Abstract
Identification and characterization of factors regulating intracellular localization of the androgen receptor (AR) are fundamentally important because nucleocytoplasmic trafficking of AR is a critical step in AR regulation by androgen manipulation. Normally, AR is localized to the cytoplasm in the absence of androgen. Upon ligand binding, AR translocates to the nucleus, where it can modulate transcription of AR-responsive genes. The withdrawal of androgen results in the export of unliganded AR from the nucleus to the cytoplasm, where it is transcriptionally inactive. Calreticulin has been implicated as a possible nuclear export factor for AR because the two proteins form a complex. In this study, we assessed whether the cytoplasmic localization of AR requires binding to calreticulin. To test this we substituted the calreticulin binding sequence (CBS) KVFFKR (residues 579–584) with the amino acids RLAARK in AR and monitored the cellular localization of a GFP-AR fusion protein in the absence of androgen. We also determined if knockdown or knockout of calreticulin expression affected the cytoplasmic localization of the AR. We found that a mutated CBS did not affect the localization of AR and that in the absence of androgen, AR is localized to the cytoplasm regardless of its ability to interact with calreticulin. Also, a reduction in the levels or loss of calreticulin did not affect the localization of AR. These data argue that calreticulin is not required for the cytoplasmic localization of AR.
Keywords: Androgen receptor, Calreticulin, Nuclear export
1. Introduction
Prostate cancer is the most common form of cancer and the second leading cause of cancer deaths in men in the United States (ACS, 2008). The androgen receptor (AR) plays a central role in prostate cancer. Thus, to gain a better understanding of this disease, many studies have focused on AR, particularly its transcriptional activation and transcription factors that affect its transactivation activity. However, AR must translocate to the nucleus as a prerequisite for its transcriptional activity, making the process of nuclear import and export an integral part of the mechanism regulating AR function. In normal prostate cells, AR remains in the cytoplasm in the absence of ligand. Upon ligand binding, AR travels to the nucleus where it accesses and regulates androgen-responsive genes (Georget et al., 1997; Roy et al., 2001; Simental et al., 1991). The withdrawal of ligand results in the export of AR from the nucleus to the cytoplasm (Tyagi et al., 2000). Previous studies have shown a possible association between the regulation of AR intracellular localization and the transition of prostate cancer cells from an androgen-sensitive to a castration-recurrent (refractory) state (Feldman and Feldman, 2001). In androgen-sensitive prostate cancer cells, the presence or absence of androgens determines the localization of AR. Conversely, AR localizes to the nucleus in androgen-refractory prostate cancer cells even in androgen-depleted conditions, in both cell culture and clinical specimens (Gregory et al., 2001a,b). In addition, studies have shown that nuclear localized AR can have transcriptional activity, even in the absence of androgens (Huang et al., 2002; Zhang et al., 2003).
Currently, the mechanisms involved in AR nucleocytoplasmic trafficking remain unclear; however, various studies have offered insights to the nuclear import of AR. For example, a classical bipartite nuclear localization sequence has been identified in the DNA binding/hinge region of the AR (Zhou et al., 1994). This NLS utilizes the importin α/importin β pathway for transport through the nuclear pore complex (Freedman and Yamamoto, 2004; Savory et al., 1999). In addition, a second, less well defined, nuclear import amino acid sequence is present in the ligand-binding domain of the receptor (Jenster et al., 1992; Poukka et al., 2000; Saporita et al., 2003). Studies on the glucocorticoid receptor (GR) indicated that a combination of importin α/importin β and importin 7 mediate its nuclear import (Freedman and Yamamoto, 2004). Since steroid receptors share many common features, the AR may utilize similar mechanisms to enter the nucleus.
In contrast to import, little information exists regarding the mechanism of nuclear export of AR and other steroid receptors. Several studies, however, have indicated that AR nuclear export occurs in a Crm-1-independent manner. Although Crm-1 serves as a major export receptor, AR does not contain the putative leucine rich nuclear export sequence (NES) necessary for Crm-1-mediated export, nor is AR’s nuclear export blocked by Leptomycin B, an inhibitor of Crm-1 (Tyagi et al., 2000, 1998; Saporita et al., 2003). We have reported the identification of a novel nuclear export signal (NES) for AR. A 75 amino acid sequence (residues 743–817) in the ligand-binding domain (LBD) of AR, which we termed NESAR, was both necessary and sufficient to promote cytoplasmic localization of AR (Saporita et al., 2003). The estrogen receptor (ER) and mineralocorticoid receptor (MR) both have similar sequences to NESAR that mediate cytoplasmic localization of receptor-GFP chimeras. In addition, the deletion of NESAR resulted in the nuclear retention of GFP-tagged AR. These results suggested that factors associated with the NESAR have potential roles in mediating the nuclear export of AR (Saporita et al., 2003).
Studies have implicated the calcium binding protein, calreticulin, as an export factor for steroid receptors, including AR (Black et al., 2001; Holaska et al., 2001, 2002). Both integrins and the DNA-binding domain (DBD) of the steroid hormone receptor family contain a calreticulin binding site (CBS) with the consensus sequence KXFFKR (Burns et al., 1994; Dedhar et al., 1994). The 46-kDa calreticulin, protein normally resides in the lumen of the endoplasmic reticulum (Gelebart et al., 2005; Michalak et al., 1999). However, additional reports have described a cytosolic form of calreticulin (Holaska et al., 2001; Burns et al., 1994; Dedhar et al., 1994). The most well documented roles for calreticulin are as a chaperone and in maintaining Ca2+ homeostasis (Gelebart et al., 2005; Johnson et al., 2001). In addition, there has been a wide range of other functions attributed to calreticulin, including roles in cell adhesion, development, and gene expression (Michalak et al., 1999; Johnson et al., 2001; Nakamura et al., 2001; Trombetta, 2003). Calreticulin has also been shown to be an essential gene as calreticulin-deficient mice exhibit embryonic lethality (Guo et al., 2002).
