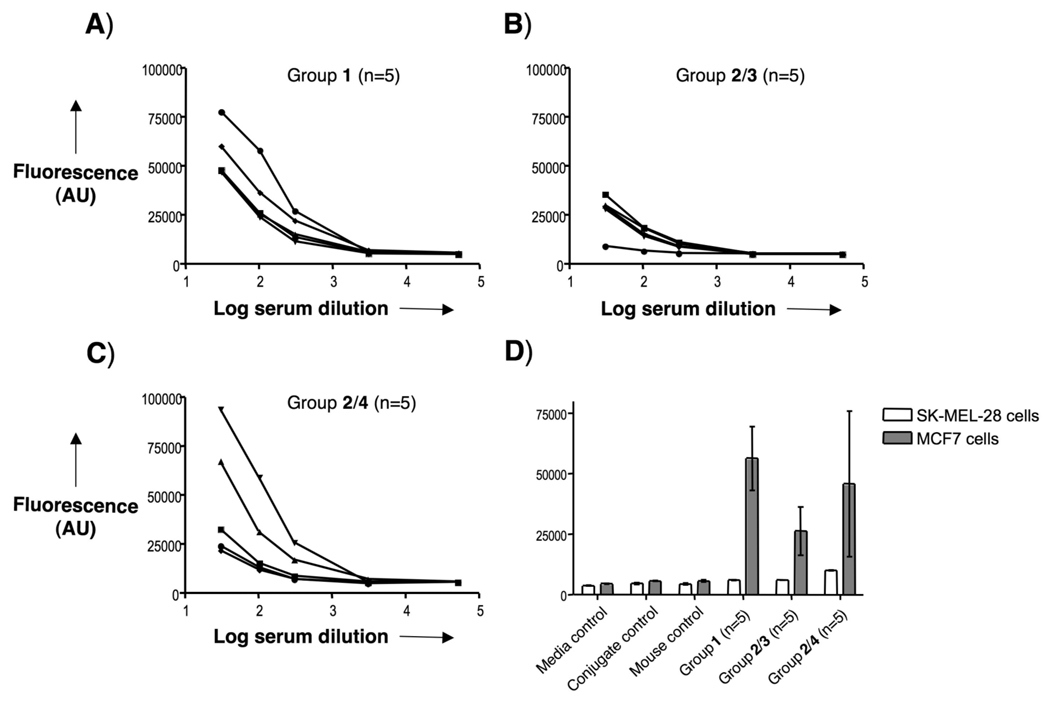
Cell recognition analysis for specific anti-MUC1 antibodies. Reactivity of sera was tested on MCF7 cells and SK-MEL-28 cells. Serial dilutions of serum samples after 4 immunizations with A) 1, B) 2/3, or C) 2/4 were incubated with MCF7 cells. D) Control sera (media, conjugate, and mouse (normal control mouse sera) controls) and the serum samples (1:30 diluted) were incubated with MCF7 and SK-MEL-28 cells. After incubation with FITC-labeled anti-mouse IgG antibody, the fluorescence intensity was assessed in cell lysates. AU indicates arbitrary fluorescence units.
Next, the ability of the mouse antisera to recognize native MUC-1 antigen present on cancer cells was established. Thus, serial dilutions of the serum samples were added to MUC-1 expressing MCF7 human breast cancer cells[57] and recognition was established using a FITC-labeled anti-mouse IgG antibody. As can be seen in Figure 4, anti-sera obtained from immunizations with the three-component vaccine 1 displayed excellent recognition of MUC-1 tumor cells whereas no binding was observed when SK-MEL-28 cells, which do not express the MUC-1 antigen, were employed.
Although sera obtained from mice immunizations with a combination of 2 and 3 elicited equally high IgG antibody titers as 1 (Table 1), a much-reduced recognition of MCF7 cells was observed. This result indicate that covalent attachment of the adjuvant 3 to the B-T epitope is important for proper antibody maturation leading to improved cancer cell recognition. Immunizations with a mixture of compound 2 and 4 led to variable results and two mice displayed excellent, and three modest, recognition of MCF7 cells.
