Abstract
Folate deficiency has been implicated in the etiology of unipolar depression. In this study, we attempted to cross-link plasma folate, depressive symptoms, and dietary quality (or dietary intake of folate) together in a comprehensive framework, while examining effect modification of those associations by sex. This was a cross-sectional, population-based study of 1681 participants aged 30–64 y (Healthy Aging in Neighborhoods of Diversity across the Lifespan Study). Participants were administered the Center for Epidemiologic Studies Depression scale (CES-D). Measures of plasma folate and dietary intakes (2 24-h recalls) from which the 2005-Healthy Eating Index (HEI) was computed were available. Multivariate logistic regression and structural equation modeling (SM) were conducted. Compared with the lowest tertile, the middle and uppermost tertiles of plasma folate were associated with a 39–40% reduced odds of elevated CES-D (≥16) among women [adjusted odds ratio (T3 vs. T1) = 0.60 (95% CI = 0.42–0.86); P = 0.006]. Confounding of this association by HEItotal was noted among both men and women, although dietary folate did not confound this association appreciably. In SM, plasma folate completely mediated the inverse HEItotal-CES-D association among men only, specifically for HEI2 (higher intakes of whole fruits), HEI3 (total vegetables), HEI5 (total grains), HEI6 (whole grains), HEI7 (milk), and HEI12 (lower discretionary energy). Among women, HEItotal and 4 components had an inverse direct effect on CES-D score, suggesting a mechanism that is independent of plasma folate. Depressive symptoms in our study may be alleviated by improving overall dietary quality, with plasma folate playing a potential mediating role only among men.
Introduction
Unipolar depression, a potentially life-long illness (1–3), is among the most prevalent diseases in the health care spectrum (4). In the US, its lifetime prevalence for men and women is 12 and 21%, respectively (4). Among many putative risk factors, folate deficiency has been recognized since the 1960s as an important contributor to the development of depression (5–8) and there is need for further research to strengthen the evidence for a protective effect of folate supplementation (7,8). Other reports suggest that folate supplementation may also enhance the effectiveness of certain antidepressant regimens (9,10).
Folate deficiency has been implicated in the etiology of numerous other disorders, such as neural tube defects (11), coronary heart disease (12), and dementia (13). Its link to neural tube defects prompted the U.S. government in 1998 to mandate fortification of the food supply with folic acid, a program that was deemed effective at reducing both folate deficiency (14,15) and the incidence of neural tube defects (16). However, at least 1 large observational study conducted among U.S. Latinos following this program's implementation suggested that despite a reduction in folate deficiency prevalence, lower relative plasma folate status may still be linked to an elevated number of depressive symptoms as measured by the Center for Epidemiologic Studies-Depression (CES-D)9 score, although only among women (17). Other U.S. studies, however, did not find such an association (18,19). Similarly, in some non-U.S. studies, an inverse association was uncovered between dietary intakes of folate and depression or depressive symptoms (20–24). The association of plasma and RBC folate status with depression or depressive symptoms is also equivocal according to several non-U.S. studies and 1 U.S. study (5,6,25–34). Inconsistent effect modification by sex emerged in a number of studies with blood or dietary folate as the main exposure (9,17,22–24). Nevertheless, to our knowledge, no study thus far has attempted to cross-link plasma folate, depressive symptoms, and dietary quality (or dietary intake of folate) together in a comprehensive framework, while examining effect modification of those associations by sex.
In this article, we present a cross-sectional analysis of the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study to examine the association between plasma folate level and depressive symptoms while accounting for the role of dietary quality and dietary intake of folate in explaining these associations and assessing effect modification of these associations by sex.
Materials and Methods
Database.