A report by Walther et al. (2003) has questioned the claim that calreticulin acts as an export factor for steroid hormone receptors. In this study, they demonstrated that the rapid calreticulin-mediated nuclear export of GR is a specific response to a transient disruption of the endoplasmic reticulum that occurs during polyethylene glycol (PEG)-mediated cell fusion. Using digitonin-permeabilized cells, they showed that in the absence of cell fusion, GR nuclear export occurs slowly over a period of many hours independently of direct interaction with calreticulin (Walther et al., 2003). These observations call for further studies to test whether calreticulin is indeed an export factor capable of modulating subcellular localization of steroid hormone receptors.
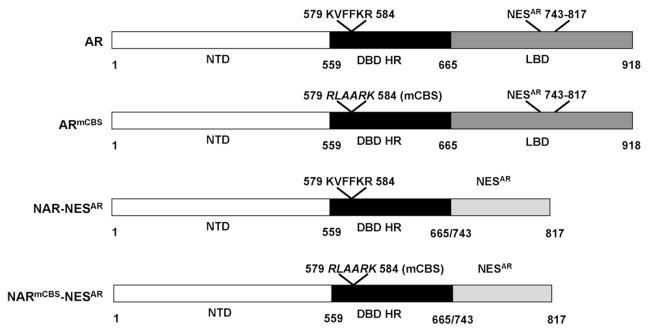
In this study, we used an alternative approach to the heterokaryon fusion assay to test if calreticulin can influence AR subcellular localization by assessing its ability to localize GFP-tagged AR and AR mutants to the cytoplasm. We constructed GFP-tagged AR mutants in which we substituted the calreticulin binding sequence (KXFFKR) with the amino acid sequence RLAARK (Fig. 1, ARmCBS). We also generated AR mutants that contained KXF-FKR or the RLAARK substitution, along with NESAR in place of the full ligand-binding domain of AR (Fig. 1, NAR-NESAR and NARmCBS-NESAR, respectively). We assessed the cytoplasmic localization of these constructs in cells that had a reduced level or loss of calreticulin to determine if calreticulin plays a role in AR cytoplasmic localization. The ability of the AR CBS mutants to localize to the cytoplasm and the cytoplasmic localization of AR upon calreticulin depletion suggest that calreticulin may not regulate AR cytoplasmic localization.
Fig. 1.
Schematic depiction of cDNA constructs encoding wild-type and mutant human androgen receptor cloned into the pEGFP-C1 vector to generate N-terminal GFP fusion proteins. AR = full-length wild-type androgen receptor; ARmCBS = full-length androgen receptor with amino acid substitution of calreticulin binding sequence KVFFKR (residues 579–584) with RLAARK, this substitution is referred to as mCBS; NAR-NESAR = N-terminal region [N-terminal domain (NTD), DNA binding region/hinge region (DBD HR)] and the nuclear export sequence (NESAR) (Saporita et al., 2003) in place of the LBD of the androgen receptor; NARmCBS-NESAR = N-terminal region containing CBS substitution and NESAR in place of the LBD.
2. Materials and methods
2.1. Generation of the sihuCRT lines
Calreticulin down-regulated lines were generated using the pSUPER vector (Brummelkamp et al., 2002). A 19-nucleotide human calreticulin siRNA target sequence, GGAGCAGTTTCTGGACGGA, was identified within 100 bp from the translational start site of calreticulin. This target sequence contains greater than 30% GC content and has AA to its 5° in the mRNA. We designed two oligonucleotides (IDT, Coralville, IA) that contain the shRNA target sequence (bolded) in both the sense and antisense directions; SUPER-FOR1:GATCCCCGGAGCAGTTTCTGGACG GATTCAAGAGATCCGTCCAGAAACTGCTCCTTTTTGGAAA, and SUPER-REV1: AGCTTTTCCAAAAAGGAGCAGTTTCT GGACGGATCTCTTGAA TCCGTCCAGAAACTGCTCCGGG. These oligonucleotides were annealed, phosphorylated using polynucleotide kinase, and ligated into pSUPER at the Bgl II and Hind III restriction sites. Positive clones were selected and sequence verified (Macrogen, Seoul, S. Korea). A single positive clone was co-transfected with pSUPER.hygro (hygromycin-resistance vector) into PC3 cells in a 6:1 ratio using FuGENE 6 (Roche, Indianapolis, IN). After selection in the presence of hygromycin, positive clones were assayed for calreticulin expression by immunoblot analysis. One of the stably transfected PC3 clones, sihuCRT.15, was used in further studies.
2.2. Expression vector construction
The constructs used in this study are depicted in Fig. 1. Cloning into the expression vector pEGFP-C1 (Clontech, Mountain View, CA) results in fusion proteins with GFP at the N-terminus, allowing for convenient visualization by fluorescence microscopy. Using this vector, we generated GFP-tagged, full-length, wild-type AR. To generate AR mutants that would fail to bind calreticulin, we substituted the amino acids KVFFKR (residues 579–584) with the amino acids RLAARK (Dedhar et al., 1994). In this study, this mutation is referred to as mCBS.
