Abstract
Background
Ventilatory efficiency, right ventricular (RV) function, and secondary pulmonary hypertension are each prognostic indicators in patients with heart failure due to left ventricular systolic dysfunction, but the relationships among these variables have not been comprehensively investigated. In this study, we hypothesized that inefficient ventilation during exercise, as defined by an abnormally steep relationship between ventilation and carbon dioxide output (VE/VCO2 slope), may be a marker of secondary pulmonary hypertension and RV dysfunction in heart failure.
Methods and Results
A cohort of patients with systolic heart failure (mean±SD age, 58±13 years; left ventricular ejection fraction, 0.27±0.05; peak oxygen uptake, 11.2±3.2 mL kg−1 min−1) underwent incremental cardiopulmonary exercise testing with simultaneous hemodynamic monitoring and first-pass radionuclide ventriculography before and after 12 weeks of treatment with sildenafil, a selective pulmonary vasodilator, or placebo. VE/VCO2 slope was positively related to rest and exercise pulmonary vascular resistance (R=0.39 and R=0.60, respectively) and rest pulmonary capillary wedge pressure (R=0.49, P<0.005 for all) and weakly indirectly related to peak exercise RV ejection fraction (R=−0.29, P=0.03). Over the 12-week study period, VE/VCO2 slope fell 8±3% (P=0.02) with sildenafil and was unchanged with placebo. Changes in VE/VCO2 slope correlated with changes in exercise pulmonary vascular resistance (R=0.69, P<0.001) and rest and exercise RV ejection fraction (R=−0.58 and −0.40, respectively, both P<0.05).
Conclusions
In patients with systolic heart failure and secondary pulmonary hypertension, ventilatory efficiency is closely related to RV function and pulmonary vascular tone during exercise.
Keywords: heart failure, exercise, ventilatory efficiency
The relationship between minute ventilation and the rate of CO2 elimination during incremental exercise (VE/VCO2 slope) is a noninvasive, reproducible measurement that is strongly related to mortality and the need for cardiovascular hospitalization in heart failure (HF) patients.1–5 However, the pathophysiologic mechanisms underlying impaired ventilatory efficiency are incompletely understood.
According to the alveolar ventilation equation, VE/VCO2 is determined by 2 variables: dead space ventilation relative to tidal volume (VD/VT) and arterial PaCO2.6 In patients with HF, inefficient ventilation (as indicated by high VE/VCO2 slope) has been ascribed to the increased ventilation required to overcome a large dead space and an increased central drive to ventilation, which results in lowering of arterial partial pressure of carbon dioxide (PaCO2).6,7 Although VD/VT is higher in patients with worsening HF, there are conflicting reports as to the degree to which increased ventilatory drive (independent of higher dead space fraction) lowers PaCO2 in patients with HF.8,9 High VD/VT in the absence of concomitant primary lung disease reflects ventilation-perfusion (V/Q) mismatch characteristic of pulmonary hypertension (PH).7
Secondary PH is present in 68% to 76% of patients with chronic left ventricular systolic dysfunction and is associated with right ventricular (RV) dysfunction and poor prognosis.10–12 The development of secondary PH in HF, due to dysregulation of pulmonary vascular tone and pulmonary vascular remodeling, may contribute to inefficient ventilation through V/Q mismatch. Previous studies of the relationship between pulmonary hemodynamics and ventilatory response to exercise have been limited by reliance on resting hemodynamics alone,13 investigation of HF patients with normal pulmonary vascular resistance,14 or reliance on instantaneous measurements of ventilation and CO2 output,15 rather than the slope of this relationship during exercise.
Sildenafil is a selective inhibitor of type 5 phosphodiesterase, the predominant phosphodiesterase isoform responsible for hydrolysis of intracellular cGMP in the pulmonary vasculature.16 Chronic sildenafil administration improves exercise capacity and decreases pulmonary vascular resistance (PVR) at rest in patients with pulmonary arterial hypertension and normal LV function.17 In patients with chronic left ventricular systolic dysfunction and secondary PH, our laboratory and others have recently shown that both one-time administration and chronic treatment with sildenafil improves exercise capacity (peak VO2).18–21 In our previous studies, the use of invasive hemodynamics during exercise demonstrated that improvement in exercise capacity with sildenafil was proportionate to reduction in exercise PVR.20,21 In this study, we used data from our 12-week randomized, double-blind placebo controlled study of sildenafil for the treatment of HF with secondary PH20 to characterize the relationship between pulmonary vasomotor tone and ventilatory efficiency. We measured VE/VCO2 slope and related it to rest and exercise pulmonary hemodynamics and RV myocardial function in a cohort of patients with systolic HF who underwent repeated cardiopulmonary exercise testing (CPET) before and after 12 weeks of treatment with the selective pulmonary vasodilator sildenafil or placebo.