The HANDLS study is an ongoing prospective cohort study of a baseline representative sample of African Americans and Whites (30–64 y old) living in Baltimore, Maryland. Participants were recruited using an area probability sampling design of 12 census segments. Screening, recruitment, and household interview were completed in phase 1 and examinations in mobile medical research vehicles were carried out in phase 2 of the study. The study was initiated in 2003 and the first follow-up wave data collection is currently under way. The protocol for HANDLS was approved by the Institutional Review Board of the National Institute on Aging.
Study population.
Of the 3724 individuals selected to participate in phase 1 (SAMPLE 1), 2436 (65.4%) so far completed their baseline phase 2 examination (sample 2a). Our data were from a subset with 2 d of dietary recall, CES-D, anthropometric, and plasma folate data (n = 1681; sample 2b). There were notable income level differences between samples 2b and 1, with participants in sample 2b having a higher percentage of poor [i.e. poverty income ratio (PIR) <125%; 48 vs. 36%; P < 0.05], although age, sex, and race/ethnicity distributions did not differ.
CES-D scale and elevated depressive symptoms.
As part of cognitive and neuropsychological testing (35), baseline depressive symptoms were measured using the CES-D scale, a 20-item self-report symptom rating scale that emphasizes the affective, depressed mood component (36). The CES-D, previously shown to have an invariant factor structure between the NHANES I and HANDLS data (37), was used both as a continuous score and a binary one, with a score ≥16 indicating moderate or severe levels of depressive symptoms, as was done in previous studies [e.g. (17)].
Plasma folate.
Fasting blood was collected from each participant during the medical research vehicle phase 2 visits. Specimens in volumes of 2 mL plasma were collected in small tubes and refrigerated. Plasma folate was measured using enzyme immunoassay. Values >50 µg/L were excluded from the main analyses, because they were considered as outliers (n = 5; range: 52–93 μg/L) (Folate, 1 μg/L = 2.266 nmol/L). Standardized Z-scores and tertiles were computed for the major parts of the analyses.
Dietary intake measurement
Dietary assessment.
Two 24-h dietary recalls were administered by trained interviewers using the USDA Automated Multiple Pass Method, a standardized 5-step process that was validated for intakes of protein, carbohydrate, fat, and energy in obese and nonobese individuals (38–40). USDA food codes were used and their amounts were estimated and then converted to equivalents by use of servings/food code databases available for the Continuing Surveys of Food Intake by Individuals 1994–98 and NHANES 1999–2000 and 2001–02 (41). Other databases were also available for converting grams of USDA food codes into nutrients consumed per day (42). Both food equivalents and nutrient intakes were summed for each individual per recall day and averaged over the 2 24-h recalls. Total intake of folate was of primary interest, although intakes naturally occurring in foods (aside from fortification) were also considered for descriptive purposes. Crude values, standardized Z-scores, and tertiles of dietary folate intakes were considered in the analyses.
Dietary quality.
The 2005 USDA Healthy Eating Index (HEI) (43) was used to measure overall dietary quality using the average intakes from the 2 24-h recalls. The 2005-HEI total score combines scores on 12 individual components and is measured on a scale of 0–100, i.e. the higher the total HEI score, the better the diet. The standards for each component considered are now based on an energy density approach (43). Each of the 12 components of the HEI was considered in the descriptive analyses and structural equation modeling (SM), while the overall score was considered for descriptive and all subsequent analyses (Supplemental Table 1).
Covariates
Covariates included in our analyses included sex, age, race/ethnicity (White vs. African American), marital status (married vs. unmarried), completed years of education [<high school (HS), HS, and >HS], PIR <125% for “poor,” measured BMI (kg/m2), lifetime use of drugs (“used opiates, marijuana or cocaine” vs. not), and smoking status (0, never or former smoker, and 1, current smoker). These covariates were considered exogenous in the SM and potential confounders in regression models.
