Abstract
PURPOSE
To assess the relationship between sibling history of myocardial infarction (MI) or stroke with cardiovascular disease (CVD) and risk factors in older adults.
METHODS
Prospective cohort study of 5,888 older adults participating to the Cardiovascular Health Study (CHS). History of MI and stroke in siblings was obtained by self-report. Participants with positive sibling histories were compared to those with negative histories to determine if prevalent or incident disease (coronary heart disease [CHD], MI, stroke, angina), subclinical CVD (carotid wall thickness, left ventricular mass, hypertension, diabetes, ankle brachial index), CVD risk factors differed between groups.
RESULTS
More than 91 percent (n=5,383) of CHS participants reported at least one sibling. Sibling history of MI was associated with increased disease prevalence (CHD, MI, angina) and incidence (CHD, angina). Sibling history of stroke was associated with increased disease prevalence (CHD, angina). Sibling history of either MI or stroke was associated with increased disease prevalence and incidence for CHD, MI and angina, more subclinical disease, and a higher CVD risk profile.
CONCLUSIONS
Sibling history of MI and stroke were markers of higher CVD risk status even in older adults. Of clinical importance, participants with positive sibling history have numerous risk factors amenable to intervention.
Keywords: Epidemiology, Cardiovascular diseases, Risk Factors, Atherosclerosis, Lifestyle
INTRODUCTION
Family history of cardiovascular disease (CVD) has been shown to be a risk factor for the subsequent development of disease, and a potential screening tool to identify individuals with increased risk who may be candidates for enhanced prevention strategies (1, 2). In the elderly, parental medical history may be difficult to obtain or is often inaccurate (3), and a positive sibling history of CVD is a stronger independent predictor of incident cardiovascular events than parental history (4). Siblings' health history has been proposed as a marker to stratify populations for genetic research (5).
Familial aggregation has been shown to occur for hypertension (6–10), myocardial infarction (10–14), ischemic stroke (15, 16), diabetes (17, 18) and obesity (19). Family history is a predictor of risk factor levels and/or disease in studies of children, young and middle-aged adults (4, 20, 21). Among twins, sibling history of coronary heart disease (CHD) death predicts increase risk of CHD death before age 65 years (22), suggesting genetic components to exert stronger effects at younger ages (23). However, less is known about the importance of family history of cardiovascular disease in the elderly.
Due to a survivorship effect in older adults it is possible that substantial differences may exist in the relationship between family history and CVD risk between middle age and older populations. A positive family history may be more predictive of the risk of early CVD events rather than events in later life (24–26). The risk from a positive family history may be diminished in older adults due to reduced survivorship in high-risk families and to potential difficulties in the assessment of familial risk. However, given the older age of the participant’s parents and siblings, an advantage of assessing family history in older adults is the relatively low level of false negatives.
This study addresses the issue of whether risk factor differences exist in an elderly population between individuals with a positive as compared to a negative sibling history of myocardial infarction (MI) and of stroke. Specifically, we examined the association between a sibling history of MI and sibling history of stroke with CVD prevalence and incidence, subclinical measures of disease, and major risk factors in this cohort of older adults.
METHODS
Study Population
The Cardiovascular Health Study (CHS) cohort consists of 5,888 elderly men and women aged 65 years and older drawn from four U.S. communities. Details of the CHS study design have been published elsewhere. (27) Demographic information, laboratory tests, physical measurements, ultrasound, measures of cognitive and functional status were collected at baseline and at annual visits thereafter. Past medical histories and reported cardiovascular events were confirmed by medical record review. (27) Sibling history was collected at baseline examinations for the original CHS cohort in 1989 (n=5201) and an additional African American cohort (n=697) in 1992. Sibling history information was obtained by self-report.
Data analysis
CVD risk factors used in these analyses were limited to "major" risk factors available in CHS and included age, gender, race, blood pressure, body size, lipids and lipoproteins, smoking status, creatinine, plasma glucose and insulin, medication use and measures of coagulation factors. Individuals were considered to have a positive sibling history for MI if any of their siblings had experienced an MI. Sibling history for stroke was constructed in a similar manner. A “combined” sibling history for MI or stroke was positive if they had a positive sibling history for MI or a positive sibling history for stroke. All measures used in the analysis were collected at baseline.
The STATA version 9.2 (StataCorp, College Station, TX) statistical package was used for data analysis. Associations between continuous and dichotomous measures of CVD and sibling history factors (i.e., MI, stroke and the combined sibling history factor) were investigated using regression analysis with robust variance estimates. Continuous measures of CVD include subclinical measures (carotid intima-media thickness [IMT], left ventricular [LV] mass, ankle-brachial index [ABI]) and other risk factors (blood pressure, lipids/lipoproteins, body size, coagulation factors, creatinine, fasting glucose and fasting insulin levels). Dichotomous measures of CVD risk factors include: gender, black race, medication use, aspirin use (greater than two times per two weeks) current smoking, ECG abnormalities, uncontrolled blood pressure (more than 140 mm Hg systolic blood pressure to 90 mm Hg diastolic blood pressure), and high low density lipoproteins (LDL > 130 mg/dL). Associations were adjusted by age, gender and race. Multivariable logistic regression with robust variance estimates was used to investigate the relationship between prevalent disease (CHD, MI, stroke, angina) and the sibling history factors. Multivariable Cox regression with robust variance estimates was used to investigate incident disease (CHD, MI, stroke, angina) and the sibling history factors. Each disease outcome was modeled separately. In logistic models, unadjusted and adjusted odds ratios (OR) were used to summarize prevalent disease associations and sibling history. In Cox models, unadjusted and adjusted hazard ratios (HR) were used to summarize the associations between incident disease associations and sibling history. Test statistics were Wald statistics. All p-values were two-sided.
