Abstract
Recent efforts in our laboratory have explored the use of polyacrylate nanoparticles in aqueous media as stable emulsions for potential applications in treating drug-resistant bacterial infections. These emulsions are made by emulsion polymerization of acrylated antibiotic compounds in a mixture of butyl acrylate and styrene (7:3 w:w) using sodium dodecyl sulfate (SDS) as a surfactant. Prior work in our group established that the emulsions required purification to remove toxicity associated with extraneous surfactant present in the media. This paper summarizes our investigations of poly(butyl acrylate-styrene) emulsions made using anionic, cationic, zwitterionic, and non-charged (amphiphilic) surfactants, as well as attachable surfactant monomers (surfmers), comparing the cytotoxicity and microbiological activity levels of the emulsion both before and after purification. Our results show that the attachment of a polymerizable surfmer onto the matrix of the nanoparticle neither improves nor diminishes cytotoxic or antibacterial effects of the emulsion, regardless of whether the emulsions are purified or not, and that the optimal properties are associated with the use of the non-ionic surfactants versus those carrying anionic, cationic, or zwitterionic charge. Incorporation of an N-thiolated β-lactam antibacterial agent onto the nanoparticle matrix via covalent attachment endows the emulsion with antibiotic properties against pathogenic bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), without changing the physical properties of the nanoparticles or their emulsions.
Keywords: polyacrylate nanoparticles, emulsions, surfactant cytotoxicity, surfmers, SDS
Background
Recent publications from our laboratory have described the preparation and in vitro microbiological properties of polyacrylate-based nanoparticle emulsions that contain antibiotic drugs.1–4 In these studies, the emulsions were prepared by pre-dissolving an antibacterial agent such as penicillin or an N-thiolated β-lactam in a warm 7:3 w:w mixture of butyl acrylate and styrene, and then creating an emulsified suspension in water using the surfactant, sodium dodecyl sulfate (SDS), prior to free radical polymerization. Typically 3 weight % of SDS is employed to form a stable emulsion, and up to 5% of the weight of the emulsion can be antibiotic drug, depending on its lipophilicity and size, either through covalent attachment to the polymer or by encapsulation during emulsion polymerization. Antimicrobial activity of these nanoparticle-bound antibiotics seemed to be dependent on the type of linkage holding the drug molecule to the polymeric matrix, and in particular, its susceptibility towards hydrolytic cleavage.1,3 The interaction of the nanoparticles in the emulsions with bacteria has not be defined but may be mediated by enzymatic degradation of the nanoparticle polymeric matrix at the bacterial membrane interface, thereby releasing the antibiotic drug into the membrane, or by endocytosis followed by drug release. Thus, altering the density or type of ionic charge on the nanoparticle surface may significantly alter these interactions, and thus the biological properties and drug delivery capabilities of the nanoparticles. It is our interest to further develop these polyacrylate nanoparticles and their aqueous emulsions for potential clinical applications5,6, such as treatment of bacterial infections, and the issue of potential toxicity naturally arose.7 In our most recent report, we noted some bactericidal and cytotoxic effects associated with the use of SDS in amounts greater than 3 weight % for the emulsion polymerization, and investigated methods for purifying the emulsions to remove unassociated SDS and other potentially toxic contaminants.7 The purification protocol we devised entailed a mild centrifugation of the crude emulsion (to remove suspended precipitates) followed by overnight dialysis in deionized water (to remove small molecular weight contaminants including unassociated SDS). This simple procedure enables us now to prepare purified aqueous emulsions of SDS-stabilized polyacrylate nanoparticles suitable for more detailed studies on their antibacterial and cytotoxic properties. Considerable work has been done before with various types of surfactants8–12 and drug delivery platforms1,4 in regards to mammalian toxicity, and since our aim is to ultimately develop these for antibiotics therapy, we wanted to explore this in the context of polyacrylate nanoparticles in aqueous media. Therefore, in this study, we expand upon our previous investigations on SDS-stabilized poly(butyl acrylate-styrene) nanoparticles in order to discern a way to remove unwanted bactericidal or cytotoxic components in the emulsion, either through purification of the initial emulsion or by replacement of the surfactant used in the emulsion polymerization.
The nanoparticle surface was modified by exchanging anionic SDS for phosphate ion by simply dialyzing the centrifuged emulsion against phosphate-buffered saline solution instead of deionized water. This was done for 24 hours, replacing the PBS buffer every 3 hours. The physical appearance of the emulsion changed from clear to milky, and dynamic light scattering indicated that the size of the nanoparticle increases almost 5-fold (200 nm) from the original SDS-stabilized nanoparticle emulsions (45 nm). Upon antibacterial and cytotoxicity testing, these phosphate-stabilized emulsions were found to be considerably more toxic. Cell viability in fibroblasts treated with the emulsions diminished by almost half, and the bacterial MIC (for MRSA) also increased substantially, relative to the original SDS-system. Thus, the attempt to switch the anionic SDS for phosphate, while successfully achieved, gave much larger particles, and failed to make the emulsion innocuous biologically. Therefore, we decided to explore some other options, by examining cationic, anionic, zwitterionic and non-ionic surfactants as stabilizers, as well as variants which bear an acrylate (polymerizable) moiety suitable for attaching the surfactant directly to the matrix. Our specific focus is on whether these types of surfactants can be used effectively in the emulsion polymerization, and what effects they may have on particle size, stability, and microbiological or cytotoxic activities.
To begin, a variety of commercially available surfactants were investigated in the formation of the poly(butyl acrylate-styrene) emulsions (Figure 1). These common agents include the anionic salt sodium dodecyl sulfate (SDS, 1), cationic salt cetyltrimethylammonium bromide (2), zwitterionic salt 3-(N,N-dimethylmyristylammonio)propanosulfonate (3), and neutral surfactant dodecanoic acid 2-(2-hydroxyethoxy)ethyl ester (4), to assess effects of surfactant charge on nanoparticle formation, particle size, emulsion stability, and biological activities (antibacterial, cytotoxic). In each case, the amount of surfactant ranged from 1–10 weight % of the total solid content of the emulsion.
Figure 1.