Statistical methods
All analyses except for SM were conducted using Stata release 10.0 (44). First, we described the HANDLS participants' study sample characteristics by sex and depressive symptoms levels (i.e. CES-D ≥16 vs. CES-D <16). Differences in means between groups were tested using ANOVA and t tests. Associations between categorical variables were tested using χ2 tests. Similarly, a comparison of tertiles of plasma folate in terms of sociodemographic and lifestyle characteristics as well as dietary factors, using χ2 test, was performed, stratifying by sex.
Second, multivariate logistic regression models with target outcome being high CES-D score (i.e. CES-D ≥16) were conducted to test the association between folate status (entered as a Z-score) and depressive symptoms, controlling for potential confounders and stratifying by sex. Potential confounding effects of total HEI score and dietary folate on the plasma folate-depressive symptoms association was examined with the change-in-estimate methodology, retaining sociodemographic and lifestyle covariates in the model. A change-in-estimate value of ≥10% was considered as appreciable confounding (45). In a separate model with main effect of sex added, the significance of the plasma exposure × sex term was tested to assess effect modification by sex.
Third, multivariate linear models were conducted in which dietary intake of folate and HEItotal were considered as alternative independent variables (X) predicting continuous CES-D score (Y) and plasma folate (M) was entered into the model to assess potential mediating effects. To this end, 3 models were run: a) Y predicted by X; b) Y predicted by X and M; and c) M predicted by X. Using findings from all 3 models, the percentage of total effect of X on Y that was mediated by M was computed as well as a Sobel-Goodman test, a Z-score indicating significant mediating effects (46). Among the findings, we focused on those in which (a) yielded at least a borderline significant total effect of X on Y. In all 3 models (a, b, and c), we controlled for the sociodemographic and lifestyle covariates listed above.
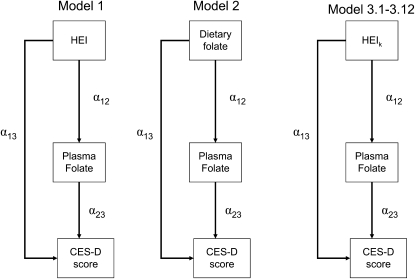
Fourth, using SAS CALIS procedure in SAS version 9.2 (47), we fit SM to determine the most likely pathway explaining the plasma folate vs. CES-D association. A theoretical model was set up in which sociodemographic, lifestyle, and metabolic factors were exogenous to the main variables of interest. The model consisted of simultaneous equations with outcomes being the dietary factor [X = HEItotal (model 1), dietary folate as Z-score (model 2), and HEIk (k = 1–12), alternatively (models 3.1–3.12)], plasma folate (Z-score), and CES-D score for each sex, separately (See Eq. 1 through 3). Significance of each path coefficient was assessed using a t test with 1 df. Global fit indices included the root mean square error of approximation (RMSEA) with its 90% CI (criterion is ≤0.06 for good fit), Goodness of Fit Index adjusted for df (AGFI), which ranges between 0 (poor fit) and 1 (model fits). Full models were reduced by removing exogenous variables with observed path coefficients that were nonsignificant at a 0.10 type I error. In all other analyses, a type I error of 0.05 was considered significant, while a level of 0.10 was considered borderline significant.
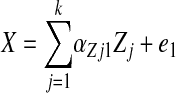 |
(Eq.1) |
 |
(Eq.2) |
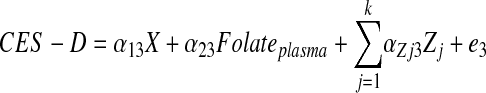 |
(Eq.3) |
where Zj is the vector of other exogenous variables (e.g. sociodemographic and lifestyle factors) and e1 through e3 are the error terms (Fig. 1).
FIGURE 1 .