RESULTS
Sibling History of Disease
The number of siblings reported by participants ranged from 0 to 10 (Table 1). The sibling questionnaire allowed information for up to 10 siblings. Participants with more than 10 siblings were effectively grouped at 10. Six CHS participants were missing sibling data; 499 participants reported no siblings and were excluded from the remaining analyses. There were more than 91 percent (n=5,383) of participants reporting one or more siblings. The presence of at least two siblings was reported by 77% of the cohort. A history of sibling MI was reported in 32% of participants. A history of sibling stroke was reported by 15% of participants. A sibling history of either MI or stroke was reported by 39% of participants.
Table 1.
Distribution of Number of Siblings, Sibling History of MI and Sibling History of Stroke for the CHS Participants.
| No. of siblings | N | Percent |
|---|---|---|
| 0 | 499 | 8.5 |
| 1 | 847 | 14.4 |
| 2 | 973 | 16.5 |
| 3 | 793 | 13.5 |
| 4 | 695 | 11.8 |
| 5 | 509 | 8.7 |
| 6 | 445 | 7.6 |
| 7 | 380 | 6.5 |
| 8 | 264 | 4.5 |
| 9 | 187 | 3.2 |
| 10 or more | 290 | 4.9 |
| Total | 5882* | 100 |
| Sibling History of MI | ||
| No | 3659 | 68 |
| Yes | 1724 | 32 |
| Total | 5383 † | 100 |
| Sibling History of Stroke | ||
| No | 4567 | 85 |
| Yes | 816 | 15 |
| Total | 5383 † | 100 |
| Sibling History of MI and/or Stroke | ||
| No | 3292 | 61 |
| Yes | 2091 | 39 |
| Total | 5383 † | 100 |
Six participants had missing values for information pertaining to their siblings.
Data exclude 499 participants with no siblings or have missing data.
Association between CVD risk factor profile and sibling history of MI or stroke
Participants who reported a positive sibling history of MI differed from participants who reported a negative sibling history for a number of CVD risk factors (Table 2). Mean internal carotid IMT, LDL, fibrinogen, glucose and creatinine were significantly higher for participants who reported a positive sibling history of MI than participants who reported a negative sibling history of MI. Mean ABI and HDL were significantly lower for participants with a positive sibling history of MI. Participants with a positive sibling history of MI were more likely to be hypertensive, have higher medication and aspirin use, less likely to be black and less likely to be male than participants who reported a negative sibling history.
Table 2.
Baseline Characteristics of CHS Participants, Comparing Positive versus Negative Sibling History of Myocardial Infarction (MI), Sibling History of Stroke, or Sibling History of either MI or Stroke.
| Sibling History | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MI | Stroke | MI or Stroke | |||||||
| Characteristic | No (N=3655) |
Yes (N=1722) |
P-val | No (N=4562) |
Yes (N=815) |
P-val | No (N=3288) |
Yes (N=2089) |
p-val |
| Mean age (years) | 72.8 ± 5.7 | 73.1 ± 5.5 | 0.075 | 72.7 ± 5.6 | 73.7 ± 5.8 | <.001 | 72.7 ± 5.7 | 73.1 ± 5.5 | 0.004 |
| Male gender (%) | 44 | 39 | 0.001 | 42 | 42 | 0.870 | 44 | 40 | 0.025 |
| African American (%) | 17 | 12 | <.001 | 15 | 16 | 0.590 | 17 | 13 | <.001 |
| Mean body mass index (kg/m2) | 26.6 ± 4.8 | 26.8 ± 4.6 | 0.260 | 26.7 ± 4.8 | 26.5 ± 4.5 | 0.207 | 26.7 ± 4.8 | 26.7 ± 4.6 | 0.624 |