Methods
Study Design
Patients referred to the Massachusetts General Hospital Heart Failure Center 18 years of age or older with left ventricular ejection fraction (LVEF) <0.40, secondary PH (mean pulmonary arterial pressure >25 mm Hg), and New York Heart Association class II to IV chronic HF despite standard therapy were eligible for study, as previously described.20 Subjects were randomized to an initial dose of 25 mg of sildenafil or placebo administered 3 times daily, titrated every 2 weeks to a maximum dose of 75 mg 3 times a day as tolerated.
Cardiopulmonary Exercise Testing
Patients underwent maximum incremental CPET (MedGraphics, St. Paul, Minn) with simultaneous hemodynamic monitoring (Witt Biomedical Inc, Melbourne, Fla) before study medication administration. CPET was repeated on completion of the 12-week randomized trial. Right atrial pressure, mean pulmonary arterial pressure, and pulmonary capillary wedge pressure (PCWP) were measured in the upright position while patients were seated on the bicycle, and cardiac output was determined at 1-minute intervals throughout exercise using the Fick oxygen technique. PVR was calculated using standard formulas. VE/VCO2 slope was determined via linear regression of VE versus VCO2 from onset of to peak exercise,22 as measured breath-by-breath and averaged every 30 seconds, with exclusion of extreme outliers. First-pass radionuclide ventriculography was performed at rest and at peak exercise to measure LVEF and RV ejection fraction (RVEF) as previously described.20
Statistical Methods
The STATA 10.0 software package (StataCorp LP, College Station, Tex) was used for statistical analysis. The sildenafil and placebo cohorts were pooled for correlation analysis and Pearson correlation coefficients are reported. Relationships between hemodynamic parameters and ventilatory efficiency were assessed by linear regression analysis. One-way ANOVA was used to assess the effect of treatment on differences in the change in continuous variables measured at baseline and at 12 weeks of study drug treatment. Reproducibility of ventilatory efficiency measurements was assessed by determining the intraclass correlation coefficient for repeated measurements at 0 and 12 weeks. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline clinical characteristics for the 30 subjects in this New York Heart Association class II to IV HF cohort are reported in Table 1. Subjects in the active treatment group were receiving 49±19 mg of sildenafil 3 times daily on completion of 12 weeks of study. Results of hemodynamic measurements, radionuclide ventriculography, and ventilatory gas exchange at rest and at peak exercise are displayed in Table 2. All patients surpassed their anaerobic thresholds and achieved respiratory exchange ratios in excess of 1.0, consistent with maximum effort during exercise (data not shown). The intraclass correlation coefficient between repeated VE/VCO2 slope measurements in the placebo group was 0.84 (95% CI, 0.7 to 0.99, P<0.0001), indicating excellent reproducibility of this measurement.
Table 1.
Clinical Characteristics of the Study Population
| Baseline Characteristic | N=30 |
|---|---|
| Demographics | |
| Age, years | 58±13 |
| Female | 3 (10) |
| White | 27 (90) |
| Etiology of heart failure | |
| Ischemic | 15 (50) |
| Nonischemic | 15 (50) |
| NYHA class | |
| II | 16 (53) |
| III | 11 (37) |
| IV | 3 (10) |
| Medical and device therapy | |
| β-blocker | 29 (97) |
| ACEI or ARB | 25 (83) |
| Aldosterone antagonist | 17 (57) |
| Diuretics | 30 (100) |
| Digoxin | 22 (73) |
| Cardiac resynchronization therapy | 8 (27) |
| Pulmonary function testing | |
| FEV1, % predicted | 66±20 |
| FVC, % predicted | 74±20 |
| DLCO, % predicted | 64±19 |
Data are presented as mean±SD for continuous variables and n (%) for dichotomous variables.
