Abstract
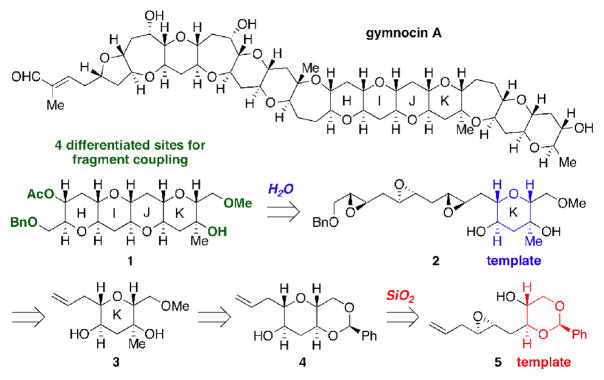
Epoxide-opening cascades offer an attractive strategy for the construction of trans-syn-fused arrays of oxygen heterocycles, a hallmark of ladder-type polyether natural products such as gymnocin A.[1] We have previously described efficient water-promoted cascades templated by a minimally functionalized tetrahydropyran (THP) ring, the products of which were not directly amenable to further elaboration.[2] To expand the utility of this methodology in target-oriented synthesis, we have now developed two highly functionalized heterocycles that not only serve as templates but also possess differentiated functional groups for modification and fragment coupling. One of these templates (THP 2), resembles ring K of the natural product gymnocin A (Figure 1). The other functionalized template (1,3-dioxane 5), while not found in ladder polyethers, is synthetically versatile and can easily be transformed into rings present in gymnocin A. The reactivity and selectivity patterns of this novel dioxane template are significantly different from and thus complementary to those of THP-based templates in water.
Keywords: cascade, ladder polyether, metathesis, natural products, template
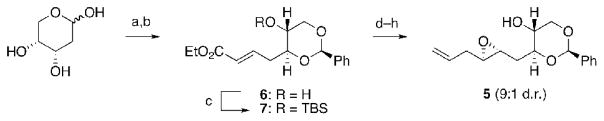
We envisioned the HIJK ring system (1) of gymnocin A could be constructed by a water-promoted cascade of triepoxide 2, where ring K of the natural product templates the reaction. In turn, this triepoxide is prepared via a cross-metathesis involving olefin 4, with the THP of 4 being assembled via an endo-selective cyclization of epoxy alcohol 5. Having not previously examined such 1,3-dioxanes as templates, we began our studies with the synthesis and evaluation of 5 in this context. 2-Deoxyribose was transformed into benzylidene acetal 7 followed by reduction and Sharpless epoxidation in good yield and diastereoselectivity (Scheme 1). [3] Conversion of the alcohol to the iodide, a copper catalyzed displacement,[4] and cleavage of the silyl ether afforded multigram quantities of 5.
Scheme 1.
Reagents and conditions: a) Ph3PCHCO2Et, THF, reflux, 83:17 E:Z; b) PhCH(OMe)2, CSA, DCM, 76% (2 steps); c) TBSCl, imid, DMF, 71%; d) DIBALH, DCM, 97%; e) D-(−)-DET, Ti(OiPr)4, TBHP, DCM, −25 °C, 95%, 9:1 d.r.; f) I2, imid, PPh3, Et2O/CH3CN, 83%; g) CuBr·DMS, CH2=CHMgBr, HMPA, THF, −25 °C, 84%; h) TBAF, THF, 0 °C, 90%.