| Diabetic (%) | 16 | 18 | 0.116 | 17 | 15 | 0.356 | 16 | 17 | 0.215 |
| Current smoking (%) | 12 | 11 | 0.362 | 12 | 13 | 0.304 | 12 | 12 | 0.768 |
| Mean common carotid IMT (mm) | 1.06 ± 0.22 | 1.07 ± 0.22 | 0.056 | 1.06 ± 0.22 | 1.08 ± 0.23 | 0.172 | 1.06 ± 0.22 | 1.07 ± 0.22 | 0.039 |
| Mean internal carotid IMT (mm) | 1.42 ± 0.57 | 1.47 ± 0.57 | 0.004 | 1.43 ± 0.56 | 1.46 ± 0.60 | 0.143 | 1.42 ± 0.56 | 1.45 ± 0.58 | 0.001 |
| Mean left ventricular mass (g) | 154 ± 36 | 155 ± 32 | 0.661 | 154 ± 35 | 156 ± 36 | 0.256 | 154 ± 36 | 155 ± 33 | 0.454 |
| ECG Abnormalities (%) | 30 | 31 | 0.436 | 30 | 32 | 0.313 | 30 | 31 | 0.751 |
| Mean systolic blood pressure (mmHg) | 136 ± 21 | 136 ± 21 | 0.921 | 136 ± 21 | 136 ± 22 | 0.520 | 136 ± 21 | 136 ± 21 | 0.751 |
| Controlled BP (< 140/90) (%) | 6 | 6 | 0.886 | 6 | 6 | 0.722 | 6 | 6 | 0.471 |
| Medication use (%) | 76 | 81 | <.001 | 77 | 80 | 0.052 | 76 | 80 | <.001 |
| Aspirin use (> 2 days/2 weeks) (%) | 33 | 37 | 0.002 | 34 | 34 | 0.889 | 33 | 36 | 0.007 |
| Hypertensive (%) | 42 | 48 | <.001 | 44 | 48 | 0.028 | 42 | 47 | <.001 |
| Mean ankle brachial index | 1.07 ± 0.17 | 1.05 ± 0.18 | 0.001 | 1.07 ± 0.17 | 1.05 ± 0.18 | 0.011 | 1.07 ± 0.17 | 1.05 ± 0.18 | 0.001 |
| Mean LDL (mg/dL) | 129 ± 35 | 131 ± 36 | 0.014 | 130 ± 36 | 130 ± 35 | 0.904 | 129 ± 35 | 131 ± 36 | 0.021 |
| LDL < 130 mg/dL (%) | 52 | 50 | 0.117 | 51 | 52 | 0.729 | 52 | 50 | 0.148 |
| Mean HDL (mg/dL) | 54.9 ± 16.0 | 52.3 ± 15.0 | <.001 | 54.2 ± 15.7 | 53.4 ± 15.5 | 0.140 | 54.9 ± 16.0 | 52.7 ± 15.2 | <.001 |
| Mean fibrinogen (mg/dL) | 322 ± 69 | 328 ± 65 | 0.005 | 324 ± 67 | 325 ± 69 | 0.553 | 322 ± 69 | 327 ± 66 | 0.010 |
| Mean Factor VII (mg/mL) | 123 ± 29 | 124 ± 30 | 0.075 | 123 ± 30 | 123 ± 29 | 0.507 | 123 ± 29 | 124 ± 30 | 0.191 |
| Mean log insulin (pmol/L) | 3.72 ± 0.79 | 3.81 ± 0.81 | 0.097 | 3.75 ± 0.79 | 3.75 ± 0.81 | 0.877 | 3.72 ± 0.79 | 3.79 ± 0.81 | 0.002 |
| Mean log glucose (mg/dL) | 6.74 ± 0.35 | 6.76 ± 0.34 | 0.041 | 6.75 ± 0.34 | 6.75 ± 0.36 | 0.855 | 6.74 ± .35 | 6.76 ± .34 | 0.073 |
| Mean log creatinine (mg/dL) | .025 ± 0.40 | .052 ± 0.41 | 0.007 | .033 ± 0.40 | .041 ± 0.41 | 0.549 | .027 ± 0.41 | .044 ± 0.40 | 0.070 |
Values represent mean ± raw SD or percent. MI indicates myocardial infarction; IMT, intima media thickness; LDL, low-density lipoprotein; HDL, high-density lipoprotein. Baseline characteristics are adjusted for age, gender and race. Summaries for age, gender and race are unadjusted.
Participants with a positive sibling history of stroke were older and their mean ABI was lower compared to participants who reported a negative sibling history. Participants with a positive sibling history of stroke were also more likely to be hypertensive and to be taking medications.
Associations between CVD risk factors and a positive sibling history for either MI or stroke were similar to the associations observed between these risk factors and a positive sibling history of MI alone. It revealed, additionally, that mean age was significantly higher for participants with a positive sibling history of either MI or stroke. Mean common carotid IMT and mean of log insulin were significantly higher for participants with a positive sibling history of either MI or stroke.