To generate the GFP-NAR-NESAR construct, we cloned the N-terminal domain (NTD, residues 1–665) of AR, which includes the DNA-binding domain and hinge region into the pEGFP-C1 vector. In addition, in place of the ligand-binding domain of AR (residues 666–918), we cloned in the AR nuclear export sequence, NESAR (residues 743–817) identified by Saporita et al. (2003). To generate GFP-NARmCBS-NESAR, the amino acids KVFFKR (residues 579–584) were substituted with the amino acids RLAARK.
2.3. Cell culture and transfection
The human prostate cancer cell line PC3 and Cos-7 cells were obtained from American Type Culture Collection (Manassas, VA). Wild-type and calreticulin-knockout MEF cells were generated as described previously (Nakamura et al., 2000). PC3 sihuCRT.15 was generated as stated above. PC3 and PC3 sihuCRT.15 were maintained in RPMI 1640 medium and Cos-7 and MEF cell lines were maintained in DMEM medium. RPMI 1640 and DMEM media were supplemented with 10% fetal bovine serum (FBS), 1% glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA).
The DNA plasmids encoding the various AR mutants were transfected into PC3, PC3 sihuCRT, Cos-7 or MEF cells using FuGENE 6 according to the manufacturer’s instructions (Roche Applied Science). Cells were transfected at >60% confluence in 6-well plates in phenol red-free RPMI or DMEM with charcoal-stripped FBS. The localization of GFP was visualized using a Nikon TE 2000U inverted microscope. In transfected cells, cytoplasmic localization was defined as when the GFP fluorescence in the cytoplasm was greater than in the nuclei (C or C > N), even distribution was defined as when GFP fluorescence was evenly distributed between the nucleus and cytoplasm (C N), and nuclear localization was defined as when GFP localization in the nucleus was greater than in the cytoplasm (N or C < N).
Quantification of cells exhibiting cytoplasmic, even, and nuclear localization of GFP-tagged fusion proteins was carried out by counting 25–100 transfected cells in each experiment. All of the experiments were reproduced at least three times.
2.4. Western blot analysis
Cells were grown to >60% confluence in maintenance media and conditions (see Cell Culture and Transfection). To prepare cell lysates, cells were trypsinized using 0.05% trypsin with EDTA (Invitrogen) for 10 min at 37 °C. Cells were then collected and washed in culture media. Cells were then resuspended in lysate buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton x-100) with the protease inhibitor cocktail (PIC) (Sigma–Aldrich, St. Louis, MO) and phenylmethylsulfonyl fluoride (PMSF) (Sigma). Next, 20 μg of each cell lysate was denatured and loaded with protein loading buffer onto a NuPAGE 4–12% Bis-Tris precast gel (Invitrogen). Samples were then transferred onto nitrocellulose membrane. Membrane was blocked in 5% milk in TBS-Tween for 1 h and incubated overnight at 4 °C in 1:1000 dilution of antibody for calreticulin (NW1, Wang lab) in blocking buffer. Membrane was then washed with TBS-Tween three times and then incubated with 1:5000 dilution of anti-rabbit horseradish peroxidase (HRP) (Amersham Pharmacia, Piscataway, NJ) in blocking buffer for 1 h. Membrane was washed with TBS-Tween and treated with enhanced chemiluminesence (ECL) (Amersham Pharmacia) and exposed to Kodak BioMax Light Film for visualization.
To verify equivalent loading of protein, the membrane was stripped and reprobed for β-actin. Membrane was blotted in the same manner as for calreticulin with the exception of use of 1:1000 dilution of antibody for β-actin (Sigma) and a 1:5000 dilution of anti-goat HRP as a secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
3. Results
3.1. Mutation of the calreticulin binding site does not affect the localization of AR
Previous studies, predominantly using cellular heterokaryon fusion assays, implicated calreticulin as a nuclear export factor for the steroid hormone receptors GR and AR (Black et al., 2001; Holaska et al., 2001, 2002); however, a subsequent study using permeabilized cells brought into question this role for calreticulin (Walther et al., 2003). Findings from this latter study suggest that the method used to induce cell fusion may have given rise to the observed calreticulin-mediated nuclear export of GR. Given the differences in experimental approaches utilized in these studies, we took an alternative approach to examine the localization of wild-type and mutant AR GFP chimeras in live cells, obviating the need for heterokaryon or cell permeabilization assays.