Discussion
Most efforts aimed at developing carbohydrate-based cancer vaccines have focused on the use of chemically synthesized tumor-associated carbohydrates linked through an artificial linker to a carrier protein.[1, 3, 58, 59] It has been established that the use of KLH as a carrier protein in combination with the powerful adjuvant QS-21 gives the best results. However, a drawback of this approach is that KLH is a very large and cumbersome protein that can elicit high titers of anti-KLH-antibodies,[60] leading to immune suppression of the tumor-associated carbohydrate epitope. Furthermore, the conjugation chemistry is often difficult to control as it results in conjugates with ambiguities in composition and structure, which may affect the reproducibility of immune responses. Also, the linker moiety can elicit strong B-cell responses.[9, 10] Not surprisingly, preclinical and clinical studies with carbohydrate-protein conjugates have led to results of mixed merit. For example, mice immunized with a trimeric cluster of Tn-antigens conjugated to KLH (Tn(c)-KLH) in the presence of the adjuvant QS-21 elicited modest titers of IgG antibodies.[61] Examination of the vaccine candidate in a clinical trial of relapsed prostate cancer patients gave low median IgG and IgM antibody titers.[62] In another study, a number of MUC-1-derived glycopeptides conjugated to KLH were investigated as immunogens.[63] It was found that only glycopeptides composed of multiple repeat units that are highly glycosylated with Tn antigens could elicit IgG antibodies that could recognize cancer cells.
The studies reported herein show that a three-component vaccine (1), in which a MUC-1 associated glycopeptide B-epitope, a promiscuous murine MHC class II restricted helper T-cell epitope, and a TLR2 agonist are covalently linked, can elicit robust IgG antibody responses. Although covalent attachment of the TLR2 ligand to the T-B glycopeptide epitope was not required for high IgG antibody titers, it was critical for optimal recognition of cancer cells expressing MUC-1. In this respect, liposomes containing compound 1 or a mixture of compound 2 and TLR2 agonist 3 elicited similar high anti-MUC-1 IgG antibody titers. However, antisera obtained from immunizations with 1 recognized MUC-1 expressing cancer cells at much higher sera dilutions than antisera obtained from immunizations with a mixture of 2 and 3. It appears that immunizations with three-component vaccine 1 lead to more efficient antibody maturation resulting in improved cancer cell recognition.
Differences in antigenic responses against the helper T-epitope were also observed. Thus, 1 elicited low titers of IgG antibodies against the helper T-epitope whereas a combination of 2 and 3 induced no antigenic responses against this part of the candidate vaccine. Thus, the covalent attachment of the TLR2 ligand makes compound 1 more antigenic resulting in low antibody responses against the helper T-epitope.
It was observed that a mixture of compound 2 with 3 or 4 induced similar high titers of total IgG antibodies. However, a bias towards a Th2 response (IgG1) was observed when the TLR2 agonist Pam3CysSK4 (3) was employed whereas mixed Th1/Th2 responses (IgG2a,b) were obtained when the TLR4 agonist MPL-A (4) was used. The difference in polarization of helper T-cells is probably due to the induction of different patterns of cytokines by TLR2 or TLR4. In this respect, it was previously observed that Pam3Cys induces lower levels of Th1 inducing cytokines Il-12(p70) and much higher levels of Th2-inducing IL-10 than E. coli LPS.[64] The differences are likely due to the ability of TLR4 to recruit the adaptor proteins MyD88 and TRIF whereas TLR2 can only recruit MyD88. The results indicate that the immune system can be tailored in a particular direction by proper selection of an adjuvant, which is significant since different IgG isotypes perform different effector functions.