Association between prevalent CVD and sibling history of MI, stroke, or MI and stroke combined
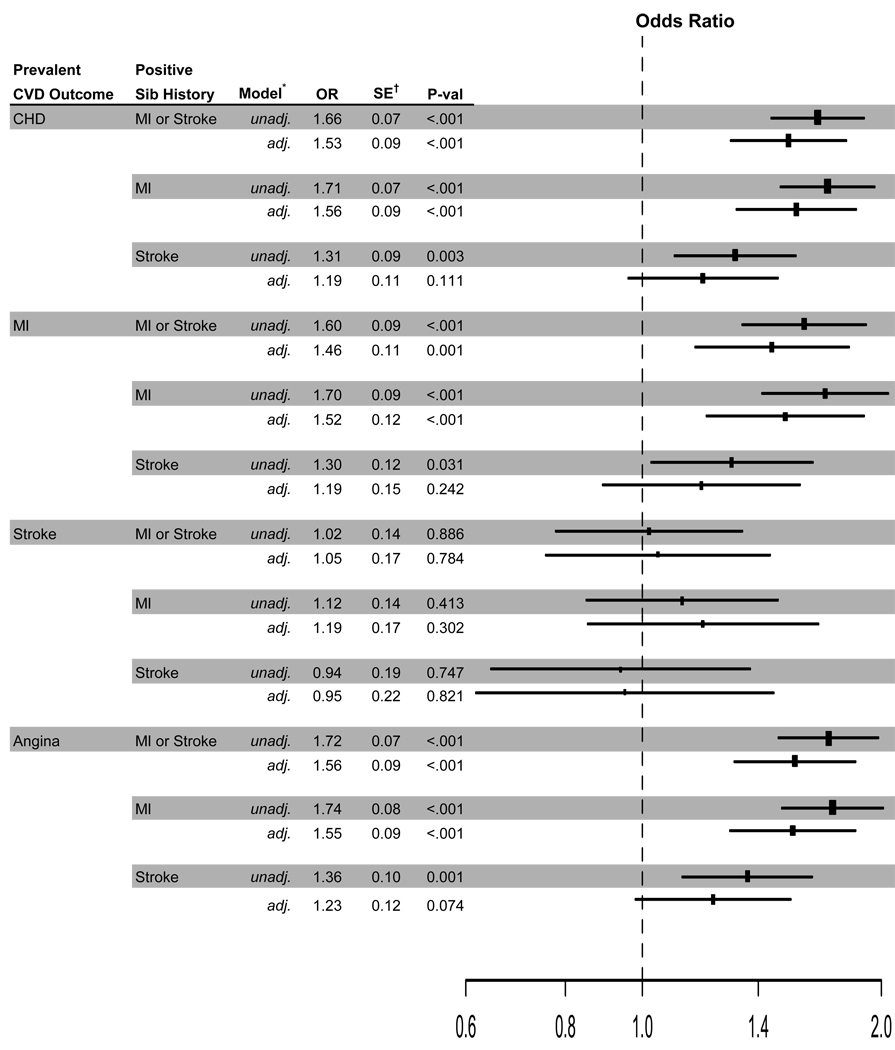
Figure 1 presents both unadjusted and adjusted ORs comparing positive with negative sibling histories to the prevalent CVD outcomes (CHD, MI, stroke, angina). Adjusted analyses included baseline characteristics presented in Table 2.
Figure 1.
Odds ratios of prevalent outcomes and sibling history of mi or sibling history of stroke. The size of the rectangles is proportional to the reciprocal of the variance of the odds ratio. *Adjusted analyses include age, gender, black race, IMT of common & internal carotid arteries, ECG LV mass, ankle-brachial index, fibrinogen, Factor VII, LDL & HDL cholesterols, body mass index, log insulin, log glucose, log creatinine, systolic blood pressure, diabetes, hypertension, medication use, aspirin use greater than two times per two weeks, ECG abnormalities. †Estimated standard errors are for log (OR) estimates.
Prevalent CHD
A positive sibling history of MI was associated with a 56% increase in the odds of having prevalent CHD relative to those with a negative sibling history of MI for participants with the same observed CVD risk profile. A positive sibling history of stroke was not associated with prevalent CHD (OR=1.19, p=0.111). A positive history of either MI or stroke was associated with a 53% increase in the odds of having prevalent CHD compared to participants with negative sibling histories.
Prevalent MI
A positive sibling history of MI was associated with a 52% increase in the odds of having prevalent MI relative to those with a negative sibling history of MI. A positive sibling history of stroke was not significantly associated with prevalent MI (OR=1.19, p=0.242). A positive sibling history of either MI or stroke was associated with a 46% increase in the odds of having prevalent MI compared to participants with negative sibling histories.
Prevalent stroke
Sibling history of MI and sibling history of stroke were not significantly associated with prevalent stroke in unadjusted or adjusted analyses.
Prevalent angina
A positive sibling history of MI was associated with a 55% increase in the odds of having prevalent angina relative to those with a negative sibling history of MI. A positive sibling history of stroke was marginally associated with 23% increase in the odds of prevalent angina compared to participants with a negative sibling history of stroke (p=0.074). A positive sibling history of either MI or stroke was associated with a 56% increase in the odds of having prevalent angina compared to participants with negative sibling histories.