NYHA indicates New York Heart Association; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing limit for carbon monoxide.
Table 2.
Hemodynamic Values Measured in the Upright Position at Rest and During Exercise Before and After 12 Weeks of Treatment With Sildenafil or Placebo
| Placebo |
Sildenafil |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Rest | Week 12 Rest | Baseline Exercise | Week 12 Exercise | Baseline Rest | Week 12 Rest | Baseline Exercise | Week 12 Exercise | |
| Heart rate, min −1 | 70 ± 7 | 73 ± 14 | 103 ± 11 | 105 ± 25 | 74 ± 11 | 78 ± 14 | 112 ± 17 | 120 ± 22 |
| MAP, mm Hg | 84 ± 14 | 82 ± 11 | 98 ± 15 | 94 ± 22 | 76 ± 22 | 78 ± 22 | 87 ± 11 | 91 ± 11 |
| Right atrial pressure, mm Hg | 8 ± 7 | 7 ± 4 | 16 ± 7 | 16 ± 7 | 6 ± 4 | 6 ± 4 | 16 ± 7 | 16 ± 7 |
| Mean PAP, mm Hg | 33 ± 11 | 31 ± 11 | 50 ± 11 | 50 ± 14 | 30 ± 7 | 28 ± 7 | 48 ± 7 | 45 ± 7 |
| PCWP, mm Hg | 19 ± 7 | 19 ± 7 | 30 ± 7 | 30 ± 7 | 18 ± 7 | 18 ± 7 | 28 ± 7 | 28 ± 7 |
| PVR, dyne/s per cm −5 | 360 ± 290 | 340 ± 05 | 300 ± 160 | 320 ± 194 | 340 ± 142 | 280 ± 146* | 250 ± 110 | 180 ± 58* |
| SVR, dyne/s per cm −5 | 1930 ± 900 | 2020 ± 790 | 1190 ± 370 | 1160 ± 470 | 2130 ± 710 | 2020 ± 630 | 850 ± 170 | 770 ± 172 |
| LV ejection fraction, % | 28 ± 4 | 28 ± 7 | 32 ± 7 | 32 ± 7 | 26 ± 4 | 27 ± 7 | 28 ± 4 | 28 ± 7 |
| RV ejection fraction, % | 35 ± 7 | 37 ± 11 | 34 ± 7 | 37 ± 11 | 33 ± 11 | 37 ± 11 | 34 ± 11 | 35 ± 11 |
| Peak VO2, mL/kg per min | 10.2 ± 2.8 | 9.9 ± 3.2 | 12.2 ± 2.3 | 13.9 ± 3.6* | ||||
| VE/VCO2 slope | 40.6 ± 9.6 | 41.4 ± 14.0 | 45.6 ± 10.7 | 41.6 ± 8.9 | ||||
| VD/VT | 0.46 ± 0.04 | 0.48 ± 0.04 | 0.39 ± 0.07 | 0.41 ± 0.07 | 0.46 ± 0.07 | 0.44 ± 0.07 | 0.39 ± 0.11 | 0.37 ± 0.07 |
| pH | 7.46 ± 0.04 | 7.46 ± 0.04 | 7.45 ± 0.04 | 7.45 ± 0.04 | 7.45 ± 0.04 | 7.45 ± 0.04 | 7.45 ± 0.04 | 7.43 ± 0.04 |
| PaO2, mm Hg | 89 ± 14 | 93 ± 14 | 92 ± 14 | 89 ± 11 | 94 ± 14 | 92 ± 11 | 93 ± 24 | 87 ± 18 |
| PaCO2, mm Hg | 38 ± 7 | 39 ± 7 | 36 ± 7 | 37 ± 7 | 37 ± 4 | 36 ± 4 | 34 ± 4 | 33 ± 4 |
| C(a–v)O2, mL O2/dL | 8.88 ± 2.11 | 9.23 ± 2.10 | 13.9 ± 2.9 | 13.5 ± 2.4 | 8.47 ± 2.4 | 7.67 ± 1.4 | 14.5 ± 2.9 | 13.6 ± 2.9 |
Data are presented as mean±SD. MAP indicates mean arterial pressure; PAP, pulmonary arterial pressures; C(a–v)O2, difference in oxygen content between arterial and venous blood.