Structural equations model for the association between dietary quality (HEItotal)/dietary folate/components of HEI and depressive symptoms (continuous CES-D score), stratified by sex: mediation effects through plasma folate
Results
The prevalence of depressive symptoms as measured by a CES-D score ≥16 was 21.2% in men and 32.1% in women (P < 0.05) (Table 1). Participants with depressive symptoms were in general less educated, poorer, more likely to be current smokers, had a lower level of plasma folate, and had a reduced HEI (overall score and selected components). Women with symptoms suggesting depression were younger, more likely to be White, and had a reduced naturally occurring folate intake (P < 0.05). The mean difference in HEI (overall score) between the depressed (CES-D score ≥16) and nondepressed (CES-D score <16) groups by sex was greater for women (P < 0.0001). Sex differences for a number of sociodemographic and lifestyle factors are also presented (Table 1). It is worth noting that among women, only 8 were postpartum or had at least 1 child <1 y old (data not shown).
TABLE 1.
Selected characteristics of HANDLS participants by sex and CES-D score1
| Men, n = 734 |
Women, n = 947 |
Men vs. women | Low vs. high CES-D score among men | Low vs. high CES-D score among women | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <16 | ≥16 | All men | <16 | ≥16 | All women | ||||||
| n | 578 | 156 | 734 | 643 | 304 | 947 | P2 | ||||
| Age, y | 47.9 ± 9.4 | 47.7 ± 8.9 | 47.9 ± 9.3 | 48.4 ± 9.4 | 47.2 ± 8.9 | 47.9 ± 9.2 | 0.9466 | 0.7809 | 0.037 | ||
| African-American, % | 60.2 | 64.1 | 61.0 | 61.1 | 54.3 | 58.9 | 0.381 | 0.3760 | 0.046 | ||
| Married, % | 53.5 | 51.3 | 53.0 | 44.3 | 36.2 | 41.0 | <0.0001 | 0.2770 | 0.060 | ||
| Education, % | |||||||||||
| <HS | 6.4 | 11.5 | 7.5 | 4.7 | 7.2 | 5.5 | 0.216 | 0.009 | 0.002 | ||
| HS | 55.4 | 62.8 | 56.9 | 51.9 | 61.8 | 55.1 | |||||
| >HS | 32.5 | 20.5 | 30.0 | 37.0 | 25.0 | 33.2 | |||||
| Missing | 5.7 | 5.1 | 5.6 | 6.4 | 5.9 | 6.2 | |||||
| PIR <125%, % | 40.3 | 59.0 | 44.3 | 44.5 | 62.5 | 50.3 | 0.015 | <0.0001 | <0.001 | ||
| Current smoking status, % | |||||||||||
| Currently smoking | 48.6 | 59.0 | 50.8 | 35.9 | 46.0 | 39.2 | <0.0001 | 0.043 | 0.009 | ||
| Missing | 6.9 | 7.7 | 7.1 | 8.6 | 8.6 | 8.6 | |||||
| Ever used illicit drugs, % | |||||||||||
| Used any type | 68.5 | 68.6 | 68.5 | 50.4 | 58.2 | 52.9 | <0.0001 | 0.992 | 0.077 | ||
| Missing | 5.5 | 5.8 | 5.6 | 7.0 | 5.6 | 6.6 | |||||