Association between incident CVD and sibling history of MI, stroke, or MI and stroke combined
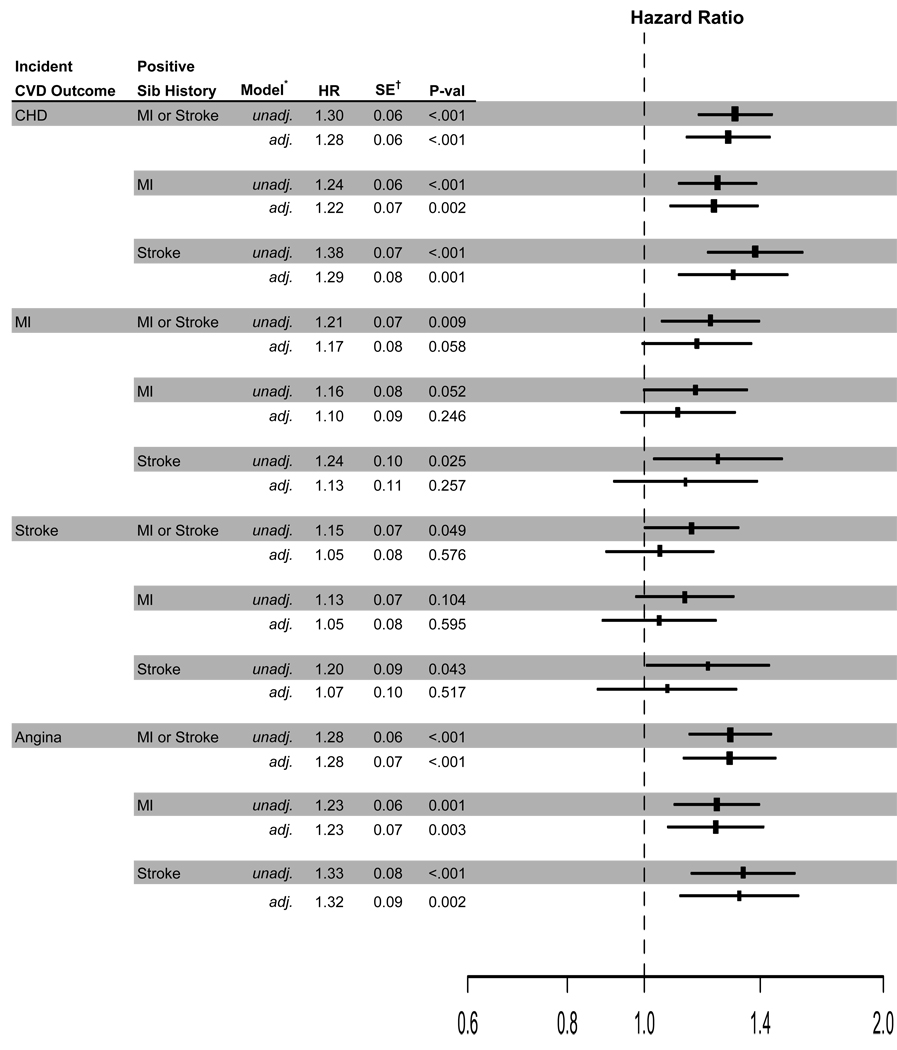
Figure 2 presents both unadjusted and adjusted hazard ratios (HRs) comparing positive to negative sibling histories to incident CVD outcomes. Adjusted analyses include the same baseline characteristics noted previously.
Figure 2.
Hazard ratios of incident outcomes and sibling history of mi or sibling history of stroke. the size of the rectangles is proportional to the reciprocal of the variance of the odds ratio. *Adjusted analyses include age, gender, black race, IMT of common & internal carotid arteries, ECG LV mass, ankle-brachial index, fibrinogen, Factor VII, LDL & HDL cholesterols, body mass index, log insulin, log glucose, log creatinine, systolic blood pressure, diabetes, hypertension, medication use, aspirin use greater than two times per two weeks, ECG abnormalities. †Estimated standard errors are for log (HR) estimates.
Incident CHD
A positive sibling history of MI was associated with a 22% increased risk of incident CHD relative to those with a negative sibling history of MI for participants with the same observed CVD risk profile. A positive sibling history of stroke was associated with a 29% increased risk of incident CHD compared to participants with a negative sibling history of stroke. A positive sibling history of either MI or stroke was associated with a 28% increased risk of incident CHD compared to participants with negative sibling histories.
Incident MI
A positive sibling history of either MI or stroke was marginally associated with a 17% increased risk of incident MI (HR=1.17, p =0.058) compared to participants with negative sibling histories. A positive sibling history of MI and a positive sibling history of stroke were associated with an increased risk of incident MI (HRs=1.11 and 1.13). These associations, however, were not significant (p=0.246 and 0.257, respectively).
Incident stroke
Sibling history for MI, sibling history for stroke and the two factors combined were not significantly associated with incident stroke in adjusted analyses.
Incident angina
A positive sibling history of MI was associated with a 23% increased risk of incident angina relative to those with a negative sibling history of MI. A positive sibling history of stroke was associated with a 32% increased risk of incident angina compared to participants with a negative sibling history of stroke. A positive sibling history of either MI or stroke was associated with a 28% increased risk of incident angina compared to participants with negative sibling histories.
DISCUSSION
Sibling history of MI and sibling history of stroke in this sample of older adults was associated with a greater prevalence of CVD risk factors, subclinical and clinical disease in the participant. Specifically, the prevalence of CHD was associated with a positive sibling history of MI and marginally associated with a positive sibling history stroke. Prevalent MI was associated with a positive sibling history of MI and was marginally associated with a positive sibling history of stroke. Prevalence of angina was associated with a positive sibling history of MI and a positive sibling history of stroke. No significant associations were observed between sibling history of MI or sibling history of stroke and prevalent stroke. Incident CHD and angina, but not incident MI, were associated with a positive sibling history of MI or a positive sibling history of stroke. A combined positive sibling history (either MI or stroke) was marginally associated with incident MI. Sibling history of MI and sibling history of stroke were not associated with incident stroke.
Other studies have shown a similar association between sibling history and CVD in adults, but these reports have not focused on this older age group (10, 14, 17). Similar to our findings, a recent report combining the Siblings With Ischemic Stroke Study (Swiss cohort) (28) and the Umeå cohort (29) observed a lack of aggregation of ischemic stroke subtypes in affected sibling pairs (30).