The results described herein also show that compound 2 alone, which contains an immuno-silent lipopeptide, elicits much lower IgG titers compared to compound 1, which is modified by a TLR2 ligand. In particular, the ability of compound 2 to elicit IgG2 antibodies was impaired. Recent studies employing mice deficient in TLR signaling have cast doubt about the importance of these innate immune receptors for adaptive immune responses.[35–38] In this respect, studies with MyD88 deficient mice showed that IgM and IgG1 are largely, but not completely, dependent on TLR signaling whereas the IgG2 isotype is entirely TLR-dependent.[35] These observations, which are in agreement with the results reported here, were attributed to a requirement of TLR signaling for B-cell maturation. However, another study found that MyD88−/−/TRIFlps/lps double knockout mice elicited similar titers of antibodies as wild type mice when immunized with trinitrophenol-hemocyanin or trinitrophenol-KLH in the presence or absence of several adjuvants.[36] It was concluded that it might be desirable to exclude TLR agonists from adjuvants. It has been noted that the importance of an adjuvant may depend on the antigenicity of the immunogen.[37, 38] In this respect, proteins conjugates of trinitrophenol are highly antigenic and may not require an adjuvant for optimal responses. However, self-antigens such as tumor-associated carbohydrates have low intrinsic antigenicity and the results reported here clearly show that much more robust antibody responses are obtained when a TLR ligand is co-administered. In addition, it is demonstrated here that the architecture of a candidate vaccine is critical for optimal antigenic responses and in particular covalent attachment of a TLR ligand to a T-B epitope led to improved cancer cell recognition.
The failure of a mixture of compounds 5 and 6 to elicit anti-MUC-1 glycopeptide antibodies indicates that covalent attachment of the T- to the B-epitope is essential for antigenic responses. In this respect, activation of B-cells by helper T-cells requires a similar type of cell-cell interaction as for helper T-cell activation by antigen presenting cells. Thus, a protein or peptide-containing antigen needs to be internalized by B-cells for transport to endosomal vesicles, where proteases will digest the protein and some of the resulting peptide fragments will be complexed with class II MHC protein. The class II MHC - peptide complex will then be transported to the cell surface of the B-lymphocyte to mediate an interaction with helper T-cell resulting in a class switch from low affinity IgM to high affinity IgG antibody production. Unlike APCs, B-cells have poor phagocytic properties and can only internalize molecules that bind to the B-cell receptor. Therefore, it is to be expected that internalization of the helper T-epitope is facilitated by covalent attachment to the B-epitope (MUC-1 glycopeptide) and as a result covalent attachment of the two epitopes will lead to more robust antigenic responses.
Recently, several other fully synthetic vaccines have been described. For example, immunizations with a compound composed of a clustered Tn-antigen, a CD4+ and CD8+ T-epitope, and a palmitoyl moiety elicited robust immune responses in mice that provided protection against a challenge with MO5 melanoma cells.[65, 66] Furthermore, Bundle and co-workers demonstrated that an antigen composed of a mannam trisaccharide derived from Candida albicans, a CD4+ helper T-epitope and an immuno-stimulatory peptide from interleukin-1β was able to elicit IgG antibody responses that could recognize C. albicans cell wall extract.[67] Kunz and co-workers described a two-component vaccine composed MUC-1 glycopetide containing a Tn and STn moiety and a helper T-epitope, which was able to elicit IgG antibodies in mice that were specific for the glycopeptide.[68] Based on the results reported here, it is to be expected that the antigenicity of these fully synthetic vaccine constructs can be enhanced by covalent attachment of a TLR agonist.
In conclusion, it has been demonstrated that antigenic properties of a fully synthetic cancer vaccine can be optimized by structure-activity relationship studies. In this respect, it has been established that a three-component vaccine in which a tumor-associated MUC-1 glycopeptide B-epitope, a promiscuous helper T-cell epitope, and a TLR2 ligand are covalently linked can elicit exceptionally high IgG antibody responses, which have an ability to recognize cancer cells. It is essential that the helper T-epitope is covalently linked to the B-epitope, probably since internalization of the helper T-epitope by B-cells requires the presence of a B-epitope. It has also been shown that incorporation of a TLR agonist is important for robust antigenic responses against tumor associated glycopeptide antigens. In this respect, cytokines induced by the TLR2 ligand are important for maturation of immune cells leading to robust antibody responses. A surprising finding was that improved cancer cell recognition was observed when the TLR2 epitope was covalently attached to the glycopeptide T-B epitope. The result presented here provides important information of the optimal constitution of three-component vaccines and will guide successful development of carbohydrate-based cancer vaccines.