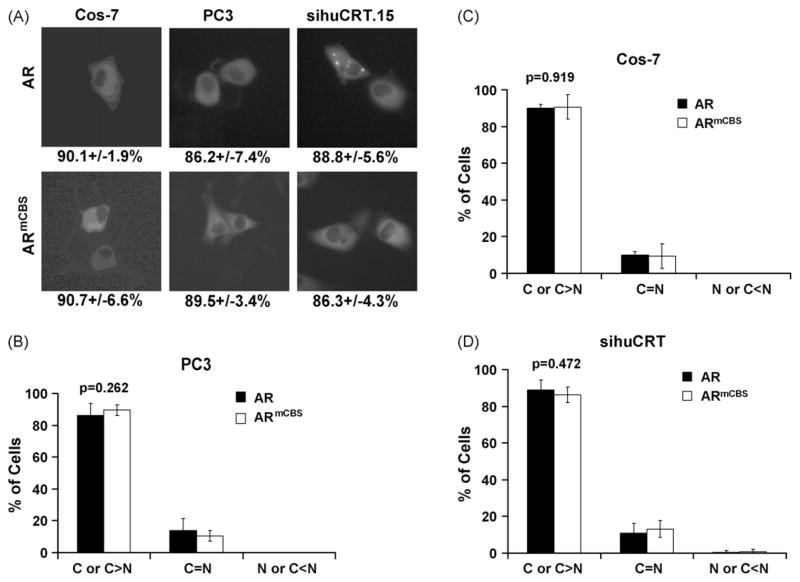
The consensus calreticulin binding sequence, KVFFKR, is found in the alpha subunit of integrin and between zinc fingers of steroid hormone receptors (Burns et al., 1994; Dedhar et al., 1994). We substituted this sequence with RLAARK, producing the chimera GFP-ARmCBS thus creating an AR unable to bind calreticulin (Fig. 1). Subsequently, it was demonstrated that the loss of either of the phenylalanine residues disrupts calreticulin binding (Black et al., 2001). In the absence of androgen, we transfected PC3 and Cos-7 cells, both of which express calreticulin, with either GFP-AR or GFP-ARmCBS. Fluorescence microscopy revealed that GFP-AR predominately localized to the cytoplasm in about 90% of GFP-expressing cells, with no significant difference between Cos-7 and PC3 cells (p = 0.226) (Fig. 2A–C). GFP-ARmCBS, which has a defective calreticulin binding site, also predominately localized to the cytoplasm (Fig. 2A–C), again with no significant difference seen between Cos-7 and PC3 cells (p = 0.851). Indeed, a comparison of cells expressing GFP-AR and GFP-ARmCBS revealed a very similar level of cytoplasmic localization of the GFP signal (Fig. 2B and C). Taken together, these results indicate that the mechanism governing AR subcellular localization is similar in prostate-derived cells (PC3) and in non-prostate-derived cells (Cos-7), and that the calreticulin binding site is not essential for the cytoplasmic localization of unliganded AR in these cells.
Fig. 2.
Subcellular localization of wild-type AR and ARmCBS. Cos-7, PC3, and PC3 sihuCRT.15 cells were transfected with expression vectors for GFP-tagged wild-type AR or ARmCBS. Cells were counted to determine the percentage of cells that displayed cytoplasmic, even, or nuclear GFP localization. (A) Fluorescence microscopy showing representative cells and GFP localization. Numbers below indicate percentage of cells that exhibited cytoplasmic GFP localization. (B) Percentage of transfected PC3 cells that displayed cytoplasmic, even, or nuclear localization. (C) Percentage of transfected Cos-7 cells that displayed cytoplasmic, even, and nuclear localization. (D) Percentage of transfected PC3 sihuCRT.15 that displayed cytoplasmic, even, and nuclear localization. Student t-tests show that there is not a statistical difference in the percentage of cells that displayed cytoplasmic localization between cells transfected with the wild-type AR and those transfected with the ARmCBS.
3.2. Reduction of calreticulin levels does not affect localization of GFP-AR
To determine if the levels of calreticulin affect the localization of a GFP-AR, we generated a subline of PC3 cells (sihuCRT.15) using siRNA technology to down-regulate the endogenous levels of calreticulin. Western blot analysis using an antibody to calreticulin showed that Cos-7 and the parental prostate cancer cell line PC3 have similar levels of calreticulin and that the subline PC3 sihu-CRT.15 has a substantial reduction in the level of calreticulin relative to PC3 and Cos-7 cells (Fig. 3).
Fig. 3.
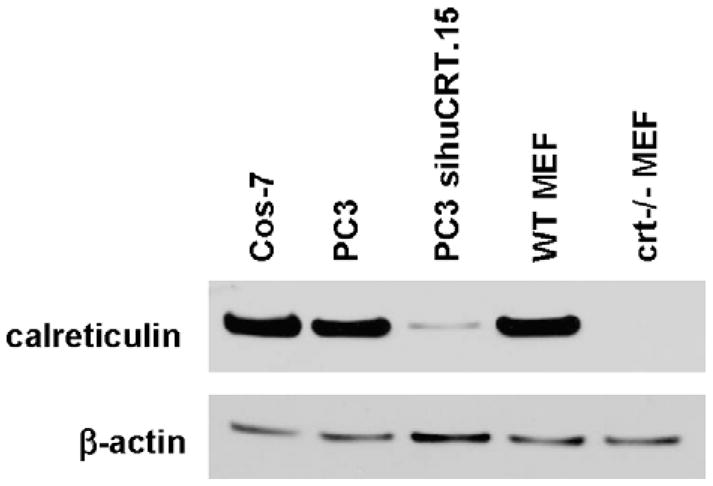
Western blot analysis of calreticulin levels in different cell lines. Cos-7, PC3, PC3 sihuCRT.15, wild-type MEF, and crt−/− MEF cells were assessed for their levels of calreticulin. Twenty micrograms of total protein was separated by SDS-PAGE. Blot was probed with anti-calreticulin antibody and re-probed with β-actin antibody as a loading control.
PC3 sihuCRT.15 cells were then transiently transfected with DNA plasmids encoding GFP-AR or GFP-ARmCBS in the absence of androgen and viewed under fluorescence microscopy to determine the subcellular localization of GFP. Over 80% of PC3 sihuCRT.15 cells displayed predominantly cytoplasmic GFP localization when expressing either GFP-AR or GFP-ARmCBS (Fig. 2A and D). The percentage of PC3 sihuCRT.15 cells displaying predominately cytoplasmic GFP-AR localization was not statistically different from that of the GFP-AR-transfected parental PC3 cells (88.8 ± 5.6% vs. 86.2 ± 7.4%, p = 0.517), nor were PC3 sihuCRT.15 cells statistically different from Cos-7 cells (p = 0.696) (Fig. 2A). Additionally, Cos-7 and PC3 sihuCRT.15 cells did not show a difference in GFP-ARmCBS cytoplasmic localization (p = 0.515), nor did PC3 and PC3 sihuCRT.15 cells (p = 0.091) (Fig. 2A). These results suggest that reduction in the levels of endogenous calreticulin has no significant effect on cytoplasmic localization of the unliganded AR.