Given the above association between sibling history of MI and participant prevalent subclinical disease, we were interested in assessing if risk factor differences could be observed between positive and negative sibling history. Our data show associations between CVD risk factor levels in these older adults and sibling history of MI. The associations are weaker for sibling history of stroke. Sibling history of MI and sibling history of stroke were associated with a more abnormal risk profile for major CVD risk factors. Similarly, an association between family history of CVD and more abnormal levels of known risk factors has been observed in children (11, 13) and also in younger and middle-aged adults (7, 10, 12, 14, 31, 32).
Our data suggest that the increased prevalence of CHD in participants with positive sibling histories of MI or stroke persisted after adjustment for known CVD risk factors. Other studies of adult populations have observed that the increased susceptibility of adults with a family history of CHD may be independent of lipids and blood pressure levels (32–34).
We did not observe an association between sibling history of stroke and stroke in this elderly population, despite prior suggestion of a genetic contribution to cerebral susceptibility to ischemia (35–37). Likewise, recent data from a population-based cohort of patients with recent transient ischemic attack indicated that family history of stroke does not predict future risk of ischemic stroke (38). The discrepancy between these findings could possibly be explained by different predisposition in subjects with different ethnicity (37), stroke subtype (36), and elderly individuals. Few subjects with prevalent stroke were recruited into this study; these subjects were probably at lower risk of having stroke events. It is also possible that due to a limited number of events, our study did not have sufficient power to detect such associations. However, the size of the effect did not appear to be very strong.
There are a number of limitations of this study. A potential drawback is the lack of medical record validation data on sibling’s disease status. However, other investigators have detected a relatively good concordance (78%) between a reported family history of MI and medical record validation, suggesting that, despite some imprecision, the reported history gives a reasonably good estimate of family history for the diseases we have assessed (39). It is possible that an ascertainment bias may have occurred with those individuals with a positive sibling history of MI having a greater likelihood of diagnoses (hypertension, diabetes, CHD) than participants with a negative sibling history due to increased awareness of the condition by participants and their medical care providers. It is also possible that recall bias might affect the results to some extent. If differential misclassification occurs, due to more active investigations in participants with positive sibling histories, this could have led to some degree of bias. However, risk factors and events in the CHS cohort were sought prospectively, at regular intervals through the study visits, and it is unlikely that participants had different likelihood of being diagnosed with risk factors for CVD or CVD. Our study emphasizes the clinical importance of sibling history as an easily ascertained risk factor for CVD, and a potential feature able to identify subjects amenable to primary prevention.
Given our definition of sibling history, individuals from large families may have a slightly higher risk of being categorized into the positive history group. We investigated confounding by family size by including a covariate for the number of siblings in all fitted logistic and Cox regression. Family size did not appear to confound the associations between the CVD outcomes and sibling history. The relative change in odds ratios was between 1–2% and the relative change in hazard ratios was between 2–4% after adjustment for the number of siblings. The conclusions drawn from the reported results were unchanged. We were also unable to identify half-sibs or step-sibs in our analyses. Given these relationships are aggregated in our data, it is likely the association between sibling history and outcome would be stronger if the half-sibs and step-sibs were excluded from the analysis if the associations observed were genetic in nature. Including non full siblings in the analyses likely bias our results toward the null.
In addition, we did not attempt to obtain family history data on parents and other CVD events. This was done due to concern about the reliability of self-reported data and the comparability of diagnostic methods for ascertaining disease 40–50 years ago.
We have shown that a positive sibling history of MI and a positive sibling history of stroke in older adults were associated with a significantly worse cardiovascular disease prevalence and incidence, more subclinical measures of CVD, and a more adverse risk factor profile. Given that this older cohort of adults represents the “survivors” from early onset of CVD events, it is interesting to note that sibling history of MI and sibling history of stroke remain associated with a more adverse risk factor profile. We have shown that differences were observed in modifiable factors such as lipoprotein levels in older adults with a positive sibling history of disease. The observed differences in prevalent and subclinical CVD remained after adjusting for the major risk factors, suggesting an independent effect of sibling history of MI and sibling history of stroke beyond the direct effects of the risk factors. These data also suggest that these sibling history factors may be markers for other genetic/familial factors that may be poorly or inadequately measured using traditional CVD risk factors in older adults. These data provide additional guidance in the interpretation of siblings' history and could help reduce barriers to the recognition of positive family history (40). Accurate history taking and increasing awareness of siblings’ cardiovascular disease, even among older adults, might promote the identification of subjects at risk of cardiovascular disease and potentially improve the access to care and motivate subjects to follow a healthier lifestyle.
ACKNOWLEDGMENTS
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Funding Sources: The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging, R01 HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827.