| BMI, kg⋅m−2 | 28.0 ± 6.3 | 27.8 ± 6.4 | 28.0 ± 6.3 | 31.3 ± 9.3 | 31.8 ± 8.9 | 31.5 ± 9.2 | <0.0001 | 0.6682 | 0.513 | ||
| CES-D score1 | 7.6 ± 4.2 | 21.7 ± 4.8 | 10.6 ± 7.2 | 7.4 ± 4.4 | 22.6 ± 5.7 | 12.3 ± 8.6 | <0.0001 | <0.0001 | <0.001 | ||
| Plasma folate status,3μg/L | 14.9 ± 7.4 | 13.4 ± 5.6 | 14.6 ± 7.1 | 14.8 ± 6.8 | 13.2 ± 6.2 | 14.3 ± 6.6 | 0.4696 | 0.0259 | <0.001 | ||
| Dietary folate, μg/d | |||||||||||
| Total | 416.8 ± 281.7 | 378.4 ± 236.4 | 408.7 ± 281.1 | 320.6 ± 202.2 | 305.5 ± 205.0 | 316.8 ± 203.1 | <0.0001 | 0.1297 | 0.285 | ||
| Naturally found in food | 206.3 ± 117.8 | 191.3 ± 112.9 | 203.1 ± 116.9 | 174.5 ± 113.2 | 156.3 ± 7.0 | 168.7 ± 116.6 | <0.0001 | 0.1557 | 0.024 | ||
| HEI components4 | |||||||||||
| HEI1:total fruit (includes 100% juice) | 2.0 ± 1.6 | 1.7 ± 1.5 | 1.9 ± 1.6 | 2.3 ± 1.7 | 2.0 ± 1.6 | 2.2 ± 1.7 | 0.0017 | 0.0171 | 0.003 | ||
| HEI2:whole fruit (not juice) | 2.2 ± 1.8 | 1.8 ± 1.7 | 2.1 ± 1.8 | 2.6 ± 1.3 | 2.3 ± 1.9 | 2.5 ± 1.9 | <0.0001 | 0.0138 | 0.005 | ||
| HEI3:total vegetables | 2.6 ± 1.3 | 2.6 ± 1.3 | 2.4 ± 1.3 | 3.0 ± 1.3 | 2.6 ± 1.4 | 2.9 ± 1.4 | <0.0001 | 0.1363 | <0.001 | ||
| HEI4:dark green and orange vegetables and legumes | 1.0 ± 1.3 | 0.8 ± 1.1 | 1.0 ± 1.2 | 1.5 ± 1.6 | 1.0 ± 1.2 | 1.3 ± 1.6 | <0.0001 | 0.0651 | <0.001 | ||
| HEI5:total grains | 4.0 ± 1.0 | 4.1 ± 1.0 | 4.0 ± 1.0 | 4.0 ± 1.1 | 4.2 ± 1.0 | 4.0 ± 1.0 | 0.8823 | 0.4398 | 0.029 | ||
| HEI6:whole grains | 1.1 ± 1.1 | 1.0 ± 1.1 | 1.1 ± 1.1 | 1.2 ± 1.3 | 1.3 ± 1.3 | 1.3 ± 1.3 | 0.0056 | 0.2976 | 0.942 | ||
| HEI7:dairy | 3.9 ± 2.6 | 3.6 ± 2.7 | 3.8 ± 2.6 | 3.9 ± 2.8 | 3.9 ± 2.8 | 3.9 ± 2.8 | 0.6707 | 0.2134 | 0.790 | ||
| HEI8:meat and beans | 9.1 ± 1.7 | 9.0 ± 1.8 | 9.1 ± 1.8 | 9.1 ± 1.7 | 8.5 ± 2.3 | 8.9 ± 2.0 | 0.0788 | 0.3929 | <0.001 | ||
| HEI9:oils | 5.6 ± 2.9 | 5.3 ± 3.2 | 5.5 ± 3.0 | 6.1 ± 3.1 | 5.8 ± 2.9 | 6.0 ± 3.0 | 0.0010 | 0.2378 | 0.189 | ||
| HEI10:saturated fat | 5.1 ± 3.2 | 5.5 ± 3.1 | 5.1 ± 3.2 | 5.4 ± 3.2 | 5.2 ± 3.3 | 5.3 ± 3.2 | 0.2417 | 0.1737 | 0.337 | ||
| HEI11:sodium | 3.9 ± 2.7 | 4.3 ± 2.7 | 4.0 ± 2.7 | 4.0 ± 2.8 | 4.0 ± 2.9 | 4.0 ± 2.8 | 0.7720 | 0.0739 | 0.803 | ||
| HEI12:energy from solid fat, alcohol, and added sugar | 7.4 ± 5.9 | 6.1 ± 5.8 | 7.1 ± 5.9 | 8.4 ± 6.2 | 6.9 ± 5.8 | 7.9 ± 6.1 | 0.0087 | 0.0154 | <0.001 | ||
| HEItotal | 47.9 ± 11.6 | 45.6 ± 10.0 | 47.4 ± 11.3 | 51.6 ± 12.8 | 47.6 ± 11.7 | 50.3 ± 12.6 | <0.0001 | 0.0210 | <0.001 | ||
Values are mean ± SD or percent.