3.3. Loss of calreticulin does not affect AR localization
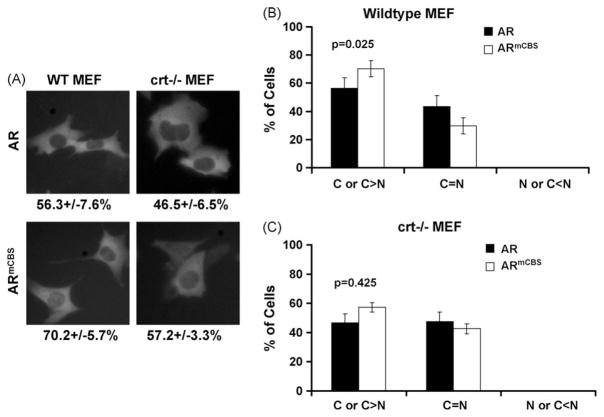
Although the subline PC3 sihuCRT.15 exhibited a significant reduction in calreticulin levels as compared with the parental PC3 and Cos-7 cell lines, there still remained a low level of calreticulin (Fig. 3). To further test if calreticulin plays a role in the cytoplasmic localization of AR, we determined if the genetic ablation of calreticulin resulted in any changes in AR localization. Western blot analysis showed that wild-type (WT) MEF cells expressed similar levels of calreticulin as PC3 and Cos-7 cells, while crt−/− MEF cells expressed no detectable levels of calreticulin (Fig. 3). We then transfected WT MEF and crt−/− MEF with plasmid DNA encoding various GFP-tagged AR constructs and examined the GFP signal. No significant difference in GFP-AR cytoplasmic localization existed between WT MEF and crt−/− MEF cells (56.3 ± 7.6% vs. 46.5 ± 6.5%, p = 0.47; Fig. 4A). However, compared to PC3 and Cos-7 cells, both WT and crt−/− MEF cells displayed a lower percentage of cells with predominantly cytoplasmic AR localization. Next, we compared localization of GFP-AR and GFP-ARmCBS in WT MEF cells. Interestingly, we found that transfection with GFP-ARmCBS led to a greater percentage of cells with GFP in the cytoplasm than cells transfected with GFP-AR (Fig. 4B). The difference, while small, was statistically significant (p = 0.025). This trend was also seen in the crt−/− MEF cells, but was not statistically significant (Fig. 4C). These results again indicate that cytoplasmic localization of unliganded AR does not require calreticulin, and further support our siRNA experiments.
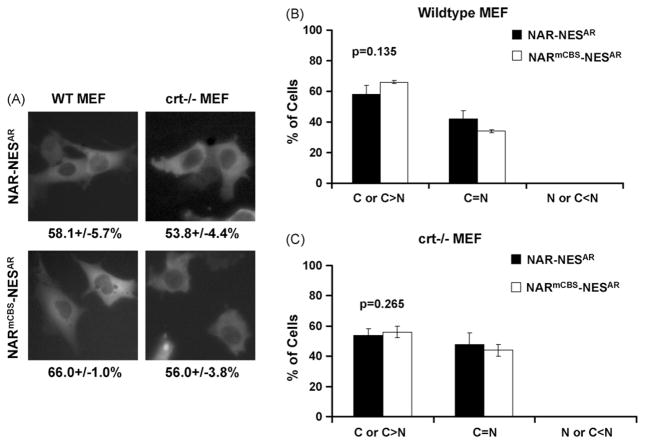
Fig. 4.
Subcellular localization of wild-type AR and ARmCBS in wild-type and crt−/− MEF cells. MEF cells were transfected with plasmid DNA encoding wild-type AR or ARmCBS. (A) Fluorescence microscopy showing representative cells and GFP localization. Numbers below indicate percentage of cells that displayed cytoplasmic GFP localization. Percentages of transfected cells displaying cytoplasmic, even or nuclear localization for indicated GFP-fusion protein are shown in (B) for WT MEF cells and in (C) for crt−/−MEF cells. Student t-tests show that there is not a statistical difference in the percentage of cells with GFP cytoplasmic localization between WT MEF and crt−/− MEF cells transfected with the wild-type AR. There is a small, but statistically significant difference between WT MEF cells transfected with WT AR and ARmCBS, but no statistical difference between crt−/− MEF cells transfected with WT AR and ARmCBS.
3.4. CBS is not required for NESAR-mediated, cytoplasmic localization of AR
NESAR is both necessary and sufficient for the nuclear export and/or cytoplasmic localization of AR. NESAR alone tagged with GFP localized to the cytoplasm, while deletion of NESAR from AR resulted in a predominately nuclear GFP signal (Saporita et al., 2003). In light of this evidence that an amino acid sequence other than the CBS might be essential for nuclear export, we tested if the loss of CBS affected the ability of NESAR to direct cytoplasmic localization of GFP-AR. We generated DNA constructs that encoded the NESAR instead of the LBD, and either the wild-type CBS (NAR-NESAR) or the CBS substitution (NARmCBS-NESAR) (Fig. 1).