List of Abbreviations and Acronyms
- ABI
Ankle Brachial Index
- CHD
Coronary Heart Disease
- CHS
Cardiovascular Health Study
- CVD
Cardiovascular Disease
- HR
Hazard Ratio
- IMT
Intima Media Thickness
- LDL
Low Density Lipoproteins
- LV
Left ventricle
- MI
Myocardial Infarction
- OR
Odds Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hengstenberg C, Holmer SR, Mayer B, Engel S, Schneider A, Lowel H, et al. Siblings of myocardial infarction patients are overlooked in primary prevention of cardiovascular disease. Eur Heart J. 2001;22:926–933. doi: 10.1053/euhj.2000.2413. [DOI] [PubMed] [Google Scholar]
- 2.McCusker ME, Yoon PW, Gwinn M, Malarcher AM, Neff L, Khoury MJ. Family history of heart disease and cardiovascular disease risk-reducing behaviors. Genet Med. 2004;6:153–158. doi: 10.1097/01.gim.0000127271.60548.89. [DOI] [PubMed] [Google Scholar]
- 3.Murabito JM, Nam BH, D'Agostino RB, Sr, Lloyd-Jones DM, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Annals of internal medicine. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 4.Murabito JM, Pencina MJ, Nam BH, D'Agostino RB, Sr, Wang TJ, Lloyd-Jones D, et al. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 5.Hauser ER, Mooser V, Crossman DC, Haines JL, Jones CH, Winkelmann BR, et al. Design of the Genetics of Early Onset Cardiovascular Disease (GENECARD) study. Am Heart J. 2003;145:602–613. doi: 10.1067/mhj.2003.13. [DOI] [PubMed] [Google Scholar]
- 6.Munger RG, Prineas RJ, Gomez-Marin O. Persistent elevation of blood pressure among children with a family history of hypertension: the Minneapolis Children's Blood Pressure Study. J Hypertens. 1988;6:647–653. doi: 10.1097/00004872-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Shear CL, Webber LS, Freedman DS, Srinivasan SR, Berenson GS. The relationship between parental history of vascular disease and cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Epidemiol. 1985;122:762–771. doi: 10.1093/oxfordjournals.aje.a114159. [DOI] [PubMed] [Google Scholar]
- 8.Clarke WR, Schrott HG, Burns TL, Sing CF, Lauer RM The Muscatine Study. Aggregation of blood pressure in the families of children with labile high systolic blood pressure. Am J Epidemiol. 1986;123:67–80. doi: 10.1093/oxfordjournals.aje.a114225. [DOI] [PubMed] [Google Scholar]
- 9.Annest JL, Sing CF, Biron P, Mongeau JG. Familial aggregation of blood pressure and weight in adoptive families. II. Estimation of the relative contributions of genetic and common environmental factors to blood pressure correlations between family members. Am J Epidemiol. 1979;110:492–503. doi: 10.1093/oxfordjournals.aje.a112830. [DOI] [PubMed] [Google Scholar]
- 10.Laskarzewski P, Morrison JA, Horvitz R, Khoury P, Kelly K, Mellies M, et al. The relationship of parental history of myocardial infarction, hypertension, diabetes and stroke to coronary heart disease risk factors in their adult progeny. Am J Epidemiol. 1981;113:290–306. doi: 10.1093/oxfordjournals.aje.a113099. [DOI] [PubMed] [Google Scholar]
- 11.Moll PP, Sing CF, Weidman WH, Gordon H, Ellefson RD, Hodgson PA, et al. Total cholesterol and lipoproteins in school children: prediction of coronary heart disease in adult relatives. Circulation. 1983;67:127–134. doi: 10.1161/01.cir.67.1.127. [DOI] [PubMed] [Google Scholar]
- 12.Glueck CJ, Laskarzewski PM, Suchindran CM, Chambless LE, Barrett-Connor E, Stewart P, et al. The Lipid Research Clinics Program Prevalence Study. Progeny's lipid and lipoprotein levels by parental mortality. Circulation. 1986;73:I51–I61. [PubMed] [Google Scholar]
- 13.Ibsen KK, Lous P, Andersen GE. Coronary heart risk factors in 177 children and young adults whose fathers died from ischemic heart disease before age 45. Acta Paediatr Scand. 1982;71:609–613. doi: 10.1111/j.1651-2227.1982.tb09483.x. [DOI] [PubMed] [Google Scholar]
- 14.Khaw KT, Barrett-Connor E. Family history of heart attack: a modifiable risk factor? Circulation. 1986;74:239–244. doi: 10.1161/01.cir.74.2.239. [DOI] [PubMed] [Google Scholar]
- 15.Jood K, Ladenvall C, Rosengren A, Blomstrand C, Jern C. Family history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2005;36:1383–1387. doi: 10.1161/01.STR.0000169944.46025.09. [DOI] [PubMed] [Google Scholar]
- 16.Meschia JF, Brott TG, Brown RD, Jr, Kissela BM, Hardy JA, Brown WM, et al. Correlation of proband and sibling stroke latency: the SWISS Study. Neurology. 2005;64:1061–1063. doi: 10.1212/01.WNL.0000154602.20719.E8. [DOI] [PubMed] [Google Scholar]
- 17.Beaty TH, Neel JV, Fajans SS. Identifying risk factors for diabetes in first degree relatives of non-insulin dependent diabetic patients. Am J Epidemiol. 1982;115:380–397. doi: 10.1093/oxfordjournals.aje.a113316. [DOI] [PubMed] [Google Scholar]
- 18.Selby JV, Newman B, King MC, Friedman GD. Environmental and behavioral determinants of fasting plasma glucose in women. A matched co-twin analysis. Am J Epidemiol. 1987;125:979–988. doi: 10.1093/oxfordjournals.aje.a114636. [DOI] [PubMed] [Google Scholar]