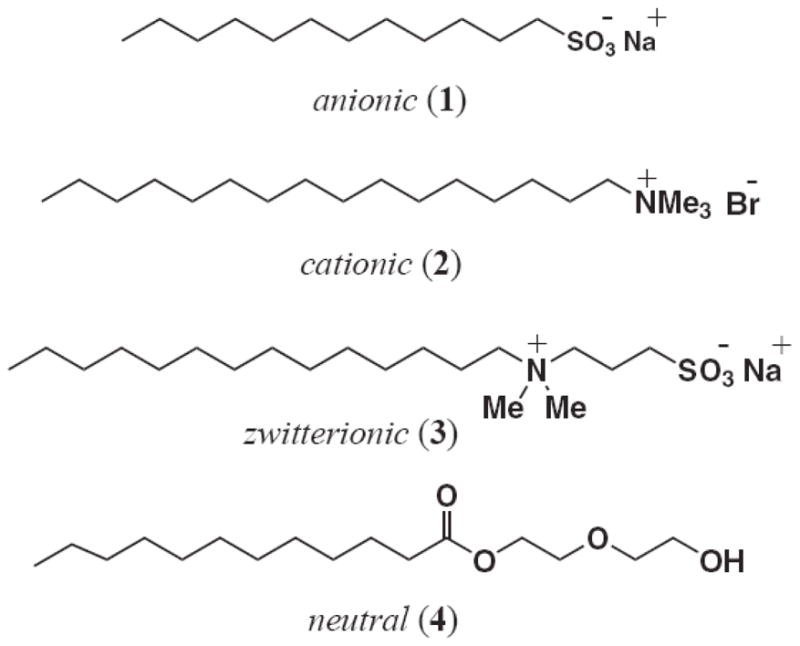
Four differentially charged surfactants 1–4 used to prepare poly(butyl acrylate-styrene) nanoparticle emulsions for these investigations.
The type of surfactant used in the emulsion is known to influence the formation and stability of the nanoparticles, but also that biological interactions, migration properties9 and cellular toxicity of the surfactant through disruption of membrane integrity are all highly dependent on the concentration and structural properties of the surfactant itself.13 Consequently, we investigating acrylated variants of these different surfactants so that the molecule could be introduced covalently into the nanoparticle matrix during emulsion polymerization. The structures of these acrylated surfactant monomers, or surfmers14, are shown in Figure 2 and include the anionic surfmer, sodium 11-(acryloloxyundecan-1-yl) sulfate (5), the two cationic surfmers, (11-acryloyloxyundecyl)dimethyl(2-hydroxyethyl)ammonium bromide (6) and (11-acryloyloxyundecyl)dimethylethylammonium bromide (7), the zwitterionic surfmer, 3-[N,N-diethyl-N-(3-sulfopropyl)ammonio]acrylate (8), and finally, the neutral surfmer, dodecanoic acid 2-(2-acryloyloxyethoxy)ethyl ester (9).9–12
Figure 2.
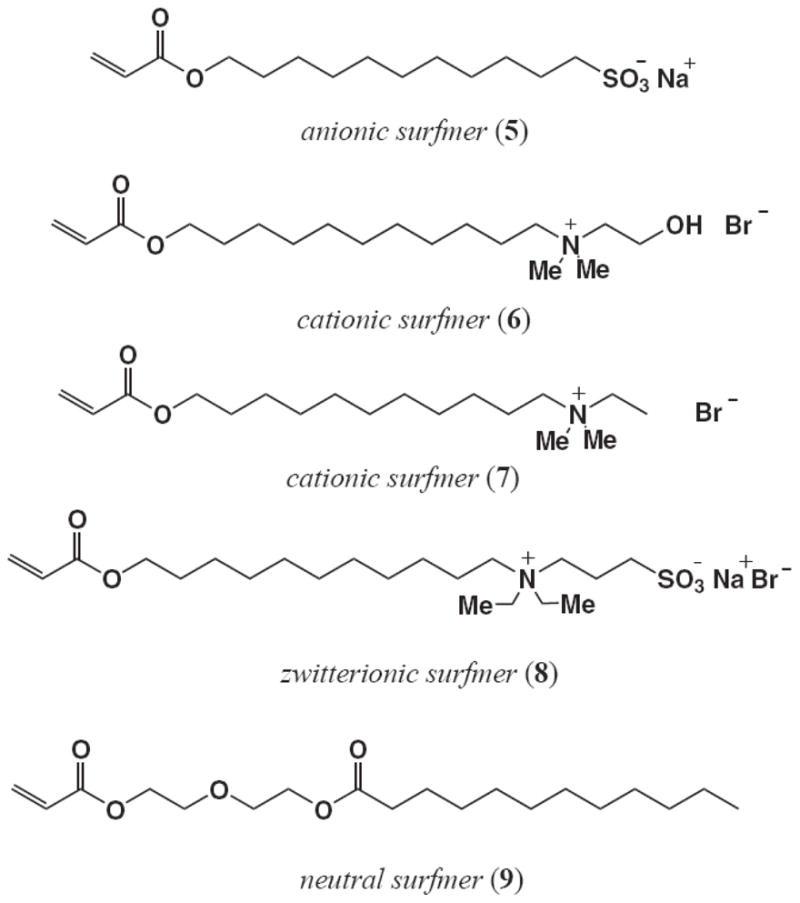
Polymerizable (acrylated) surfactant monomers 5–9 used to prepare poly(butyl acrylate-styrene) nanoparticle emulsions for these investigations.
Methods
All commercially-available reagents including surfactants 1–3 were purchased from Sigma-Aldrich Chemical Company or Acros Organic and used without further purification. Solvents were obtained from Fisher Scientific Company. Thin-layer chromatography (TLC) was performed using EM Reagent plates. Products were purified by flash chromatography using Silicycle Chemical Division flash chromatography silica gel (40–63 μm). NMR spectra were recorded in a 400-MHz Varian Instrument using CDCl3. 13C NMR spectra were proton-broad band decoupled.