P<0.05 for comparison of baseline with week 12 measurements between groups by 1-way ANOVA.
Correlations between ventilatory efficiency (VE/VCO2 slope), right heart hemodynamics, ventricular function, and gas exchange parameters are shown in Table 3. VE/VCO2 slope was weakly correlated with resting hemodynamic indices of PH, including pulmonary arterial pressure and PVR. VE/VCO2 slope did not correlate with LVEF or systemic vascular resistance but did correlate with PCWP (Table 3). Higher VE/VCO2 slope correlated with a lower arterial PaCO2 (R = −0.56, P<0.001) and higher dead space fraction (VD/VT; R=0.26, P=0.048) at rest, consistent with increased ventilatory drive and increased ventilation perfusion mismatch, respectively.
Table 3.
Coefficients of the Correlations of Hemodynamic, Ventricular Function, and Ventilatory Gas Exchange Parameters at Rest and at Peak Exercise With VE/VCO2 Slope Measured Throughout Exercise and the Change in VE/VCO2 Slope Between 0 and 12 Weeks
| VE/VCO2 Slope Correlation With Parameters |
Change in VE/VCO2 Slope Correlation With Parameters |
|||
|---|---|---|---|---|
| Parameter | At Rest | At Peak Exercise | At Rest | At Peak Exercise |
| Hemodynamic | ||||
| Cardiac index | −0.26 (0.05) | −0.32 (0.01) | −0.29 (0.12) | −0.43 (0.02) |
| Mean right atrial pressure | −0.05 (0.72) | −0.07 (0.59) | 0.12 (0.51) | 0.28 (0.14) |
| Mean pulmonary arterial pressure | 0.52 (<0.001) | 0.30 (0.02) | 0.54 (0.002) | 0.47 (0.01) |
| Mean PCW pressure | 0.43 (<0.001) | −0.07 (0.61) | 0.51 (0.004) | 0.06 (0.77) |
| Pulmonary vascular resistance | 0.39 (0.002) | 0.60 (<0.001) | 0.31 (0.10) | 0.69 (<0.001) |
| Systemic vascular resistance | 0.24 (0.06) | 0.30 (0.02) | 0.24 (0.21) | 0.30 (0.11) |
| Ventricular function | ||||
| RVEF | −0.20 (0.12) | −0.29 (0.03) | −0.58 (<0.001) | −0.40 (0.03) |
| LVEF | −0.07 (0.63) | −0.15 (0.28) | −0.21 (0.28) | −0.08 (0.66) |
| Ventilatory gas exchange | ||||
| PaCO2 | −0.56 (<0.001) | −0.69 (<0.001) | −0.24 (0.23) | −0.46 (0.01) |
| VD/VT | 0.26 (0.048) | 0.42 (0.001) | 0.04 (0.84) | −0.57 (0.002) |
| PaO2 | 0.23 (0.08) | −0.01 (0.93) | −0.06 (0.76) | 0.06 (0.76) |
| pH | 0.26 (0.05) | 0.30 (0.02) | −0.19 (0.33) | −0.17 (0.38) |
| C(a-v)O2 | 0.20 (0.13) | 0.01 (0.96) | 0.30 (0.11) | 0.03 (0.86) |
Data are presented as R (P value).
PCW indicates pulmonary capillary wedge, VD/VT, fractional dead space volume relative to tidal volume.
PVR and RVEF at peak exercise were more closely correlated with VE/VCO2 slope than at rest (Table 3). Left ventricular function at peak exercise remained unrelated to VE/VCO2 slope. To examine the relationship between pulmonary vascular tone during exercise and ventilatory efficiency further, we assessed the correlation between exercise PVR and the determinants of VE/VCO2 slope (arterial PaCO2 and VD/VT).6 Peak exercise PVR was related to both exercise VD/VT (R=0.29, P=0.03) and exercise arterial PaCO2 (R = −0.31, P=0.02).