Experimental Section
Peptide synthesis
Peptides were synthesized by established protocols on an ABI 433A peptide synthesizer (Applied Biosystems), equipped with a UV-detector using Nα-Fmoc-protected amino acids and HBTU/HOBt as the activating reagents. Single coupling steps were performed with conditional capping. The following protected amino acids were used: Nα-Fmoc-Arg(Pbf)-OH, Nα-Fmoc-Asp(OtBu)-OH, Nα-Fmoc-Asp-Thr(ΨMe,MePro)-OH, Nα-Fmoc-Ile-Thr(ΨMe,MePro)-OH, Nα-Fmoc-Lys(Boc)-OH, Nα-Fmoc-Ser(tBu)-OH, Nα-Fmoc-Thr(tBu)-OH, and Nα-Fmoc-Tyr(tBu)-OH. The coupling of glycosylated amino acid Nα-Fmoc-Thr-(AcO3-α-d-GalNAc)[51] was carried out manually using HATU/HOAt as a coupling agent. The coupling of Nα-Fmoc-lipophilic amino acid (Nα-Fmoc-d,l-tetradeconic acid)[52, 53] and Nα-Fmoc-S-(2,3-bis(palmitoyloxy)-(2R-propyl)- (R)-cysteine,[54, 69] which was prepared from R)-glycidol, were carried out using Py BOP/HOBt as coupling agent. Progress of the manual couplings was monitored by standard Kaiser test.
Liposome preparation
Egg PC, egg PG, Chol, and compound 1 or 2 (15 mmol, molar ratios 65:25:50:10) or PC/PG/Chol/2/3 or 4 (15 mmol, molar ratios 60:25:50:10:5) or PC/PG/Chol/5/6 (15 mmol, molar ratios 65:25:50:5:5) were dissolved in a mixture of trifluoroethanol and methanol (1:1, v/v, 5 mL). The solvents were removed in vacuo to give a thin lipid film, which was hydrated by shaking in HEPES buffer (10 mm, pH 6.5) containing NaCl (145 mm) (1 mL) under argon atmosphere at 41 °C for 3 h. The vesicle suspension was sonicated for 1 min and then extruded successively through 1.0, 0.4, 0.2, and 0.1 µm polycarbonate membranes (Whatman, Nucleopore Track-Etch Membrane) at 50 °C to obtain SUVs. The GalNAc content was determined by heating a mixture of small unilamellar vesicles (50 µL) and aqueous trifluoroacetic acid (2 M, 200 µL) in a sealed tube for 4 h at 100 °C. The solution was then concentrated in vacuo and analyzed by high-pH anion exchange chromatography using a pulsed amperometric detector (Methrome) and CarboPac columns PA-10 and PA-20 (Dionex).
Dose and immunization schedule
Groups of five mice (female BALB/c, age 8–10 weeks; The Jackson Laboratory) were immunized four times at weekly intervals. Each boost included 3 µg of saccharide in the liposome formulation. Serum samples were obtained before immunization (pre-bleed) and one week after the final immunization. The final bleeding was done by cardiac bleed.
Serologic assays
Anti-MUC-1 IgG, IgG1, IgG2a, IgG2b, IgG3, and IgM antibody titers were determined by enzyme-linked immunosorbent assay (ELISA), as described previously.[9] Briefly, ELISA plates (Thermo Electron Corp.) were coated with a conjugate of the MUC-1 glycopeptide conjugated to BSA through a maleimide linker (BSA-MI-MUC-1). Serial dilutions of the sera were allowed to bind to immobilized MUC-1. Detection was accomplished by the addition of phosphatase-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.), IgG1 (Zymed), IgG2a (Zymed), IgG2b (Zymed), IgG3 (BD Biosciences Pharmingen), or IgM (Jackson ImmunoResearch Laboratories Inc.) antibodies. After addition of p-nitrophenyl phosphate (Sigma), the absorbance was measured at 405 nm with wavelength correction set at 490 nm using a microplate reader (BMG Labtech). Antibody titers against the T (polio)-epitope were determined as follows. Reacti-bind NeutrAvidin coated and pre-blocked plates (Pierce) were incubated with biotin-labeled T-epitope 7 (a stock solution of 7 in DMSO (4 mg mL−1) was diluted to 10 µg mL−1; 100 µL) for 2 h. Next, serial dilutions of the sera were allowed to bind to immobilized T-epitope. Detection was accomplished as described above. The antibody titer was defined as the highest dilution yielding an optical density of 0.1 or greater relative to normal control mouse sera. Experiments were performed in triplicate.