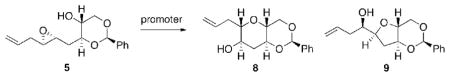
We quickly discovered that the behavior of the 1,3-dioxane template is markedly different than that of the THP templates we have reported.[2] Water, which proved effective for THP templates, did not promote the cyclization of 5 at ambient temperature and only hydrolyzed the acetal at elevated temperatures (Table 1, entries 1–2). Since both solvent and promoter can significantly affect selectivity, we examined a variety of conditions in order to identify an efficient promoter for this dioxane template (entries 3–8). Low selectivity or low conversion was generally observed, yet gratifyingly, silica gel exhibited high endo-selectivity albeit in low yield (entry 9).[5] Recognizing that silica is hygroscopic, it was unclear to us whether trace H2O or the silanol surface of the silica gel was promoting the reaction. Rigorous drying of the silica prior to use had no effect on conversion or selectivity (entry 10), suggesting that indeed the silanol was the promoting species.[6] Complete conversion was obtained by simply increasing the promoter loading (entry 11). Silicic acid (SiO3H2) also promoted the cyclization (entry 12). Due to the mild nature of silica promoters, only the major diastereomer of 5 cyclized at 40 °C, allowing for facile removal of the unwanted stereoisomer by chromatography. Conveniently, microwave heating reduced the reaction time from days to minutes with no appreciable drop in yield or selectivity (entry 13).
Table 1.
Screen of promoters for endo-selective cyclization of 5.
 | ||||||
|---|---|---|---|---|---|---|
| entry | solvent | promoter | T (°C) | t | 8 : 9a | yield 8 |
| 1 | H2O | none | 23 | 13 d | --b | -- |
| 2 | H2O | none | 60 | 3 d | --c | -- |
| 3 | H2O | NaOH | 23 | 12 h | 2.0:1 | 58% |
| 4 | MeOH | Cs2CO3 | 23 | 12 h | 1.2:1 | 45% |
| 5 | MeOH | imidazole | 23 | 12 h | --b | -- |
| 6 | MeOH | none | 23 | 9 d | --b | -- |
| 7 | C6H6 | CSA | 23 | 1 h | 1.9:1c | 51% |
| 8 | CH2Cl2 | none | 40 | 2 d | --b | -- |
| 9 | CH2Cl2 | SiO2(35)d | 40 | 2 d | >9:1e | 45% |
| 10 | CH2Cl2 | SiO2(35)d,f | 40 | 2 d | >9:1e | 47% |
| 11 | CH2Cl2 | SiO2(90)d,f | 40 | 2 d | >9:1 | 78% |
| 12 | CH2Cl2 | SiO3H2(90)d,f | 40 | 2 d | >9:1 | 75% |
| 13 | CH2Cl2 | SiO3H2(90)d,f | 135 | 0.2 h | >9:1 | 72% |
Determined by 1H-NMR.
<5% cyclization of 5.
1,3-dioxane cleaved.
mg silica promoter/mg 5.
50–60% cyclization of 5 observed.
Promoter dried at 140 °C for 12 h prior to use.
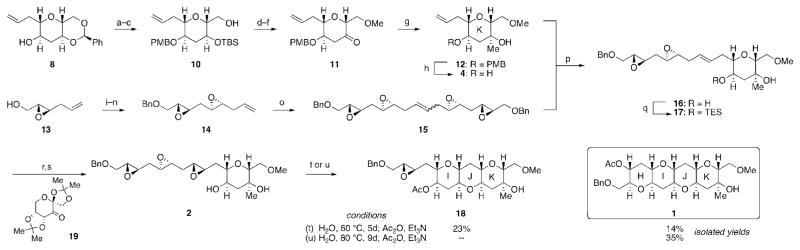
Having identified a new and efficient promoter for the cyclization of the 1,3-dioxane template onto a single epoxide,[7] we turned our attention towards elaboration of the newly formed THP in 8 into ring K of the natural product, and the template for the next cascade. Protection of alcohol 8 as a PMB ether was followed by acetal solvolysis to provide a diol, the secondary alcohol of which was protected as a silyl ether (Scheme 2).[8] At this stage we chose to protect primary alcohol 10 as a methyl ether which would serve as an orthogonally protected fragment coupling site following the epoxide-opening cascade. Silyl group cleavage, oxidation and a stereoselective addition of methyl Grignard[9] afforded 12, ring K of the natural product, in 38% overall yield from 8.
Scheme 2.