P-value was based on 1-way ANOVA when row variable was continuous and χ2 test when row variable was categorical.
1 ng/mL folate = 2.266 nmol/L.
Note that total HEI score may range between 0 and 100, whereas each component may range between 0 and 5, 0 and 10, or 0 and 20 depending on the component in question. A higher score indicated better dietary quality given the 2005 dietary guidelines (63).
Further, we examined associations between tertiles of plasma folate on one hand and sociodemographic, lifestyle factors, and HEI scores on the other hand (Table 2). In general, among both men and women, participants in the upper tertile of folate compared with the lowest were older, less likely to be African-American, more likely to have >HS education, less likely to be poor (PIR <125%), and had a lower prevalence of current smoking, a lower mean CES-D score, a higher mean total and naturally occurring dietary folate intake, and a higher total HEI score. Among men, mean BMI was higher in the upper folate tertile compared with the lowest, whereas the opposite pattern was observed in women. Among HEI components, HEI11 (sodium as percent of energy intake) in men but not in women was inversely related to plasma folate tertiles. All other components, when significantly associated with folate tertiles, followed the same pattern as the total HEI score.
TABLE 2.
| Men, n = 734 |
Women, n = 947 |
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P3 | T1 | T2 | T3 | P2 | |
| Age, y | 46.9 ± 9.5 | 47.2 ± 9.2 | 49.7 ± 9.0 | 0.0012 | 46.0 ± 8.7 | 47.6 ± 9.6 | 50.2 ± 8.9 | <0.001 |
| African-American, % | 69.5 | 61.0 | 53.2 | 0.002 | 63.6 | 65.0 | 48.6 | <0.001 |
| Married, % | 51.3 | 55.0 | 52.3 | 0.413 | 39.1 | 41.6 | 44.6 | 0.403 |
| Education, % | ||||||||
| <HS | 7.1 | 5.9 | 9.7 | 0.001 | 5.4 | 4.8 | 6.2 | <0.001 |
| HS | 62.8 | 58.3 | 49.8 | 62.4 | 59.1 | 43.9 | ||
| >HS | 24.8 | 27.3 | 38.0 | 26.3 | 29.9 | 43.3 | ||
| Missing | 5.3 | 8.5 | 2.5 | 6.0 | 6.2 | 6.5 | ||
| PIR <125%, % | 50.9 | 44.3 | 38.0 | 0.020 | 60.0 | 49.5 | 40.8 | <0.001 |
| Current smoking status, % | ||||||||
| Currently smoking | 62.4 | 50.6 | 40.1 | <0.0001 | 53.4 | 36.1 | 27.1 | <0.001 |
| Missing | 10.2 | 5.2 | 6.3 | 9.0 | 8.9 | 7.8 | ||
| Ever used illicit drugs, % | ||||||||
| Used any type | 68.1 | 69.0 | 68.3 | 0.209 | 57.9 | 50.2 | 50.2 | 0.124 |