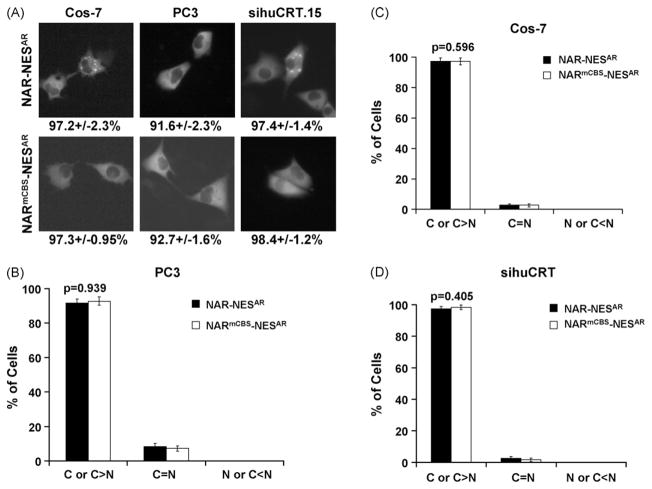
GFP-NAR-NESAR or GFP-NARmCBS-NESAR were transfected into PC3, Cos-7, and PC3 sihuCRT.15 in the absence of androgen. Transfected cells were then viewed under fluorescence microscopy to determine GFP localization. Cells expressing GFP-NAR-NESAR or GFP-NARmCBS-NESAR showed predominately cytoplasmic localization of GFP (Fig. 5). This was similar to cells expressing GFP-AR or GFP-ARmCBS (Fig. 2). The percentage of GFP-NAR-NESAR-expressing cells displaying cytoplasmic GFP localization did not significantly differ from those expressing GFP-NARmCBS-NESAR in these cell lines (Fig. 5). GFP-NAR-NESAR or GFP-NARmCBS-NESAR were also expressed in WT MEF or crt−/− MEF cells. Similarly, there was no significant difference between WT and crt−/− MEF cells expressing GFP-NAR-NESAR or GFP-NARmCBS-NESAR construct (Fig. 6). These results suggest that CBS is not necessary for the NESAR-mediated cytoplasmic localization of AR.
Fig. 5.
Subcellular localization of AR constructs with NESAR in place of LBD. Cos-7, PC3, and PC3 sihuCRT.15 cells were transfected with plasmids encoding the N-terminal region and NESAR from the AR (NAR-NESAR) or N-terminal region and NESAR from the AR with CBS substitution (NARmCBS-NESAR). (A) Fluorescence microscopy showing representative cells and GFP localization of indicated AR mutant. Numbers below indicate percentage of cells that displayed cytoplasmic GFP localization. Percentages of transfected cells that displayed cytoplasmic, even or nuclear localization are shown in (B) for PC3, (C) for Cos-7, and (D) for PC3 sihuCRT.15. Student t-tests show that there is not a statistical difference in the percentage of cells that displayed cytoplasmic localization between cells expressing NAR-NESAR and those expressing NARmCBS-NESAR.
Fig. 6.
Subcellular localization of AR with NESAR in place of LBD in WT and crt−/− MEF cells. (A) Fluorescence microscopy showing representative transfected cells and GFP localization of indicated AR mutants. Numbers below indicate percentage of cells that displayed cytoplasmic GFP localization. Percentage of transfected cells with the cytoplasmic, even or nuclear localization of the indicated GFP-fusion protein are shown in (B) for WT MEF and (C) for crt−/− MEF cells. Student t-tests show that there are no statistical differences in the percentage of cells that displayed cytoplasmic localization between cells expressing NAR-NESAR and those expressing NARmCBS-NESAR, nor was there a difference between WT MEF and crt−/− MEF expressing NAR-NESAR.
4. Discussion
The subcellular localization of the androgen receptor is an integral component of its transcriptional regulation and may play an important role in transition of prostate cancer from the treatable androgen-sensitive state to the deadly castration-recurrent state. Knowledge of the mechanism(s) and the proteins involved in the nuclear export/import and cytoplasmic localization of AR remain very limited. Studies have suggested that the well defined nuclear export factor, Crm-1, does not appear to play a role in AR transport (Tyagi et al., 2000; Saporita et al., 2003). On the other hand, the calcium binding protein, calreticulin, has been proposed to be a potential nuclear export factor for both GR and AR (Black et al., 2001; Holaska et al., 2001, 2002).
Several characteristics and/or functions have been attributed to calreticulin that make it an attractive candidate for a nuclear export factor for steroid hormone receptors. One is that the calreticulin binding amino acid sequence, KXFFKR, is conserved among the steroid hormone receptors (Burns et al., 1994; Dedhar et al., 1994). Another is that calreticulin appears to control steroid-sensitive gene expression (Burns et al., 1994; Dedhar et al., 1994). The calreticulin gene also is an androgen responsive gene in the prostate (Zhu et al., 1998). The evidence that calreticulin acts as an export factor for AR comes from experiments that utilized heterokaryon assays. In these assays PEG was used to permeabilize the cells to allow for cell fusion (Black et al., 2001). Subsequent reports indicated that this method of cell fusion may affect the integrity of the endoplasmic reticulum, resulting in a transient efflux of calreticulin, and thus it is difficult to properly interpret the results from the heterokaryon assays (Walther et al., 2003). Defining the importance of calreticulin in AR intracellular trafficking is critical to our understanding of AR nuclear export and may help dictate the direction of future AR nuclear export studies.