Reagents and conditions: a) KH; PMBCl, THF, 94%; b) CSA, MeOH/THF, 90%; c) TBSOTf, 2,6-lut, CH2Cl2; CSA, MeOH, 91%; d) Ag2O, MeI, CH3CN, 60 °C, 76%; e) TBAF, THF, 98%; f) Dess-Martin periodinane, CH2Cl2, 96%; g) MeMgBr, PhCH3, −78 °C, 75%; h) DDQ, CH2Cl2/H2O, 0 °C, 93%; i) NaH; BnBr, THF, 87%; j) CH2=CHCHO, 2nd Hoveyda-Grubbs cat., DCM, 40 °C, 83%, >10:1 E:Z; k) NaBH4, MeOH; l) D-(−)-DET, Ti(OiPr)4, TBHP, DCM, −25 °C, (64% 2 steps), 5:1 d.r.; m) I2, imid, PPh3, Et2O/CH3CN, 87%; n) CuBr DMS, CH2=CHMgBr, HMPA, THF, −25 °C, 84%; o) 2nd Hoveyda-Grubbs cat., DCM, 40 °C, 85%; p) 2nd Hoveyda-Grubbs cat., DCM, 40 °C, 74%, 2.6:1 E:Z; q) TESCl, Imid, DMF, 82%; r) 19, Oxone, Bu4NHSO4, K2CO3, pH=10.5, DMM/CH3CN, 82%, 93:7 d.r.; s) TBAF, THF, 77%. t) H2O, 60 °C, 5 d; Ac2O, Et3N; u) H2O, 80 °C, 9 d; Ac2O, Et3N.
Olefin metathesis to join the template and epoxide-bearing fragment 14 (prepared from known epoxy alcohol 13[10] in 6 steps) initially proved challenging. Attempts to couple olefins 12 and 14 with ruthenium-based catalysts gave mainly the self-metathesis product (15) of 14 and minimal cross-metathesis. Suspecting that the PMB ether of 12 may be interfering with the desired reaction,[11] this group was removed, and under reaction conditions identical to those investigated with 12, diol 4 underwent cross-metathesis to give 16 in moderate yield and E:Z selectivity. The yield was improved significantly by replacing olefin 14 with an excess of self-metathesis product 15.[12] Greater than 90% of unreacted 15 was recovered and could be reused in the metathesis reaction without event. Furthermore, while the cross-metathesis proceeds with moderate E:Z selectivity, the olefin isomers are separable allowing for recycling of the undesired Z-olefin by resubjection to the metathesis reaction. In all, this metathesis strategy afforded several hundred milligrams of 16.[13] Finally, protection of alcohol 16 prevented undesired cyclization during the subsequent asymmetric epoxidation with fructose-derived ketone 19.[14]
Having convergently assembled polyepoxide 2, we were eager to explore the water-promoted cascade. Cognizant of how changes to the template composition can radically affect endo-selectivity,[15] it was challenging to predict a priori whether ring K, bearing a methoxymethyl substituent at the 2-position as well as a tertiary alcohol and axial methyl group at the 3-position, would template the reaction in the desired fashion. Incubation of 2 in H2O at 60 °C for 5 days followed by acetylation afforded a mixture of the desired tetrad (1) and a compound in which rings IJ had formed, but the final epoxide had remained intact. This triad (18) intrigued us for two reasons. In previous cascades, complete conversion was typically observed after 3 days at 60 °C. Second, we had not previously isolated an epoxide-containing intermediate en route to the final cascade product. The attenuated reactivity of the remaining epoxide is likely due to the presence of the electron-withdrawing oxygen atom in the benzyl ether. A higher temperature and longer reaction time (80 °C, 9 d) surmounted this stalled cascade and after acetylation afforded 1, the desired HIJK fragment of gymnocin A in 35% overall yield, corresponding to approximately 70% yield per newly formed ring.