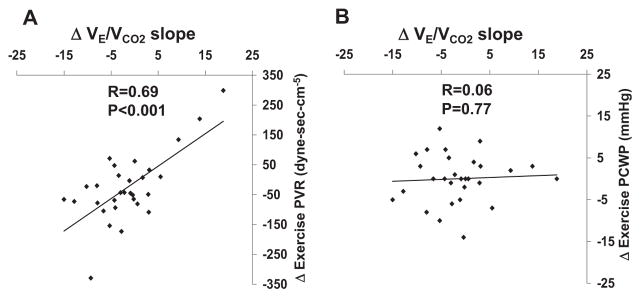
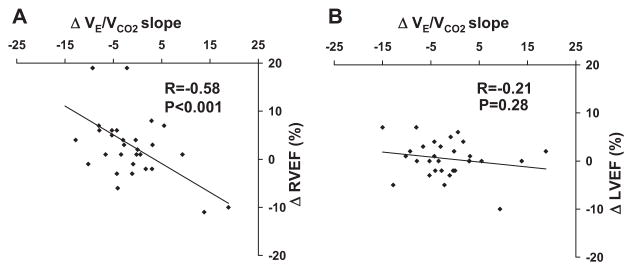
We examined whether changes in VE/VCO2 slope over the 12-week study period tracked with changes in right heart hemodynamics, ventricular function, and ventilatory parameters (Table 3). Over the 12-week study period VE/VCO2 slope fell 8±3% (P=0.02) with sildenafil and was unchanged with placebo. Between group analysis indicated a trend toward a reduction in VE/VCO2 slope in the sildenafil group (45.6±10.7 to 41.6±8.9) compared with placebo group (40.6±9.6 to 41.4±14, P=0.07). Changes in VE/VCO2 slope over 12 weeks were highly correlated with changes in exercise PVR (R=0.69, P<0.001; Figure 1), but did not correlate with changes in exercise PCWP (Figure 1) or systemic vascular resistance (Table 3). There was a significant relationship between change in VE/VCO2 slope and change in resting PCWP (R=0.51, P=0.004) suggesting that a higher starting point for PCWP at rest may influence pulmonary vascular reactivity and ventilatory efficiency during exercise. Changes in VE/VCO2 slope were inversely correlated with changes in RVEF as measured at rest (R= −0.58, P<0.001; Figure 2) and during exercise (R= −0.4, P=0.03). Finally, changes in VE/VCO2 slope were directly related to changes in dead space fraction (VD/VT; R=0.57, P=0.002) and indirectly related to changes in PaCO2 (R= −0.46, P=0.01). Adjustment for treatment group did not alter the observed relationships between VE/VCO2 slope, hemodynamic, radionuclide, and gas exchange variables.
Figure 1.
Change in VE/VCO2 slope from week 0 to week 12 versus change in PVR (A) and PCWP (B).
Figure 2.
Change in VE/VCO2 slope from week 0 to week 12 versus change in RVEF (A) and LVEF (B).
Previous studies have reported VE/VCO2 slope values including exercise data only up to the anaerobic threshold (pre-AT) to account for potential acidosis-induced dislinearity in the VE/VCO2 relationship beyond the AT. In our cohort pre-AT VE/VCO2 slope values were predictably slightly lower than values derived from the entire duration of exercise (pre-AT VE/VCO2 slope, 40.1±12.4; all-inclusive VE/VCO2 slope, 42.3±11.7; P=0.002). However, pre-AT VE/VCO2 slope values were highly correlated with values for all- inclusive VE/VCO2 slope (R >0.9, P<0.0001). Hence substitution of pre-AT VE/VCO2 slope for all-inclusive VE/VCO2 slope did not significantly alter any of the reported relationships between VE/VCO2 slope and hemodynamic, ventriculo-graphic, and gas exchange variables.
Discussion
To investigate the pathophysiologic mechanisms underlying abnormal VE/VCO2 slope in HF, we simultaneously assessed ventilatory parameters, invasive hemodynamics, and myocardial function at rest and during exercise. We observed a significant correlation between VE/VCO2 slope and pulmonary vascular tone as assessed by exercise PVR and a negative correlation between VE/VCO2 slope and exercise RVEF, neither of which has previously been reported. In contrast, there was no relationship between VE/VCO2 slope and either systemic vascular tone or LVEF. Serial analysis of VE/VCO2 slope over the 12-week study period indicated that the magnitude of improvement in ventilatory efficiency was directly related to the magnitude of improvement in resting and exercise PVR and RVEF.