| Missing | 8.4 | 3.7 | 5.1 | 7.2 | 6.9 | 5.6 | ||
| BMI, kg⋅m−2 | 27.1 ± 6.1 | 28.6 ± 6.6 | 28.1 ± 6.0 | 0.0307 | 32.3 ± 9.1 | 31.9 ± 10.1 | 30.2 ± 8.1 | 0.007 |
| CES-D score | 11.5 ± 7.5 | 10.7 ± 7.0 | 9.7 ± 7.1 | 0.0212 | 13.8 ± 8.4 | 12.0 ± 9.0 | 10.8 ± 8.2 | <0.001 |
| CES-D score ≥16, % | 23.9 | 22.1 | 17.7 | 0.242 | 40.6 | 28.2 | 26.8 | <0.001 |
| Plasma folate,4μg/L | 7.9 ± 2.0 | 13.3 ± 1.6 | 22.3 ± 6.7 | <0.0001 | 7.8 ± 1.9 | 13.3 ± 1.6 | 22.1 ± 4.2 | <0.001 |
| Dietary folate, μg/d | ||||||||
| Total | 336.3 ± 194.7 | 406.5 ± 294.5 | 480.1 ± 316.1 | <0.0001 | 270.6 ± 182.1 | 295.4 ± 162.4 | 381.3 ± 237.8 | <0.001 |
| Food | 178.9 ± 95.0 | 204.1 ± 115.9 | 225.1 ± 131.9 | <0.0001 | 149.7 ± 111.4 | 166.9 ± 113.4 | 190.1 ± 121.5 | <0.001 |
| HEI components5 | ||||||||
| HEI1:total fruit (includes 100% juice) | 1.7 ± 1.6 | 2.0 ± 1.6 | 2.1 ± 1.6 | 0.0182 | 1.8 ± 1.5 | 2.2 ± 1.7 | 2.7 ± 1.7 | <0.001 |
| HEI2:whole fruit (not juice) | 1.8 ± 1.7 | 2.1 ± 1.7 | 2.3 ± 1.8 | 0.0048 | 2.0 ± 1.7 | 2.4 ± 1.8 | 3.1 ± 1.9 | <0.001 |
| HEI3:total vegetables | 2.4 ± 1.2 | 2.5 ± 1.2 | 2.7 ± 1.3 | 0.0157 | 2.8 ± 1.3 | 2.8 ± 1.4 | 3.0 ± 1.4 | 0.073 |
| HEI4:dark green and orange vegetables and legumes | 0.8 ± 1.0 | 1.1 ± 1.3 | 1.1 ± 1.4 | 0.0157 | 1.1 ± 1.5 | 1.3 ± 1.5 | 1.6 ± 1.6 | 0.002 |
| HEI5:total grains | 3.8 ± 1.1 | 4.1 ± 1.0 | 4.2 ± 0.9 | <0.0001 | 3.9 ± 1.1 | 4.1 ± 1.0 | 4.2 ± 1.0 | <0.001 |
| HEI6:whole grains | 0.8 ± 0.8 | 1.0 ± 1.1 | 1.4 ± 1.3 | <0.0001 | 0.9 ± 1.1 | 1.3 ± 1.3 | 1.6 ± 1.4 | <0.001 |
| HEI7:dairy | 3.3 ± 2.4 | 3.7 ± 2.6 | 4.5 ± 2.7 | <0.0001 | 3.2 ± 2.3 | 3.9 ± 2.8 | 4.5 ± 3.1 | <0.001 |
| HEI8:meat and beans | 9.2 ± 1.5 | 9.1 ± 1.9 | 8.9 ± 1.9 | 0.1170 | 9.0 ± 1.8 | 9.0 ± 1.9 | 8.7 ± 2.1 | 0.077 |
| HEI9:oils | 5.3 ± 3.0 | 5.6 ± 3.1 | 5.7 ± 2.9 | 0.2897 | 5.7 ± 2.9 | 6.1 ± 3.1 | 6.2 ± 3.1 | 0.092 |
| HEI10:saturated fat | 5.1 ± 3.2 | 5.4 ± 3.2 | 5.0 ± 3.2 | 0.3659 | 5.2 ± 3.3 | 5.4 ± 3.2 | 5.4 ± 3.2 | 0.580 |
| HEI11:sodium | 4.4 ± 2.9 | 3.9 ± 2.6 | 3.6 ± 2.6 | 0.0046 | 4.2 ± 2.9 | 3.8 ± 2.9 | 4.0 ± 2.7 | 0.370 |
| HEI12:energy from solid fat, alcohol, and added sugar | 5.6 ± 5.5 | 7.2 ± 5.8 | 8.6 ± 6.0 | <0.0001 | 6.3 ± 5.5 | 8.0 ± 6.2 | 9.5 ± 6.3 | 0.036 |
| HEItotal | 44.2 ± 10.4 | 47.6 ± 11.0 | 50.2 ± 11.9 | <0.0001 | 46.1 ± 10.8 | 50.5 ± 12.1 | 54.6 ± 13.4 | <0.001 |
Values are mean ± SD or percent.