Cell culture
RAW 264.7 γNO(−) cells, derived from the RAW 264.7 mouse monocyte/macrophage cell line, were obtained from ATCC. The cells were maintained in RPMI 1640 medium with l-glutamine (2 mm), adjusted to contain sodium bicarbonate (1.5 g L−1), glucose (4.5 g L−1), HEPES (10 mm) and sodium pyruvate (1.0 mm) and supplemented with penicillin (100 u mL−1) / streptomycin (100 µg mL−1; Mediatech) and FBS (10%; Hyclone). Human breast adenocarcinoma cells MCF7,[57] obtained from ATCC, were cultured in Eagle’s minimum essential medium with L-glutamine (2 mm) and Earle’s balanced salt solution (BSS), modified to contain sodium bicarbonate (1.5 g L−1), non-essential amino acids (0.1 mm) and sodium pyruvate (1 mm) and supplemented with bovine insulin (0.01 mg mL−1; Sigma) and FBS (10%). Human skin malignant melanoma cells SK-MEL-28 were obtained from ATCC and grown in Eagle’s minimum essential medium with l-glutamine (2 mm) and Earle’s BSS, adjusted to contain sodium bicarbonate (1.5 g L−1), non-essential amino acids (0.1 mm) and sodium pyruvate (1 mm) and supplemented with FBS (10%). All cells were maintained in a humid 5% CO2 atmosphere at 37 °C.
TNF-α and IFN-β assays
RAW 264.7 γNO(−) cells were plated on the day of the exposure assay as 2 × 105 cells/well in 96-well plates (Nunc) and incubated with different stimuli for 5.5 h in the presence or absence of polymyxin B. Culture supernatants were collected and stored frozen (−80 °C) until assayed for cytokine production. Concentrations of TNF-α were determined using the TNF-α DuoSet ELISA Development kit from R&D Systems. Concentrations of IFN-β were determined as follows. ELISA MaxiSorp plates were coated with rabbit polyclonal antibody against mouse IFN-β (PBL Biomedical Laboratories). IFN-β in standards and samples was allowed to bind to the immobilized antibody. Rat anti-mouse IFN-β antibody (USBiological) was then added, producing an antibody-antigen-antibody “sandwich”. Next, horseradish peroxidase (HRP) conjugated goat anti-rat IgG (H+L) antibody (Pierce) and a chromogenic substrate for HRP 3,3’,5,5’-tetramethylbenzidine (Pierce) were added. After the reaction was stopped, the absorbance was measured at 450 nm with wavelength correction set to 540 nm. Concentration-response data were analyzed using nonlinear least-squares curve fitting in Prism (GraphPad Software, Inc.). These data were fit with the following four parameter logistic equation: Y = Emax / (1 + (EC50/X)Hill slope), where Y is the cytokine response, X is the concentration of the stimulus, Emax is the maximum response and EC50 is the concentration of the stimulus producing 50% stimulation. The Hill slope was set at 1 to be able to compare the EC50 values of the different inducers. All cytokine values are presented as the means ± SD of triplicate measurements, with each experiment being repeated three times.
Evaluation of materials for contamination by LPS
To ensure that any increase in cytokine production was not caused by LPS contamination of the solutions containing the various stimuli, the experiments were performed in the absence and presence of polymyxin B, an antibiotic that avidly binds to the lipid A region of LPS, thereby preventing LPS-induced cytokine production. TNF-α and IFN-β concentrations in supernatants of cells preincubated with polymyxin B (30 µg mL−1; Bedford Laboratories) for 30 min before incubation with E. coli O55:B5 LPS for 5.5 h showed complete inhibition, whereas preincubation with polymyxin B had no effect on TNF-α synthesis by cells incubated with the synthetic compounds 1 and 3. Therefore, LPS contamination of the latter preparations was inconsequential.
Cell recognition analysis by fluorescence measurements
Serial dilutions of pre-and post-immunization sera were incubated with MCF7 and SK-MEL-28 single-cell suspensions for 30 min on ice. Next, the cells were washed and incubated with goat anti-mouse IgG γ-chain specific antibody conjugated to FITC (Sigma) for 20 min on ice. Following three washes, cells were lysed in passive lysis buffer (Promega). Cell lysates were analyzed for fluorescence intensity (485 ex / 520 em) using a microplate reader (BMG Labtech). Data points were collected in triplicate and are representative of three separate experiments.