Our findings extend results from previous studies that have coupled invasive hemodynamics with measurement of VE/VCO2 slope. Reindl et al13 demonstrated a positive correlation between resting pulmonary arterial pressure, PCWP, PVR, and VE/VCO2 slope, all of which were corroborated by our study. However, Reindl et al did not perform hemodynamic monitoring during exercise, whereby our study identified a stronger relationship between exercise PVR and VE/VCO2 slope and no significant relationship between exercise PCWP and VE/VCO2 slope. In contrast to our findings, Metra et al14 did not find a significant correlation between rest or exercise PVR and VE/VCO2 slope. The likely explanation for the discrepancy between these findings and ours is that Metra et al studied patients with less severe HF and relatively normal rest and exercise PVR (140±73 and 102±62 dyne/s per cm−5, respectively) compared with our study in which patients had more advanced HF and a greater degree of pulmonary vasoconstriction and remodeling (rest and exercise PVR, 347±218 and 262±155 dyne/s per cm−5, respectively).
Our results also suggest an important relationship between ventilatory efficiency and RV performance during exercise. Previous studies in patients with LV systolic dysfunction and secondary PH have demonstrated that acute administration of inhaled nitric oxide (a direct, selective pulmonary vasodilator without systemic effects) can decrease PVR and VD/VT at rest23 and improve VE/VCO2 slope.24 On the other hand, administration of the systemic vasodilator, isosorbide dinitrate, did not improve ventilatory efficiency,23 which further suggests a predominant role of RV afterload in mediating ventilatory efficiency during exercise. In our study, sildenafil, a selective pulmonary vasodilator, tended to improve VE/VCO2 slope after 12 weeks of drug exposure, in agreement with prior observations with single dose administration of sildenafil in similar patient cohorts.18,21,25 Taken together, these results implicate poor RV performance and higher exercise pulmonary vascular tone in the pathogenesis of inefficient ventilation, and suggest that therapies directed at reducing PVR may improve ventilatory efficiency. Moreover, given the technical challenges posed by measuring RVEF during exercise and the invasive nature of hemodynamic monitoring during exercise, these results suggest that VE/VCO2 slope, which is easily obtained during submaximal exercise testing, may serve as a surrogate for hemodynamic parameters in following response to treatment in patients with HF with secondary PH. The mechanism by which dynamic changes in PVR during exercise mediate inefficient ventilation remains debated. According to the alveolar ventilation equation VE/VCO2 is determined by 2 variables, VD/VT and arterial PaCO2.6 Inefficient ventilation in patients with advanced HF has been ascribed to abnormalities in gas exchange (higher VD/VT) as well as an independent, “primary” drive to hyperventilation that results in low PaCO2.7 We observed both a positive correlation between PVR and VD/VT at peak exercise and a negative correlation between PVR and arterial PaCO2 at peak exercise. Though pulmonary vasoconstriction or remodeling leading to a higher VD/VT has been proposed to account for increased VE/VCO2 slope in HF,13,14 our results suggest that, even in the setting of a higher VD/VT, elevated PVR may provide a distinct physiological stimulus for exercise hyperventilation, resulting in lower arterial PaCO2.
This study and others have observed that rest PCWP correlates with VE/VCO2 slope.13,14 Chronically elevated resting left sided filling pressures may contribute to inadequate pulmonary vasodilation and high VD/VT during exercise through pulmonary vascular remodeling. Indeed, resting PCWP was correlated with exercise PVR in this study (R=0.58, P=0.007). However, the lack of relationship between exercise PCWP and VE/VCO2 slope indicates that ventilatory efficiency is not simply dictated by acute changes in left-sided filling pressures during exercise. Reducing resting PCWP and directly targeting high PVR in HF may each reduce the propensity of patients with left ventricular systolic dysfunction to develop inefficient ventilation.
A putative mechanism for the excess ventilatory drive leading to reductions in arterial PaCO2 in HF is a heightened “ergoreflex,” a complex metabolic reflex by which peripheral chemoreceptors in muscle register local metabolic byproducts during exercise and initiate a neural reflex that drives hyper-ventilation.26–28 A heightened ergoreflex has been observed in numerous studies of patients with HF,2,29–31 but the relationship between the degree of secondary PH in HF and the extent of heightened ergoreflex has not been determined. Interestingly, however, long-term sildenafil administration to patients with HF has been shown to attenuate abnormal ergoreflex proportionate to improvement in VE/VCO2 slope,19 but invasive hemodynamics were not measured during exercise in that study. Additional studies directed at defining the relative contribution of PVR and ergoreflex to exercise hyperventilation are needed to further define this relationship.