2-(2-Hydroxyethoxy)ethyl dodecanoate (4)
100 mg (5 mmol) of lauric acid was placed in a round bottom flask. 5 ml of dry CH2Cl2 was added, followed by the addition of 1.244 g (6.48 mmol) of 1-ethyl-3-(3-dimethylamino)propylcarbodiimide hydrochloride (EDCI), and a catalytic amount of DMAP were added, this solution was placed in a ice bath and stirred for 30 min, then 430 μl (21.4 mmol) of 2-hydroxyethyl ether was added. The reaction progress was followed by TLC. The reaction mixture was stirred overnight, the solvent was evaporated and the product was purified using flash chromatography (hexanes:ethyl acetate, starting by 4:1 to 2:1) to yield ester 4 as a colorless oil in 39 % yield. 1H NMR (400 MHz, CDCl3): 4.21 (2H, t, J= 4.8 Hz), 3.68 (4H, m), 3.57 (2H, t, J= 4.8 Hz), 2.30 (2H, t, J= 7.6 Hz), 2.15 (1H, broad), 1.59 (2H, q, J= 7.2 Hz), 1.25 (2H, s), 1.22 (14H, s), 0.84 (3H, t, J= 7.2 Hz). 13C NMR (100 MHz, CDCl3): δ 174.1, 72.5, 69.4, 63.4, 61.9, 34.4, 32.1, 29.8, 29.6, 29.5, 29.4, 29.3, 25.1, 22.9, 14.4.
The surfactant monomers 5–9 were prepared by O-acrylation of the commercially-available alcohol precursors, as described below.
Sodium 11-acryloyloxyundecan-1-yl sulfate (5)
Chlorosulfonic acid (8.65 g, 74.0 mmol) was placed in a three-necked round bottom flask fitted with a mechanical stirrer, a dropping funnel and nitrogen inlet. 11-Acryloyloxyundecan-1-ol (19.0 g, 78.0 mmol) was added drop wise over one hour with vigorous stirring. The reaction mixture was then stirred for two hours and purged with nitrogen for two hours more. The mixture at this point was a brown viscous liquid, which was added drop wise to an ice-cold, saturated NaHCO3 solution (20 ml) with vigorous stirring. During the addition process, the mixture was kept basic (pH paper) by adding solid NaHCO3, as required. 2-Propanol (56 ml) and water (90 ml) were added and the mixture was filtered, and the filtrate was washed two times with 40 ml of petroleum ether (boiling range of 40–60°C). The sample was then lyophilized, yielding 35.0 g of a light yellow waxy solid. The proton NMR spectrum of this compound matches the one reported.9
N-(11-Acryloyloxyundecyl)-N-(2-hydroxyethyl)-N,N-dimethylammonium bromide (6)
This procedure is based on the published protocol of Sanderson with modification as noted below.12 11-Bromoundecan-1-ol (0.70 g, 2.8 mmol) was placed in a round-bottom flask, 5 ml of CH2Cl2 was added, and the mixture was stirred at 0°C for 15 min. To this was added NaHCO3 (0.34 g, 4.0 mmol) and 1 mg of hydroquinone (as radical inhibitor), then acryloyl chloride (340 μl, 4.0 mmol) was added drop wise. After stirring overnight, the reaction mixture was evaporated and the product was purified by flash chromatography to yield 0.68 g (88%) of a colorless oil used for the following step.
11-Bromoundecyl acrylate (0.57 g, 2.07 mmol), dimethylethanolamine (333 ml, 3.31 mmol) and 1 mg of hydroquinone were placed in a round-bottom flask fitted with a condenser. The setup was immersed in an oil bath and vigorously stirred at 50°C for 3 h, to yield a brownish solid. This was washed several times with diethyl ether, yielding a pale brown powdery product. This was then dried under vacuum overnight, recrystallized in hot ethyl acetate, filtered and dried overnight under vacuum to yield 0.61 g (76%) of 6 as a pale yellowish solid. 1H NMR data match the reported values in the literature.12
N-(11-Acryloyloxyundecyl)-N,N-dimethyl-N-ethylammonium bromide (7)
Pale yellow powder, 28%, mp 100 ± 1°C. The 1H NMR spectrum matches the one reported12: 1H NMR (400 MHz, CDCl3): δ 6.36 (1H, d, J= 17.2 Hz), 6.09 (1H, dd, J= 10.8, 6.8 Hz), 5.79 (1H, d, J= 10.4 Hz), 4.12 (2H, t, J= 6.4 Hz), 3.62 (2H, q, J= 7.2 Hz), 3.47 (2H, t, J= 8.4 Hz), 1.65 (6H, m), 1.33 (18H, m). 13C NMR (100 MHz, CDCl3): δ 130.7, 128.8, 64.9, 63.7, 59.5, 50.9, 29.5, 29.4, 28.8, 26.5, 26.0, 22.9
3-[N,N-Diethyl-N-(3-sulfopropyl)ammonio] acrylate (8)
Yellow solid, 15%, mp 90 ± 1°C. 1H NMR (400 MHz, CDCl3): δ 6.35 (1H, d, J= 16.0 Hz), 6.07 (1H, dd, J= 10.4, 7.2 Hz), 5.78 (1H, d, J= 9.2 Hz), 4.10 (2H, d, J= 6.8 Hz), 3.65 (2H, m), 3.08 (4H, m), 2.96 (4H, t, J= 7.2 Hz), 2.25 (2H, q, J= 7.2 Hz), 1.62 (2H, m), 1.36 (24H, m).
2-(2-Acryloyloxyethoxy)ethyl dodecanoate (9)
47 μl (1.95 mmol) of 2-(2-hydroxyethoxy)ethyl dodecanoate (4) was mixed with 3 ml of dry CH2Cl2 followed by addition of 153 μl (1.95 mmol) of acryloyl chloride and 1.0 ml (5.85 mmol) of Hunig’s base. The reaction mixture was stirred overnight, washed with 5% HCl, and the organic layer was dried over Na2SO4. Evaporation of the solvent gave an oil which was purified by flash chromatography to give a colorless oily liquid in a 71 % yield. 1H NMR (400 MHz, CDCl3): δ 6.40 (1H, d, J= 16 Hz), 6.102 (1H, dd, J= 10.0, 7.2 Hz), 5.80 (1H, d, J= 9.2 Hz), 4.29 (2H, t, J= 4.8 Hz), 4.20 (2H, t, J= 4.8 Hz), 3.71 (2H, t, J= 4.8 Hz), 3.67 (2H, t, J= 4.8 Hz), 2.30 (2H, t, J= 7.6 Hz), 1.59 (2H, q, J= 6.8 Hz), 1.25 (2 H, s), 1.22 (14H, s), 0.84 (3H, t, J= 6.8 Hz). 13C NMR (100 MHz, CDCl3): δ 174.0, 166.3, 131.3, 128.4, 69.36, 69.2, 63.8, 63.4, 34.4, 32.1, 29.8, 29.7, 29.5, 29.4, 29.3, 25.1, 22.9, 14.9.