Supplementary Material
SCHEME 1.
Chemical structures of A) synthetic antigens and B) synthetic building blocks.
Acknowledgements
This research was supported by the National Cancer Institute of the US National Institutes of Health (Grant No. RO1 CA88986). The authors thank Ms. Jidnyasa Gaekwad for performing the cytokine assays and Dr. Yanghui Zhang for synthesizing the E. coli lipid A.
Footnotes
Supporting information for this article is available on the WWW under http://www.chembiochem.org or from the author.
References
- 1.Springer GF. J. Mol. Med. 1997;75:594-–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S. Acta Anat. 1998;161:79–90. doi: 10.1159/000046451. [DOI] [PubMed] [Google Scholar]
- 3.Dube DH, Bertozzi CR. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DSA, Kerr MA. J. Clin. Pathol. Mol. Pathol. 1999;52:174–178. doi: 10.1136/mp.52.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston PO, Ragupathi G. Cancer Immunol. Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragupathi G. Cancer Immunol. 1996;43:152–157. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- 7.von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FG, van Kamp GJ, Schol DJ, Reis CA, Price MR, Livingston Po, Hilgers J. Int. J. Cancer. 2000;86:702–712. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Finn OJ. Nat. Rev. Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 9.Buskas T, Li YH, Boons GJ. Chem. Eur. J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 10.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Bioconjug. Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 11.Reichel F, Ashton PR, Boons GJ. Chem. Commun. 1997;21:2087–2088. [Google Scholar]
- 12.Alexander J, del Guercio MF, Maewal A, Qiao L, Fikes J, Chesnut RW, Paulson J, Bundle DR, DeFrees S, Sette A. J. Immunol. 2000;164:1625–1633. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- 13.Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3264–3269. doi: 10.1073/pnas.051623598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang ZH, Koganty RR. Curr. Med. Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 15.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, Smith C, Zeng W, Brown LE. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 17.Buskas T, Ingale S, Boons GJ. Angew. Chem. Int. Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 18.Dziadek S, Hobel A, Schmitt E, Kunz H. Angew. Chem. Int. Ed. 2005;44:7630–7635. doi: 10.1002/anie.200501594. [DOI] [PubMed] [Google Scholar]
- 19.Krikorian D, Panou-Pomonis E, Voitharou C, Sakarellos C, Sakarellos-Daitsiotis M. Bioconjug. Chem. 2005;16:812–819. doi: 10.1021/bc049703m. [DOI] [PubMed] [Google Scholar]
- 20.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. J. Med. Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasare C, Medzhitov R. Semin. Immunol. 2004;16:23–26. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Pashine A, Valiante NM, Ulmer JB. Nat. Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill LA. Curr. Opin. Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Lee HK, Iwasaki A. Semin. Immunol. 2007;19:48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Apetoh L, Housseau F, Kroemer G, Zitvogel L. Curr. Opin. Immunol. 2007;19:224–231. doi: 10.1016/j.coi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Lin WW, Karin M. J. Clin. Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dabbagh K, Lewis DB. Curr. Opin. Infect. Dis. 2003;16:199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Wiesmuller KH, Jung G, Hess G. Vaccine. 1989;7:29–33. doi: 10.1016/0264-410x(89)90007-8. [DOI] [PubMed] [Google Scholar]
- 30.Bessler WG, Heinevetter L, Wiesmuller KH, Jung G, Baier W, Huber M, Lorenz AR, Esche UV, Mittenbuhler K, Hoffmann P. Int. J. Immunopharmacol. 1997;19:547–550. doi: 10.1016/s0192-0561(97)00054-4. [DOI] [PubMed] [Google Scholar]
- 31.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingale S, Buskas T, Boons GJ. Org. Lett. 2006;8:5785–5788. doi: 10.1021/ol062423x. [DOI] [PubMed] [Google Scholar]