In this study we assessed whether the cytoplasmic localization of AR requires calreticulin in live cells. We generated AR constructs with a mutated calreticulin amino acid binding sequence and determined if the mutation could affect cytoplasmic localization of the AR constructs. In addition, we tested whether a reduction or the loss of calreticulin resulted in a modification of AR cytoplasmic localization.
We show here that the mutation of the calreticulin binding site in AR does not prevent the receptor from being localized to the cytoplasm, arguing against a role for calreticulin binding in AR nuclear export. When constructs containing the mCBS mutation were transfected into various cell lines, the percentage of cells that displayed cytoplasmic localization remained virtually the same with the exception of in WT MEF cells. If calreticulin mediates the export of AR by binding to its CBS, then one would predict that cells expressing WT AR would have a greater percentage of cells displaying cytoplasmic GFP-AR than cells expressing ARmCBS. Interestingly, in WT MEF cells, ARmCBS displayed a small but statistically significant increase in the percentage of cells that displayed cytoplasmic localization of the chimera. Although the reason for the small increase is not clear, this observation is in contrast to what would be expected if calreticulin was an AR export factor.
We further investigated the role of calreticulin by expressing different variants of tagged AR in cells with calreticulin knockdown or knockout. A comparison of parental PC3 cells and the subline PC3 sihuCRT.15, which had a substantial knockdown in calreticulin levels, showed no significant difference in the percentage of cells with a predominate cytoplasmic localization of GFP-AR variants. Also, the localization of the GFP-AR constructs in crt−/− MEF cells was virtually the same as that in the WT MEF cells. One noticeable difference was both WT and crt−/− MEF cell lines showed a significantly lower percentage of cells that displayed a cytoplasmic localization when compared to Cos-7 and PC3 lines. The possibility exists that whatever mechanism directs cytoplasmic localization of AR may be cell-type specific. Furthermore, it may reflect the difference in the ability to transport AR in cells that are epithelial in origin (Cos-7, PC3) vs. those that are fibroblast in origin (MEF). These experiments strongly argue that calreticulin does not influence AR cytoplasmic localization in living cells and thus may not play a role in AR nuclear export, corroborating the finding of Walther et al. (2003). We recognize that our study did not assay nuclear export of the GFP-tagged constructs directly. Cytoplasmic localization of AR does not definitively exclude the possibility of calreticulin as an export factor; however, it does raise the question that if calreticulin is an AR export factor, why is it not required for AR’s cytoplasmic localization in living cells?
The present study suggests that region(s) other than the calreticulin binding site may govern AR nuclear export. Previous studies have shown that the LBD alone is responsive to ligand in that it can be imported into the nucleus in the presence of ligand and exported upon ligand withdrawal (Saporita et al., 2003). This would suggest that amino acid sequences within the LBD play a critical role in the localization of the AR. Indeed, a 75 amino acid sequence within the LBD, termed NESAR, was shown to be both necessary and sufficient for androgen receptor cytoplasmic localization. Given the evidence for the NESAR as an export sequence, it seems that future studies to determine nuclear export factors should focus on elements associated with NESAR.
Mechanisms other than known nuclear transport pathways may contribute to the balance of the nuclear and cytoplasmic pools of AR. Some recent work has provided additional insights into steroid hormone receptor import (Freedman and Yamamoto, 2004). Another study demonstrated that stress kinase signaling regulates AR nuclear export and activity by phosphorylation of AR at Ser 650. A serine to alanine mutation at this site resulted in reduced AR nuclear export while a mutation to aspartate, a phosphomimetic amino acid, rescued the AR nuclear export function (Gioeli et al., 2006). Another study has implicated a DNA-dependent protein kinase activity as a regulator of AR nuclear export in vitro. Addition of okadaic acid, a phophotase inhibitor resulted in an approximately 2-fold enhancement in AR nuclear export which was similar to the results seen in the GR (DeFranco et al., 1991; Shank et al., 2008). Interestingly, the phosphorylation of Ser 650 does not regulate ds-DNA dependent export of AR (Shank et al., 2008) Curiously, in this study, agonist-bound AR underwent both import and export and ligand dissociation, which is widely thought to be required, was not a prerequisite for AR export in digitonin-permeabilized cells (Shank et al., 2008). Another protein implicated to play a role in AR transport is the chaperone Hsp90. In experiments comparing the localization of AR in androgen-sensitive cells (LNCaP) and androgen-refractory cells (C4-2), the addition of 17-AAG, a Hsp90 inhibitor, inhibited basal PSA expression and nuclear localization of AR in C4-2 cells. This study suggested that Hsp90 plays a key role in ligand-independent nuclear localization and AR activity in castration-recurrent prostate cancer cells (Saporita et al., 2007).
The regulation of AR trafficking and subcellular localization is undoubtedly very complex. Multiple proteins and regions of the AR play a role in AR intracellular trafficking, and the interplay of nuclear export and import activities determines AR subcellular localization. Clearly defining the factors essential in the regulation of AR nuclear export will guide future studies to elucidate the mechanism of AR nucleorcytoplasmic trafficking.
Acknowledgments
Special thanks to Moira Hitchens for critical reading of this manuscript and Dr. Reuven Agami (Netherland Cancer Institute) for his generous gift of the pSUPER vectors. This work was supported by NIH Training Grant T32 CA080621, NIH R01 CA108675-01, ACS #PF-05-229-01-CSM, NIH R37 DK51193, NIH 1 P50 CA90386, DOD DAMD17-01-1-0088 CIHR MT-15291, and the Mellam Foundation.