Preparation of the polyacrylate nanoparticle emulsions
Poly(butyl acrylate-styrene) nanoparticles were prepared by emulsion polymerization as described in our previous publications.1–5 Briefly, a 7:3 w:w mixture of butyl acrylate and styrene (total volume 1084 uL) was heated at 80°C for 10 min, followed by pre-emulsification in deionized water (4.0 mL) with simultaneous addition of the desired amount of surfactant (10–100 mg, 1–10 weight %) with rapid stirring. After 30 min, K2S2O8 (10 mg, 1 weight %), was added to the homogeneous emulsion to induce polymerization. The mixture was then stirred for 6 hours at 80°C and cooled to rt prior to purification as described below.
Purification of the emulsions
We subjected each of the freshly-prepared emulsions to our previously described protocol.7 Briefly, 1.0 mL of the above emulsion was initially centrifuged using an Eppendorf Centrifuge 5415 D at 13.2K rpm during 30 minutes in a 2.0 ml Eppendorf Safe-Lock centrifugation tube, then the centrifugate was dialyzed for 24 h in a 3″ section of 50K Spectra/Por® dialysis tubing (Sigma) in 800 mL of DI water. The water was changed after 2 h, 4h, 6h, and 12 h. The contents of the dialysis bag were then transferred into a 2.0 ml Eppendorf Safe-Lock centrifugation tube and centrifuged at 13.2K rpm for 10 min. prior to physical analysis.
Measuring the solid content of the emulsions
The % solid content of each nanoparticle emulsion was determined by freeze-drying a weighed, 2 ml volume of purified emulsion (as described above) on a Virtix Sentra Freezemobile 12XL instrument for 24 h, and the dried residue was then carefully weighed on a Sartorius CP124S balance. The weight % was calculated by dividing the dried weight by the initial weight of the emulsion, and multiplying by 100.
Dynamic light scattering analysis of the emulsions
Particle size analysis of the emulsions was determined using an UPA 150 Honeywell MicroTrac instrument, after diluting the emulsions with deionized water to about 100 μg/ml. Analysis was performed in triplicate (180 seconds per run per sample). Determination of the particle size was calculated directly by the instrument with the respective standard deviation value. Zeta potential measurements were likewise done in triplicate by micro electrophoresis on a Brookhaven ZetaPALS instrument. For these measurements, the emulsion was first diluted to 1.5% of its initial solid content (20%). For each sample, 2 ×10 runs were performed and averaged.
Assay of in vitro microbiological activity of the nanoparticle emulsions against MRSA
The minimum inhibitory concentration (MIC) of each emulsion was determined in broth by serial dilution; according to NCCLS protocols.15 The test medium was prepared in 100 mm glass test tubes by adding the test emulsion to the appropriate volume of Mueller-Hinton broth. The total volume in each tube was reduced to 1.0 ml, wherein each sequential tube contained half the concentration of emulsion. Bacterial cultures of MRSA (ATCC 43300) were grown overnight at 37°C on Trypticase Soy Agar (TSA) plates. The inoculum was then prepared by inoculating the Mueller-Hinton broth with several colonies to just under 0.5 McFarland Standard (~1.5 × 108 cfu/ml). Bacterial cultures were incubated at 37°C for approximately 2 h. The absorbance of each culture was determined at 625 nm, and increasing the incubation time or diluting with broth until the absorbance was equal to 0.08–0.10 adjusted the cultures. The cultures were then diluted 1:100 in Mueller-Hinton broth to reach approximately 1.5 × 106 cfu/ml. The dilution tubes were inoculated with an equal volume of inoculum (1.0 ml), resulting in a final concentration of 5 × 10 cfu/ml. The tubes were incubated at 37°C for 16–20 h. After incubation, the absorbance was read at 625 nm to determine the MIC. The MIC was determined as the lowest concentration of emulsion (0 absorbance) that completely inhibited bacterial growth in the tubes.
In vitro cytotoxicity assay of the nanoparticle emulsions
Cytotoxicity was evaluated using human keratinocytes cells, which were grown in Dubelco’s Modified Eagle Medium (DMEM) at 37°C with a 5% CO2 atmosphere for several days until cells were confluent. The cells were harvested and re-suspended in DMEM containing 10% fetal bovine serum (FBS) and 0.1% gentamycin. The cells were counted using a hemocytometer, the total number of cells was determined and the cells were seeded into 96-well plates at 50,000 cells per well. Each well contained 150 μl DMEM with 10% FBS and 0.1% gentamycin. Cells were allowed to grow for 4–6 hours prior to treatment with the nanoparticle emulsions. The emulsions being assayed were added directly to the media in each well at the following dilutions (volume of emulsion to volume of media): 1:150, 1:125, 1:100, 1:75, 1:50 and 1:25. Testing of each emulsion at each concentration was performed in triplicate. On each 96-well plate, three wells were left untreated for use in calculating the 100% absorbance value. The plates were then incubated for 48 hours and observed under the microscope at various time points. A 5 mg/ml solution of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) in phosphate buffered saline (PBS) was prepared and 15 μl (10% of the total culture volume) was added to each well except those designated as instrument blanks. The plates were incubated for 4 hours to allow sufficient time for the conversion of the MTT dye (yellow liquid) to the water-insoluble formazan derivative, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (purple solid) by the mitochondrial dehydrogenases in the living cells. After incubation, purple crystals were observed and the media was removed from each well by aspiration. The crystals were then dissolved by adding 100 μl of dimethylsulfoxide (DMSO) to each well. DMSO was also added to the wells designated as reference blanks. Viable cell count was determined spectrophotometrically using a microplate reader by measuring the absorbance at two discrete wavelengths (595 and 630 nm). For each emulsion at each concentration, the absorbance values were averaged and the percent cell viability was determined as a percentage of the average absorbance obtained from the untreated cells.