- 33.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. J. Exp. Med. 1997;185:1951. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spohn R, Buwitt-Beckmann U, Brock R, Jung G, Ulmer AJ, Wiesmuller KH. Vaccine. 2004;22:2494–2499. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 35.Blander JM, Medzhitov R. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 36.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer-Bahlburg A, Khim S, Rawlings DJ. J. Exp. Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulendran B. N Engl J Med. 2007;356:1776–1778. doi: 10.1056/NEJMcibr070454. [DOI] [PubMed] [Google Scholar]
- 39.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell J. J. Biol. Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 40.Burchell J, Taylor-Papadimitriou J, Boshell M, Gendler S, Duhig T. Int. J. Cancer. 1989;44:691–696. doi: 10.1002/ijc.2910440423. [DOI] [PubMed] [Google Scholar]
- 41.Price MR, Hudecz F, O'Sullivan C, Baldwin RW, Edwards PM, Tendler SJ. Mol. Immunol. 1990;27:795–802. doi: 10.1016/0161-5890(90)90089-i. [DOI] [PubMed] [Google Scholar]
- 42.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. J. Biol. Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 43.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. J. Biol. Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 44.von Mensdorff-Pouilly S, Kinarsky L, Engelmann K, Baldus SE, Verheijen RH, Hollingsworth MA, Pisarev V, Sherman S, Hanisch FG. Glycobiology. 2005;15:735–746. doi: 10.1093/glycob/cwi058. [DOI] [PubMed] [Google Scholar]
- 45.Hanisch FG, Muller S. Glycobiology. 2000;10:439–449. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- 46.Leclerc C, Deriaud E, Mimic V, van der Werf S. J. Virol. 1991;65:711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toth I, Danton M, Flinn N, Gibbons WA. Tetrahedron Lett. 1993;34:3925–3928. [Google Scholar]
- 48.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 49.Paulsen H, Adermann K. Liebigs Ann. Chem. 1989:751–769. [Google Scholar]
- 50.Liebe B, Kunz H. Helv. Chim. Acta. 1997;80:1473–1482. [Google Scholar]
- 51.Cato D, Buskas T, Boons GJ. J. Carbohydr. Chem. 2005;24:503–516. [Google Scholar]
- 52.Gibbons WA, Hughes RA, Charalambous M, Christodoulou M, Szeto A, Aulabaugh AE, SMascagani P, Toth I. Liebigs Ann. Chem. 1990:1175–1183. [Google Scholar]
- 53.Koppitz M, Huenges M, Gratias R, Kessler H, Goodman SL, Jonczyk A. Helv. Chim. Acta. 1997;80:1280–1300. [Google Scholar]
- 54.Metzger JW, Wiesmuller KH, Jung G. Int. J. Pept. Protein Res. 1991;38:545–554. doi: 10.1111/j.1399-3011.1991.tb01538.x. [DOI] [PubMed] [Google Scholar]
- 55.Kawai T, Akira S. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. J. Am. Chem. Soc. 2007;129:5200–5216. doi: 10.1021/ja068922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horwitz KB, Costlow ME, McGuire WL. Steroids. 1975;26:785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- 58.Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Expert Rev. Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 59.Slovin SF, Keding SJ, Ragupathi G. Immunol. Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 60.Cappello S, Liu SX, Musselli C, Brezicka F-T, Livingston PO, Ragupathi G. Cancer Immunol. Immunother. 1999;48:483–492. doi: 10.1007/s002620050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuduk SD, Schwarz JB, Chen XT, Glunz PW, Sames D, Ragupathi G, Livingston PO, Danishefsky SJ. J. Am. Chem. Soc. 1998;120:12474–12485. [Google Scholar]
- 62.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, Scher HI. J. Clin. Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 63.Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 64.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 65.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. ChemMedChem. 2008;3:737–741. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 66.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, Benmohamed L. Cancer Immunol. Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dziadek S, Jaques S, Bundle DR. Chem. Eur. J. 2008;14:5908–5917. doi: 10.1002/chem.200800065. [DOI] [PubMed] [Google Scholar]
- 68.Westerlind U, Hobel A, Gaidzik N, Schmitt E, Kunz H. Angew. Chem. Int. Ed. 2008;47:7551–7556. doi: 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]
- 69.Roth A, Espuelas S, Thumann C, Frisch B, Schuber F. Bioconj. Chem. 2004;15:541–553. doi: 10.1021/bc034184t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.