References
- ACS. Cancer Statistics. American Cancer Society; 2008. [Google Scholar]
- Black BE, Holaska JM, Rastinejad F, Paschal BM. DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr Biol. 2001;11:1749–1758. doi: 10.1016/s0960-9822(01)00537-1. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Burns K, Duggan B, Atkinson EA, Famulski KS, Nemer M, Bleackley RC, Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;367:476–480. doi: 10.1038/367476a0. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Rennie PS, Shago M, Hagesteijn CY, Yang H, Filmus J, Hawley RG, Bruchovsky N, Cheng H, Matusik RJ, et al. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994;367:480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- DeFranco DB, Qi M, Borror KC, Garabedian MJ, Brautigan DL. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol. 1991;5:1215–1228. doi: 10.1210/mend-5-9-1215. [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15:2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Georget V, Lobaccaro JM, Terouanne B, Mangeat P, Nicolas JC, Sultan C. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol Cell Endocrinol. 1997;129:17–26. doi: 10.1016/s0303-7207(97)04034-3. [DOI] [PubMed] [Google Scholar]
- Gioeli D, Black BE, Gordon V, Spencer A, Kesler CT, Eblen ST, Paschal BM, Weber MJ. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001a;61:4315–4319. [PubMed] [Google Scholar]
- Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001b;61:2892–2898. [PubMed] [Google Scholar]
- Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, Michalak M. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem. 2002;277:50776–50779. doi: 10.1074/jbc.M209900200. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Black BE, Love DC, Hanover JA, Leszyk J, Paschal BM. Calreticulin is a receptor for nuclear export. J Cell Biol. 2001;152:127–140. doi: 10.1083/jcb.152.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska JM, Black BE, Rastinejad F, Paschal BM. Ca2+-dependent nuclear export mediated by calreticulin. Mol Cell Biol. 2002;22:6286–6297. doi: 10.1128/MCB.22.17.6286-6297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZQ, Li J, Wong J. AR possesses an intrinsic hormone-independent transcriptional activity. Mol Endocrinol. 2002;16:924–937. doi: 10.1210/mend.16.5.0829. [DOI] [PubMed] [Google Scholar]
- Jenster G, van der Korput JA, Trapman J, Brinkmann AO. Functional domains of the human androgen receptor. J Steroid Biochem Mol Biol. 1992;41:671–675. doi: 10.1016/0960-0760(92)90402-5. [DOI] [PubMed] [Google Scholar]
- Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11:122–129. doi: 10.1016/s0962-8924(01)01926-2. [DOI] [PubMed] [Google Scholar]
- Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344 (Pt 2):281–292. [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR, Michalak M. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Robertson M, Liu G, Dickie P, Nakamura K, Guo JQ, Duff HJ, Opas M, Kavanagh K, Michalak M. Complete heart block and sudden death in mice overexpressing calreticulin. J Clin Invest. 2001;107:1245–1253. doi: 10.1172/JCI12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poukka H, Karvonen U, Yoshikawa N, Tanaka H, Palvimo JJ, Janne OA. The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J Cell Sci. 2000;113 (Pt 17):2991–3001. doi: 10.1242/jcs.113.17.2991. [DOI] [PubMed] [Google Scholar]
- Roy AK, Tyagi RK, Song CS, Lavrovsky Y, Ahn SC, Oh TS, Chatterjee B. Androgen receptor: structural domains and functional dynamics after ligand-receptor interaction. Ann N Y Acad Sci. 2001;949:44–57. doi: 10.1111/j.1749-6632.2001.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem. 2003;278:41998–42005. doi: 10.1074/jbc.M302460200. [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Ai J, Wang Z. The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. Prostate. 2007;67:509–520. doi: 10.1002/pros.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank LC, Kelley JB, Gioeli D, Yang CS, Spencer A, Allison LA, Paschal BM. Activation of the DNA-dependent protein kinase stimulates nuclear export of the androgen receptor in vitro. J Biol Chem. 2008;283:10568–10580. doi: 10.1074/jbc.M800810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- Trombetta ES. The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology. 2003;13:77R–91R. doi: 10.1093/glycob/cwg075. [DOI] [PubMed] [Google Scholar]
- Tyagi RK, Amazit L, Lescop P, Milgrom E, Guiochon-Mantel A. Mechanisms of progesterone receptor export from nuclei: role of nuclear localization signal, nuclear export signal, and ran guanosine triphosphate. Mol Endocrinol. 1998;12:1684–1695. doi: 10.1210/mend.12.11.0197. [DOI] [PubMed] [Google Scholar]
- Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–1174. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- Walther RF, Lamprecht C, Ridsdale A, Groulx I, Lee S, Lefebvre YA, Hache RJ. Nuclear export of the glucocorticoid receptor is accelerated by cell fusion-dependent release of calreticulin. J Biol Chem. 2003;278:37858–37864. doi: 10.1074/jbc.M306356200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Johnson M, Le KH, Sato M, Ilagan R, Iyer M, Gambhir SS, Wu L, Carey M. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63:4552–4560. [PubMed] [Google Scholar]
- Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–13123. [PubMed] [Google Scholar]
- Zhu N, Pewitt EB, Cai X, Cohn EB, Lang S, Chen R, Wang Z. Calreticulin: an intracellular Ca++-binding protein abundantly expressed and regulated by androgen in prostatic epithelial cells. Endocrinology. 1998;139:4337–4344. doi: 10.1210/endo.139.10.6242. [DOI] [PubMed] [Google Scholar]