Results
Each of the freshly-prepared (raw) emulsions obtained with the different surfactants were evaluated in terms of their physical properties (size, surface charge) and in vitro cytotoxicities using human keratinocytes cells cultures due to their high sensitivity to the surfactant’s toxic effects as observed from previous studies.7 Samples were also purified by centrifugation of the crude emulsions to remove residual sedimentation followed by dialysis into water using a 50 K molecular weight cut-off membrane tubing to remove small molecule contaminants.7 This is the same procedure that we reported previously for purification of SDS-stabilized polyacrylate emulsions, which removes most of the contaminants that give rise to bactericidal and cytotoxic behavior.7 Both the freshly-prepared (raw) and purified emulsions were analyzed by dynamic light scattering (DLS) to compare average particle size, size distribution, and surface charge concentration (zeta potential) for each sample. Figure 1 shows the average particle size of the nanoparticles in the emulsions as a function of surfactant used in the emulsion polymerization. In each case, 3 weight % of the surfactant was used for the reaction. The smallest particles, measuring around 40 nm, were obtained for the anionic surfactant SDS, while the largest particles (around 400 nm) were formed in emulsions made with the zwitterionic (3) and nonionic (amphiphilic) surfactants (4). The particle size distributions and zeta potential values were also analyzed after purification of the emulsions (Figure 1). Purification does remove large colloidal precipitates in the emulsions prepared from the surfactants 2–4, as reflected by the diminished particle sizes following purification. The zeta potential values of these emulsions, a measure of surface charge and particle stability, do not change appreciably upon purification for emulsions prepared from different surfactants 1–4 (Table 1).
Table 1.
Average particle sizes and zeta potential values of poly(butyl acrylate-styrene) nanoparticle emulsions prepared using surfactants, as measured by dynamic light scattering before and after purification.
| Surfactant | Particle size (nm) | Zeta potential (eV) | ||
|---|---|---|---|---|
| Unpurified | Purified | Unpurified | Purified | |
| Anionic | ||||
| Conventional (1) | 41 | 42 | −97 | −80 |
| Surfmer (5) | 120 | 125 | −50 | −49 |
| Cationic | ||||
| Conventional (2) | 128 | 54 | 48 | 42 |
| Surfmer (6) | 39 | nt | 3 | nt |
| Zwitterionic | ||||
| Conventional (3) | 118 | 85 | −46 | −49 |
| Uncharged | ||||
| Conventional (4) | 408 | 332 | −8 | 2 |
| Surfmer (9) | 552 | 561 | 3 | −2 |
Next, we investigated whether attaching the surfactant agent covalently to the polymeric chain of the nanoparticle could alter stability or toxicity of the emulsions. The surfactant studied in this case carry an acrylate moiety for attachment to the polymer matrix during emulsion polymerization, as described for SDS. In general, we observed that the emulsions prepared with conventional surfactants are visibly clearer than those prepared with the acrylated surfactant monomers (surfmers), and are also more stable in that they do not settle out or coagulate over time. This may be related to particle size, in that the average diameter of the nanoparticles in the emulsion increases when a polymerizable (attachable) surfmer is used in place of a conventional surfactant. For instance, while SDS-stabilized emulsions show average particle sizes around 40 nm, the corresponding emulsion made with anionic surfmer 5 contained nanoparticles around 110 nm in diameter. Purification did not alter the size distribution in this case. For the non-ionic surfactant systems, the emulsions were much milkier and show average particle sizes of over 300 nm (after purification) for conventional agent 4 and over 500 nm for the surfmer variant. A plausible explanation for this size difference could be related with the grade of mobility that the surfactant has to reorganize to form the micelle. The covalently-bound surfactant molecule loses its freedom because of its linkage to the polymer backbone, increasing the physical distance between charged surfactant monomers during the formation of the nanoparticle. For the non-charged agents, where the surfmer gives smaller particles, the absence of charge on the surface and hydrophobic effects between surfactant molecules could account for the reduction in overall particle dimensions. This effect has previously been observed for reversible-addition-fragmentation chain transfer (RAFT) emulsion polymerizations of methyl methacrylate.10 As expected, when anionic surfactants such as SDS (1) and surfmer 5 are employed, the nanoparticle surface is negatively charged, while positive zeta potential values are obtained for emulsions made with the cationic surfactants 2, 6, and 7 even though their net values are diminished due to anionic sulfate from the persulfate initiation step (Table 1). The zeta potential of the emulsions prepared from a conventional surfactant is generally larger than that of its surfmer analogue, which may be due to the surfactant molecule mobility being reduced when the surfactant is covalently attached to the polymer backbone, lowering the electrical potential between the Stern layer and the diffuse layer. As expected, emulsions prepared with non-ionic surfactants 4 and 9 exhibit low surface charge.
Although the emulsification process requires a tensoactive component13,14 to ensure that the mixture is homogeneous and stable, surfactants are known to have inherent antimicrobial and cytotoxic properties at elevated levels due to their powerful detergent effects on cellular membranes.16–29 For assessing drug delivery capabilities of surfactant-stabilized nanoparticles, it is important to reduce this undesired property as much as possible to ensure that any observed antibacterial activity is due to the drug itself, not to the delivery vehicle. The toxicity associated with surface-active agents such as SDS and other commonly used surfactants (in the absence of nanoparticles) has been widely studied in various eukaryotic cell lines by a number of different methods, such as fathead minnow-sp19, human fibroblasts16–21, epithelial cells22, keratinocytes23–26, gingival cells27, and by measuring hemolytic activity.28 Furthermore, our preliminary experiments found elevated cytotoxicity for the emulsions prepared using more the 3 weight % of SDS, and that this toxicity could be significantly reduced by removing excess (unassociated) SDS by purification.7 Investigating this further, we examined each of the emulsions prepared with surfactants 1–4 for in vitro antibacterial properties and cytotoxicity.
Table 2 summarizes the minimum inhibitory concentrations for emulsions prepared from surfactants 1–4 that we obtained in testing against MRSA (ATCC 43300), using 3–7 weight % of surfactant in the emulsion polymerization of butyl acrylate and styrene (7:3 w:w). These results confirm that all of the surfactant types, except for the non-ionic surfactants (4 and 9), are cidal to MRSA, suggesting that the stronger detergent activity of the charged, non-associated surfactants can induce lysis of the cellular membrane. Interestingly, our data shows no discernible difference in microbiological efficacies between the non-covalently attached (conventional) surfactants and the polymerizable surfmers.
Table 2.