Limitations
These results were derived from a relatively small patient cohort, however, our study population was extensively characterized with respect to clinical, hemodynamic, and ventilatory parameters. The severely impaired exercise capacity and cardiac index in our cohort indicates that these patients had relatively advanced HF that may have been underestimated by their New York Heart Association class. Hence, the generalizability of our findings to patients with less severe HF requires further investigation. We did not adjust for multiple exploratory analyses investigating the relationships between hemodynamic and gas exchange parameters and ventilatory efficiency. However, relationships between related indicators of RV performance (eg, pulmonary arterial pressure, PVR, RVEF) and VE/VCO2 slope were highly concordant, supporting the validity of our conclusion that dynamic changes in PVR and RV function modulate ventilatory efficiency. In addition, although this study consisted of subjects receiving either active sildenafil or placebo, over the 12-week exposure period we observed changes in hemodynamic parameters that allowed us to capture intraindividual relationships between ventilatory efficiency and hemodynamics. Finally, our study was confined to patients with HF and secondary PH; however, the high prevalence of PH in patients with chronic symptomatic LV systolic dysfunction suggests that our findings may have broad applicability.
Conclusions
In this study, we found that alterations in pulmonary vasomotor tone during exercise may have a critical role in determining ventilatory efficiency and dyspnea in patients with advanced HF. Targeting pulmonary vascular resistance and RV function therapeutically may improve ventilatory efficiency, a key prognostic indicator in patients with chronic LV dysfunction.
Acknowledgments
The authors thank the staff of the cardiopulmonary exercise laboratory for helping with data collection.
Sources of Funding
This work was supported by a grant from the Heart Failure Society of America (to G.D.L.), the American Heart Association Fellow-to-Faculty Award (to G.D.L.), the Harvard/MIT Clinical Investigator Training Program in collaboration with Pfizer Inc and Merck & Company Inc (to G.D.L.), and grant HL091106 (to G.D.L.) and grant HL04021 (to M.J.S.) from the National Heart Lung and Blood Institute.
Footnotes
Disclosures
Dr Semigran has a sponsored research agreement with Pfizer Inc and serves on the Scientific Advisory Board for INO Therapeutics LLC.
CLINICAL PERSPECTIVE
In patients with heart failure, inefficient ventilation, as indicated by an abnormally high minute ventilation to eliminate CO2 during incremental exercise (VE/VCO2 slope), contributes to dyspnea and purports a poor prognosis. To investigate the pathophysiologic mechanisms underlying abnormal VE/VCO2 slope in heart failure, Lewis et al simultaneously assessed ventilatory parameters, invasive hemodynamics, and myocardial function at rest and during exercise in a cohort of heart failure patients undergoing repeated cardiopulmonary exercise testing before and after 12 weeks of treatment with the pulmonary vasodilator sildenafil. VE/VCO2 slope was directly related to pulmonary vascular resistance and indirectly related to right ventricular ejection fraction during exercise; changes in VE/VCO2 slope over the course of the study correlated with changes in these indicators of right ventricular performance. Alterations in pulmonary vasomotor tone during exercise may have a critical role in determining ventilatory efficiency in patients with advanced heart failure. Therefore, targeting pulmonary vascular resistance and right ventricular function therapeutically may improve ventilatory efficiency, a key prognostic indicator in patients with chronic left ventricular dysfunction.