In summary, we have employed two different functionalized templates for the synthesis of the HIJK rings of gymnocin A. The first template (1,3-dioxane of 5) provides high endo-selectivity in the presence of silicon dioxide-based promoters. The product (8) of this cyclization, a synthetically versatile intermediate, was facilely elaborated into a second template (ring K of gymnocin A) facilitating a water-promoted cascade of triepoxide 2 into tetrad 1. Noteworthy is that this polyether subunit enjoys a total of 4 differentiated functional groups, 2 at each end, thus allowing for elaboration of both termini and significantly increasing the synthetic utility of products obtained from epoxide-opening cascades. The use of dioxane and other functionalized templates towards the total synthesis of ladder polyether natural products is under investigation.
Supplementary Material
Figure 1.
Gymnocin A and retrosynthetic analysis of rings HIJK.
Footnotes
The authors thank the NIGMS (GM72566) for support of this work, Wyeth Research for a fellowship (to A.V.D.), Dr. Jeffery A. Byers and Mr. Christopher J. Morten (MIT) for insightful discussions, and Dr. Li Li (MIT) for obtaining mass spectrometric data.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For an excellent and recent review of the ladder polyether natural products, see Nicolaou KC, Frederick MO, Aversa RJ. Angew Chem. 2008;120:7292–7335. doi: 10.1002/anie.200801696.Nicolaou KC, Frederick MO, Aversa RJ. Angew Chem Int Ed. 2008;47:7182–7225. doi: 10.1002/anie.200801696.
- 2.Vilotijevic I, Jamison TF. Science. 2007;317:1189–1192. doi: 10.1126/science.1146421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolaou KC, Nugiel DA, Couladouros E, Hwang CK. Tetrahedron. 1990;46:4517–4552. [Google Scholar]
- 4.Nicolaou KC, Duggan ME, Ladduwahetty T. Tetrahedron Lett. 1984;25:2069–2072. [Google Scholar]
- 5.Silica gel has been used for endo-selective epoxysilane opening but not, to our knowledge, for electronically unbiased epoxides such as 5. We confirmed that the allyl substituent was not electronically biasing the epoxide as studies with fully saturated analogues gave comparable results. See: Adiwidjaja G, Florke H, Kirschning A, Schaumann E. Tetrahedron Lett. 1995;36:8771–8774.
- 6.The solution concentration of 5 dropped by an order of magnitude after addition of silica (UV analysis), indicating significant adsorption onto SiO2.
- 7.Given the mildly acidic nature of silica, we did not predict it would be an effective promoter for an epoxide-opening cascade. Indeed, in studies of templates bearing two epoxides, silica-based promoters led to the formation of undesired oxetane and tetrahydrofuran rings.
- 8.Koch G, Loiseleur O, Altmann KH. Synlett. 2004;4:693–697. [Google Scholar]
- 9.Fuwa H, Sasaki M, Tachibana K. Tetrahedron. 2001;57:3019–3033. [Google Scholar]
- 10.Sabitha G, Sudhakar K, Reddy NM, Rajkumar M, Yadav JS. Tetrahedron Lett. 2005;46:6567–6570. [Google Scholar]
- 11.Connon SJ, Blechert S. Angew Chem Int Ed. 2003;42:1900–1923. doi: 10.1002/anie.200200556. [DOI] [PubMed] [Google Scholar]
- 12.a) Blackwell HE, O’Leary DJ, Chatterjee AK, Washenfelder RA, Bussmann DA, Grubbs RH. J Am Chem Soc. 2000;122:58–71. [Google Scholar]; b) O’Leary DJ, Blackwell HE, Washenfelder RA, Grubbs RH. Tetrahedron Lett. 1998;39:7427–7430. [Google Scholar]
- 13.While cross-metathesis afforded ample quantities of 16 in order to evaluate ring K as a template in epoxide-opening cascades, we are investigating other more general strategies for the construction of similar “skipped” polyepoxides.
- 14.Tu Y, Wang Z-X, Shi Y. J Am Chem Soc. 1996;118:9806–9807. [Google Scholar]
- 15.Byers JA, Jamison TF. unpublished results. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.