Broth MIC values for polyacrylate emulsions prepared with different surfactants, tested against MRSA either before or after purification.
| Surfactant used to prepare poly(butyl acrylate-styrene) nanoparticle emulsions | Minimum inhibitory concentration (μg/ml) against MRSA (ATCC 43300) |
|
|---|---|---|
| Unpurified emulsion | Purified emulsion | |
| Anionic (1) | 64 | 128 |
| Cationic (2) | 4 | 8 |
| Zwitterionic (3) | 128 | 256 |
| Nonionic (4) | 256 | 256 |
| Anionic surfmer (5) | 64 | 128 |
| Cationic surfmer (6) | 16 | 32 |
| Nonionic surfmer (9) | 256 | 256 |
In follow-up to this, we investigated the effects of purification of the emulsions (by centrifugation and dialysis, as previously described) on their in vitro antibacterial activities. Our objective was to determine if surfactant and residual impurities from the polymerization process in the bulk media were causing bacteriocidal effects, and whether this could be obviated by purification as we reported previously for SDS-stabilized emulsions. Table 2 gives the observed minimum inhibitory concentration values for each emulsion prepared from surfactants 1–9, before and after purification, using a representative MRSA strain as a test microorganism.
From Table 2, it is clear that bioactivities vary greatly depending on the type of surfactant used, its charge, as well as whether the emulsions are purified or not. The smaller the MIC values, the greater the bactericidal effect. The cationic systems (made from 2 and 6) have the strongest cidal effects on these bacteria, much more so than anionic or zwitterionic systems, while no microbiological activity is observed for the emulsions from nonionic surfactants 4 and 9 even in the absence of purification. Furthermore, it is noted that purification does significantly reduce antimicrobial activity of emulsions prepared from charged surfactants, which we attribute to the removal of excess (toxic) surfactant and impurities from the polymerization process. For preparing the emulsions, 3 weight % of SDS or 3–7 weight % of the other surfactants (except for non-ionic surfactants 4 and 9, which were 7 weight %) was used as required to make stable, purifiable emulsions. The non-ionic agents, whether used as conventional or polymerizable surfactants, do not cause toxicity in the resulting emulsions, and thus would be ideal for potential pharmaceutical preparations even though more is needed to form stable emulsions.
The emulsions prepared from surfactants 1–9 were then individually evaluated for cytotoxicity against human keratinocytes. These experiments involved exposing the cultured keratinocytes cells to different dilutions of the emulsions (crude versus purified) on 96-well plates over a 48 hour growth period. Cell viability was determined by MTT assay (Figure 3). What we observed, as anticipated, was that purification of the emulsions prior to biological testing led to an increase in the percentage of viable keratinocyte cells, but only in the case of emulsions prepared from anionic surfactants 1 and 5. For those from cationic surfactants 2, 6, and 7, cell viability was appreciably reduced even at low concentrations, indicative of elevated cellular toxicity. Purification did not improve cell viability. The emulsions obtained from zwitterionic surfactants 3 and 8 were substantially better than those from either anionic or cationic agents, but again, purification did not seem to alter this. However, non-ionic surfactants 4 and 9 gave excellent results that parallel the bacterial MIC data, regardless of whether the samples were purified prior to testing or not.
Figure 3.
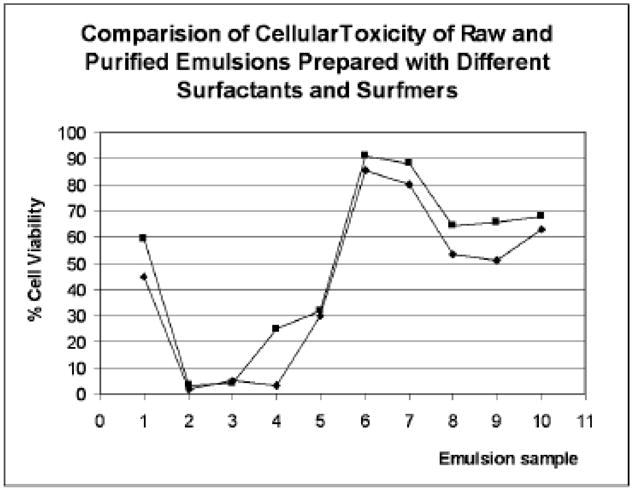
Comparative study of cytotoxicity in raw and purified emulsions prepared with different surfactants tested against MRSA (ATCC 43300) and S. aureus (ATCC 25923) by MIC in broth with 3% of drug (β-lactam 10). (1) 7% Tween 20 and 1% KPS, pH 3.6, raw (blue) and purified (pink), (2) 3% cationic surfactant without drug, raw (blue) and purified (pink), (3) 5% cationic surfactant without drug, raw (blue) and purified (pink), (4) 7% cationic surfmer (hydroxy) without drug, raw (blue) and purified (pink), (5) 3% zwitterionic surfactant without drug, raw (blue) and purified (pink), (6) 3% anionic surfmer with β-lactam 49, raw (blue) and purified (pink), (7) 7% non-ionic valeric surfactant, 1% KPS, with β-lactam 10, raw (blue) and purified (pink), (8) 3% zwitterionic surfactant with β-lactam, raw (blue) and purified (pink), (9) 50% Pluronic F68, (10) 50% Pluronic F 68 with β-lactam 10, raw (blue) and purified (pink).
We have previously reported the preparation of SDS-stabilized polyacrylate emulsions as a means to water-solubilize N-thiolated β-lactams for use as anti-MRSA agents.1 In view of what we have learned in this current study, it became of immediate interest to us to determine whether even a relatively weak antibacterial agent such as lactam 10 (MIC 128 μg/ml against MRSA)1,30 could provide antimicrobial activity to a biologically-innocuous nanoparticle (Figure 4). Thus, having selected the nonionic surfactants as the preferred ones to use to prepare toxicity-free nanoparticles, we embarked on a final experiment in which an antibiotic agent (lactam 10) is introduced into the nanoparticle emulsion. For this, we examined the nonionic surfactants 4 and 9 which we employed at 7 weight % for the emulsion polymerization, and 3 weight % of lactam 10. For both cases, the bioactivity of the nanoparticle emulsions against MRSA improve measurably, from 256 ug/ml for the drug-free samples to 32 ug/mL (when the drug is included).
Figure 4.
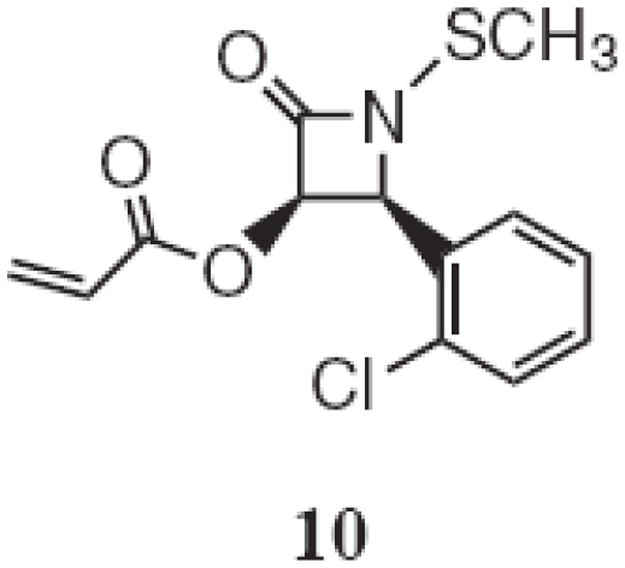
Structure of lactam 10.