- 19.Garn SM. Family-line and socioeconomic factors in fatness and obesity. Nutr Rev. 1986;44:381–386. doi: 10.1111/j.1753-4887.1986.tb07581.x. [DOI] [PubMed] [Google Scholar]
- 20.Perkins KA. Family history of coronary heart disease: is it an independent risk factor? Am J Epidemiol. 1986;124:182–194. doi: 10.1093/oxfordjournals.aje.a114377. [DOI] [PubMed] [Google Scholar]
- 21.Ten Kate LP, Boman H, Daiger SP, Motulsky AG. Familial aggregation of coronary heart disease and its relation to known genetic risk factors. Am J Cardiol. 1982;50:945–953. doi: 10.1016/0002-9149(82)90400-3. [DOI] [PubMed] [Google Scholar]
- 22.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. The New England journal of medicine. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 23.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. Journal of internal medicine. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 24.Deutscher S, Ostrander LD, Epstein FH. Familial factors in premature coronary heart disease--a preliminary report from the Tecumseh Community Health Study. Am J Epidemiol. 1970;91:233–237. doi: 10.1093/oxfordjournals.aje.a121133. [DOI] [PubMed] [Google Scholar]
- 25.Higgins M. Epidemiology and prevention of coronary heart disease in families. The American journal of medicine. 2000;108:387–395. doi: 10.1016/s0002-9343(99)00409-x. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins PN, Williams RR. Human genetics and coronary heart disease: a public health perspective. Annual review of nutrition. 1989;9:303–345. doi: 10.1146/annurev.nu.09.070189.001511. [DOI] [PubMed] [Google Scholar]
- 27.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 28.Meschia JF, Brown RD, Jr, Brott TG, Chukwudelunzu FE, Hardy J, Rich SS. The Siblings With Ischemic Stroke Study (SWISS) protocol. BMC medical genetics. 2002;3:1. doi: 10.1186/1471-2350-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stegmayr B, Asplund K. Measuring stroke in the population: quality of routine statistics in comparison with a population-based stroke registry. Neuroepidemiology. 1992;11:204–213. doi: 10.1159/000110933. [DOI] [PubMed] [Google Scholar]
- 30.Wiklund PG, Brown WM, Brott TG, Stegmayr B, Brown RD, Jr, Nilsson-Ardnor S, et al. Lack of aggregation of ischemic stroke subtypes within affected sibling pairs. Neurology. 2007;68:427–431. doi: 10.1212/01.wnl.0000252955.17126.6a. [DOI] [PubMed] [Google Scholar]
- 31.Burke GL, Savage PJ, Sprafka JM, Selby JV, Jacobs DR, Jr, Perkins LL, et al. The CARDIA study. Relation of risk factor levels in young adulthood to parental history of disease. Circulation. 1991;84:1176–1187. doi: 10.1161/01.cir.84.3.1176. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–1069. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
- 33.Sholtz RI, Rosenman RH, Brand RJ. The relationship of reported parental history to the incidence of coronary heart disease in the Western Collaborative Group Study. Am J Epidemiol. 1975;102:350–356. doi: 10.1093/oxfordjournals.aje.a112171. [DOI] [PubMed] [Google Scholar]
- 34.Snowden CB, McNamara PM, Garrison RJ, Feinleib M, Kannel WB, Epstein FH. Predicting coronary heart disease in siblings--a multivariate assessment: the Framingham Heart Study. Am J Epidemiol. 1982;115:217–222. doi: 10.1093/oxfordjournals.aje.a113293. [DOI] [PubMed] [Google Scholar]
- 35.Flossmann E, Rothwell PM. Family history of stroke in patients with transient ischemic attack in relation to hypertension and other intermediate phenotypes. Stroke. 2005;36:830–835. doi: 10.1161/01.STR.0000158920.67013.53. [DOI] [PubMed] [Google Scholar]
- 36.Lisabeth LD, Smith MA, Brown DL, Uchino K, Morgenstern LB. Family history and stroke outcome in a bi-ethnic, population-based stroke surveillance study. BMC Neurol. 2005;5:20. doi: 10.1186/1471-2377-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisabeth LD, Kardia SL, Smith MA, Fornage M, Morgenstern LB. Family history of stroke among Mexican-American and non-Hispanic white patients with stroke and TIA: implications for the feasibility and design of stroke genetics research. Neuroepidemiology. 2005;24:96–102. doi: 10.1159/000081056. [DOI] [PubMed] [Google Scholar]
- 38.Flossmann E, Rothwell PM. Family history of stroke does not predict risk of stroke after transient ischemic attack. Stroke. 2006;37:544–546. doi: 10.1161/01.STR.0000198879.11072.f2. [DOI] [PubMed] [Google Scholar]
- 39.Forde OH, Thelle DS. The Tromso heart study: risk factors for coronary heart disease related to the occurrence of myocardial infarction in first degree relatives. Am J Epidemiol. 1977;105:192–199. doi: 10.1093/oxfordjournals.aje.a112375. [DOI] [PubMed] [Google Scholar]
- 40.Yoon PW, Jorgensen C, Hariri S. Awareness of Family Health History as a Risk Factor for Disease -- United States, 2004. MMWR. 2004;53:1044–1047. [PubMed] [Google Scholar]