References
- 1.Arena R, Myers J, Aslam S, Varughese E, Peberdy M. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354–360. doi: 10.1016/j.ahj.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Chua T, Ponikowski P, Harrington D, Anker S, Webb-Peploe K, Clark A, Poole-Wilson P, Coats A. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Abella J, Peberdy M, Bensimhon D, Chase P, Guazzi M. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 4.Gitt A, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Scheider S, Schwarz A, Senges J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 5.Corra U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J. 2002;143:418–426. doi: 10.1067/mhj.2002.120772. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R. Gas exchange efficiency in congestive heart failure. Circulation. 2000;101:2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 7.Wensel R, Gerogiadou P, Francis D, Bayne S, Scott A, Genth-Zotz S, Anker S, Coats A, Piepoli M. Differential contribution of dead space ventilation and low arterial pCO2 to exercise hyperventilation in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93:318–323. doi: 10.1016/j.amjcard.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Franciosa J, Leddy C, Wilen M, Schwartz D. Relation between hemody-namic and ventilatory responses in determining exercise capacity in severe congestive heart failure. Am J Cardiol. 1984;53:127–134. doi: 10.1016/0002-9149(84)90696-9. [DOI] [PubMed] [Google Scholar]
- 9.Jondeau G, Katz S, Zohman L, Goldberger M, McCarthy M, Bourdarias J, LeJemtel T. Active skeletal muscle mass and cardiopulmonary reserve. Failure to attain peak aerobic capacity during maximal bicycle exercise in patients with severe congestive heart failure. Circulation. 1992;86:1351–1356. doi: 10.1161/01.cir.86.5.1351. [DOI] [PubMed] [Google Scholar]
- 10.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 11.Costard-Jackle A, Fowler M. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Chomsky D, Wilson J. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 13.Reindl I, Wernecke K, Opitz C, Wensel R, Konig D, Dengler T, Schimke I, Kleber F. Impaired ventilatory efficiency in chronic heart failure: possible role of pulmonary constriction. Am Heart J. 1998;136:778–785. doi: 10.1016/s0002-8703(98)70121-8. [DOI] [PubMed] [Google Scholar]
- 14.Metra M, Dei Cas L, Panina G, Visioli O. Exercise hyperventilation chronic congestive heart failure, and its relation to functional capacity and hemodynamics. Am J Cardiol. 1992;70:622–628. doi: 10.1016/0002-9149(92)90202-a. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan M, Higginbotham M, Cobb F. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite he-modynamic and pulmonary abnormalities. Circulation. 1998;77:552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 16.Rabe K, Tenor H, Dent G, Schudt C, Nakashima M, Magnussen H. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. Am J Physiol. 1994;266:L536–L543. doi: 10.1152/ajplung.1994.266.5.L536. [DOI] [PubMed] [Google Scholar]
- 17.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;20:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 18.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–2348. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 20.Lewis G, Shah R, Shahzad K, Camuso J, Pappagianopoulous P, Hung J, Tawakol A, Gerszten R, Systrom D, Bloch K, Semigran M. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 21.Lewis G, Lachmann J, Camuso J, Lepore J, Shin J, Martinovic M, Systrom D, Bloch K, Semigran M. Sildenafil improves exercise hemo-dynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59 – 66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 22.Bard R, Gillespie B, Clarke N, Egan T, Nicklas J. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant. 2006;25:589–595. doi: 10.1016/j.healun.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 23.Mastsumoto A, Momomura S, Sugiura S, Fujita H, Aoyagi T, Sata M, Omata M, Hirata Y. Effect of inhaled nitric oxide on gas exchange in patients with congestive heart failure: a randomized, controlled trial. Ann Intern Med. 1999;130:40–44. doi: 10.7326/0003-4819-130-1-199901050-00008. [DOI] [PubMed] [Google Scholar]
- 24.Bocchi E, Auler J, Gimaraes G, Carmona M, Wajngarten M, Bellotti G, Pileggi F. Nitric oxide inhalation reduces pulmonary tidal volume during exercise in severe chronic heart failure. Am Heart J. 1997;134:737–744. doi: 10.1016/s0002-8703(97)70058-9. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Casali M, Berti F, Rossoni G, D’Gennaro Colonna V, Guazzi MD. Endothelium-mediated modulation of ergoreflex and improvement in exercise ventilation by acute sildenafil in heart failure patients. Clin Pharmacol Ther. 2008;83:336–341. doi: 10.1038/sj.clpt.6100306. [DOI] [PubMed] [Google Scholar]
- 26.Scott A, Francis D, Davies L, Ponikowski P, Coats A, Piepoli M. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol. 2000;529:863–870. doi: 10.1111/j.1469-7793.2000.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott A, Wensel R, Davos C, Kemp M, Kaczmarek A, Hooper J, Coats A, Piepoli M. Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation. 2002;106:214–220. doi: 10.1161/01.cir.0000021603.36744.5e. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 29.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 30.Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 31.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]