These investigations have revealed that polyacrylate nanoparticles can be prepared conveniently by emulsion polymerization using anionic, cationic, zwitterionic, and uncharged surfactant. The resulting size and biological properties of these nanoparticles are highly dependent on the surfactant, with uncharged systems affording the largest particles in the range of 300–400 nm and lowest toxicities to human and microbial cells. By comparison, the anionic, cationic, and zwitterionic nanoparticle emulsions are much smaller, and in general, more cytotoxic than the non-ionic nanoparticle systems. Antibacterial and tissue toxicity experiments indeed indicate that the cationically-charged surfactants produce emulsions that are much more toxic to bacterial and mammalian cells compared to stabilized by anionic, zwitterionic, or uncharged surfactants, even with purification of the samples prior to testing. We have determined in this regard that for the anionically-stabilized nanoparticles, most of the observed toxicity of the crude polyacrylate emulsions can be diminished by a simple purification procedure of benchtop centrifugation and overnight dialysis.
Discussion
In attempts to devise new methodologies for drug delivery, the avoidance or elimination of toxicity associated with emulsion components or intermediates formed during the emulsion polymerization process is a key concern. It is important to understand what, if anything in the emulsion, may cause unwanted toxicity, and if so, how to minimize it by purification or use of a non-toxic substitute. In so doing, we need to know how these alterations may also affect deliverability, loading, targeting, drug release, and cell permeability, which depends keenly on particle size, stability, and surface charge. We found that the use of chemically-attachable (polymerizable) surfactants such as those demonstrated for surfmers 5–9 do not adequately solve the problem. On the contrary, the size of particle increases for the charged surfmers (relative to the conventional surfactants) and the stability of the emulsions are notably affected. On the other hand, surfmers do not seem to introduce additional burdens associated with toxic effects of the nanoparticle emulsion, which bodes well for the design of more advanced variants that may allow for bacterial cell targeting and biodegradability. We also observed from the bacterial MIC and cytotoxicity data that the charge of the surfactant used for the emulsion polymerization affects to a large degree the biological activity of the emulsions. In fact, the cationic surfactant exhibited an unacceptably high level of bacterial growth inhibition and cytotoxicity, which is reflective of the strong interactions the nanoparticles likely have with the anionic cell membrane. Similar activity was observed for the emulsions prepared with zwitterionic surfactants, albeit to a somewhat lesser degree. However, emulsions prepared with the non-ionic surfactants exhibit no inhibitory effect on bacteria (MIC >256 ug/mL) when no drug was incorporated in the final formulation. Using the non-ionic surfactant for the nanoparticle formation, incorporation of the antibiotic into the emulsification lowered significantly the bacterial MICs compared to the drug-free system. This indicates that the microbiological activity comes exclusively from the antibiotic contained within the nanoparticle and not to the nanoparticle itself or other emulsion components.
In summary, these investigations identified a simple procedure for obtaining polyacrylate nanoparticle emulsions with little if any inherent bioactivity or toxicity, either through purification of the emulsions or by choice of a suitable stabilizing surfactant. This enables an accurate assessment of inhibitory effects produced by antibiotic-containing polyacrylate nanoparticles and thus a valid determination of how effective these systems may ultimately be for treatment of bacterial infections.
Acknowledgments
Sources of Support: National Institutes of Health (R01 AI01535) and National Science Foundation (NSF 0419903, NSF 0620572), University of South Florida and the Florida Center of Excellence in Biomolecular Identification and Targeted Therapeutics (for a Graduate Multidisciplinary Scholarship to JG), and the University of South Florida Office of Technology Development for a Florida High Tech Corridor matching grant.
We thank the Florida Center of Excellence in Biomolecular Identification and Targeted Therapeutics for conducting the mammalian cytotoxicity studies.
Footnotes
Conflict of Interest Statement: Edward Turos is co-inventor on a US patent application by the University of South Florida for the polyacrylate nanoparticle antibiotics, the subject of this publication. Dr. Turos is also co-founder, chief scientific advisor, and shareholder of Nanopharma Technologies, Inc., a University of South Florida spin-out company. Nanopharma Technologies, Inc., has licensed the nanoparticles technology from University of South Florida for potential commercial development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julio C. Garay-Jimenez, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry, CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
Danielle Gergeres, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry, CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
Ashley Young, Nanopharma Technologies, Inc., 3802 Spectrum Boulevard, Suite 151, Tampa, FL 33612 USA.
Sonja Dickey, Department of Biology, IDRB 404, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620, USA.
Daniel V. Lim, Department of Biology, IDRB 404, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620, USA.
Edward Turos, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry, CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
References
- 1.Turos E, Shim JY, Wang Y, Greenhalgh K, Reddy GS, Dickey S, et al. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17:53–6. doi: 10.1016/j.bmcl.2006.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeylath S, Turos E. Glycosylated polyacrylate nanoparticles by emulsion polymerization. Carb Polym. 2007;70:32–7. doi: 10.1016/j.carbpol.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turos E, Reddy GSK, Greenhalgh K, Ramaraju P, Abeylath SC, Jang S, et al. Penicillin-bound polyacrylate nanoparticles: Restoring the activity of β-lactam antibiotics against MRSA. Bioorg Med Chem Lett. 2007;17:3468–72. doi: 10.1016/j.bmcl.2007.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeylath S, Turos E, Dickey S, Lim DV. Novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Biorg Med Chem. 2008;16:2412–8. doi: 10.1016/j.bmc.2007.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhalgh K, Turos E. In vivo studies of polyacrylate nanoparticle emulsions for topical and systemic applications. Nanomed. 2008 doi: 10.1016/j.nano.2008.07.004. In press. [DOI] [PubMed] [Google Scholar]
- 6.Abeylath S, Turos E. Drug delivery approaches to overcome bacterial resistance of β-lactam antibiotics. Exp Opin Drug Deliv. 2008;5:931–49. doi: 10.1517/17425247.5.9.931. [DOI] [PubMed] [Google Scholar]
- 7.Garay-Jimenez J, Young A, Gergeres D, Greenhalgh K, Turos E. Methods for purifying and detoxifying sodium dodecyl sulfate stabilized nanoparticles. Nanomed. 2008;4:98–105. doi: 10.1016/j.nano.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoonbrood H, Asua J. Reactive surfactants in heterophase polymerization. Emulsion copolymerization mechanism involving three anionic polymerizable surfactants (surfmers) with styrene-butyl acrylate-acrylic acid. Macromolecules. 1997;30:6024–33. [Google Scholar]
- 9.Unzue M, Schoonbrood H, Asua J, Goni A, Sherrington D, Staehler K, et al. Reactive surfactant in heterophase polymerization. VI. Synthesis and screening of polymerizable surfactants (surfmers) with varying reactivity in high solids styrene-butyl acrylic acid emulsion polymerization. J Appl Polym Sci. 1997;66:1803–20. [Google Scholar]
- 10.Matahwa H, McLeary JB, Sanderson RD. Comparative study of classical surfactants and polymerizable surfactants (surfmers) in the reversible addition-fragmentation chain transfer mediated mini-emulsion polymerization of styrene and methyl methacrylate. J Polym Sci Part A: Polym Chem. 2006;44:427–42. [Google Scholar]
- 11.Brunel S, Chevalier Y, Le Perchec P. Synthesis of new zwitterionic surfactants with improved solubility in water. Tetrahedron. 1989;45:3363–70. [Google Scholar]
- 12.Samakande A, Hartmann PC, Sanderson RD. Synthesis and characterization of new cationic quaternary ammonium polymerizable surfactants. J Colloid Interface Sci. 2006;296:316–23. doi: 10.1016/j.jcis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert RG. Emulsion Polymerization: A Mechanistic Approach. Academic Press; London: 1995. [Google Scholar]
- 14.Paleos CM, Malliaris A. Polymerization of micelle-forming surfactants. JMS Rev Macromol Chem Phys. 1998;C28:403–18. [Google Scholar]
- 15.NCCLS (National Committee for Clinical Laboratory Standards) Methods for Dilution of Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. NCCLS Document M7-A4, Vol. 17, No. 2, 1997.
- 16.Verhulst C, Coiffard C, Coiffard L, Rivalland P, de Roeck-Holzhauer Y. In vitro correlation between two colorimetric assays and the pyruvic acid consumption by fibroblasts cultured to determine the sodium lauryl sulfate cytotoxicity. J Pharmacol Toxicol Methods. 1998;39:143–6. doi: 10.1016/s1056-8719(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 17.Mueller R, Ruehl D, Runge S, Schulze-Forster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharma Res. 1997;14:458–62. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- 18.Craig S, Newby C, Barr R, Greaves M, Mallet A. Cytokine release and cytotoxicity in human keratinocytes and fibroblast induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. 2000;115:292–8. doi: 10.1046/j.1523-1747.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 19.Mori M, Kawakubo N, Wakabayashi M. Cytotoxicity of surfactants to the FHM-sp cell line. Fisheries Sci. 2002;68:1124–8. [Google Scholar]
- 20.Arechabala B, Coiffard C, Rivalland P, Coiffard LJ, de Roeck-Holtzhauer Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J Appl Toxicol. 1999;19:163–5. doi: 10.1002/(sici)1099-1263(199905/06)19:3<163::aid-jat561>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Newby C, Barr R, Greaves M, Mallet A. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. 2000;115:292–8. doi: 10.1046/j.1523-1747.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 22.Elmore E, Siddiqui S, Desai N, Moyer M, Steele E, Redpath L. The human epithelial cell cytotoxicity assay for determining tissue specific toxicity: method modifications. Meth Cell Sci. 2002;24:145–53. doi: 10.1023/a:1024453300493. [DOI] [PubMed] [Google Scholar]
- 23.Wei T, Geijer S, Lindberg M, Berne B, Törmä H. Detergents with different chemical properties induce variable degree of cytotoxicity and mRNA expression of lipid-metabolizing enzymes and differentiation markers in cultured keratinocytes. Toxicol in vitro. 2006;20:1387–94. doi: 10.1016/j.tiv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Varani J, Astrom A, Griffiths C, Voorhees J. Induction of proliferation of growth-inhibited keratinocytes and fibroblasts in monolayer culture by sodium lauryl sulfate: comparison with all-trans retinoic acid. J Invest Dermatol. 1991;97:917–21. doi: 10.1111/1523-1747.ep12491682. [DOI] [PubMed] [Google Scholar]
- 25.Törmä H, Geijer S, Gester T, Alpholm K, Berne B, Lindberg M. Variations in the mRNA expression of inflammatory mediators, markers of differentiation and lipid-metabolizing enzymes caused by sodium lauryl sulphate in cultured human keratinocytes. Toxicol in vitro. 2006;20:472–9. doi: 10.1016/j.tiv.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Van Ruissen F, Le M, Carroll J, van der Valk P, Schalkwijk J. Differential effects of detergents on keratinocyte gene expression. J Invest Dermatol. 1998;110:358–63. doi: 10.1046/j.1523-1747.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 27.Babich H, Babich J. Sodium lauryl sulfate and triclosan: in vitro cytotoxicity studies in gingival cells. Toxicol Lett. 1997;91:189–96. doi: 10.1016/s0378-4274(97)00022-2. [DOI] [PubMed] [Google Scholar]
- 28.Gould L, Lansley A, Brown M, Forbes B, Martin G. Mitigation of surfactant erythrocyte toxicity by egg phosphatidylcholine. J Pharma Pharmacol. 2000;52:1203–9. doi: 10.1211/0022357001777333. [DOI] [PubMed] [Google Scholar]
- 29.Brown D, Lydon J, McLaughlin M, Stuart-Tilley AA, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS) Histochem Cell Biol. 1996;105:261–7. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 30.Turos E, Coates C, Shim J-Y, Wang Y, Leslie M, Long TE, et al. N-Methylthio β-lactam antibacterials: effects of the C3/C4 ring substituents on anti-MRSA activity. Bioorg Med Chem. 2005;13:6289–308. doi: 10.1016/j.bmc.2005.08.011. [DOI] [PubMed] [Google Scholar]