T, Tertile.
P-value was based on 1-way ANOVA test when row variable was continuous and χ2 test when row variable was categorical.
1 ng/mL folate = 2.266 nmol/L.
Note that total HEI score may range between 0 and 100, whereas each component may range between 0 and 5, 0 and 10, or 0 and 20 depending on the component in question. A higher score indicated better dietary quality given the 2005 dietary guidelines (63).
Unadjusted and multivariate adjusted logistic regression models were conducted, with the main exposures being plasma folate (tertiles) and outcome being CES-D score ≥16 (Table 3). In the unadjusted model, the middle tertile compared with the lowest tertile of plasma folate was associated with a reduced odds of elevated depressive symptoms among women by 43% [adjusted OR (tertile (T)2 vs. T1) = 0.57 (95% CI = 0.41–0.80); P = 0.001]. Similarly, the uppermost tertile compared with the lowest was associated with a reduced odds of this outcome by 47% (P < 0.05). After adjusting for sociodemographic and lifestyle factors (adjusted model 1), plasma folate remained associated with a reduced odds of depression among women only [adjusted OR (T3 vs. T1) = 0.60 (95% CI = 0.42–0.86); P = 0.006]. Adding the total HEI score into this model appreciably attenuated the plasma folate-depression association among women (LogeOR for plasma folate was attenuated by 18% compared with adjusted model 1). A similar attenuation of an initially nonsignificant association was noted among men. This attenuation, however, did not occur when dietary folate was added to the model (change-in-estimate of LogeOR < 10%). In all models, there was no significant effect modification of the folate-CES-D association by sex, as evidenced by a nonsignificant interaction term sex × folate in a separate model that included sex among the main effects.
TABLE 3.
OR for CES-D score ≥16 and plasma folate (tertiles), stratified by sex: confounding effects of dietary quality and dietary intake of folate (HANDLS Study)
| Men, n = 734 |
Women, n = 947 |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P | % CIE for Loge(OR)2 | OR | (95% CI) | P | % CIE for Loge(OR)2 | |
| Unadjusted model with folate | ||||||||
| Plasma folate,1μg/L | ||||||||
| T2 vs. T1 | 0.90 | (0.59, 1.38) | 0.643 | __ | 0.57b | (0.41, 0.80) | 0.001 | __ |
| T3 vs. T1 | 0.69 | (0.44, 1.08) | 0.103 | 0.53b | (0.38, 0.74) | <0.001 | ||
| Adjusted model 1: | ||||||||
| Plasma folate,1μg/L | ||||||||
| T2 vs. T1 | 0.97 | (0.63, 1.48) | 0.876 | __ | 0.61b | (0.43, 0.86) | 0.005 | __ |
| T3 vs. T1 | 0.77 | (0.48, 1.24) | 0.282 | 0.60b | (0.42, 0.86) | 0.006 | ||
| Adjusted model 2: Model 1 + HEItotal | ||||||||
| Plasma folate,1μg/L | ||||||||
| T2 vs. T1 | 0.99 | (0.64, 1.53) | 0.972 | −16.0% | 0.64b | (0.45, 0.91) | 0.012 | −17.7% |
| T3 vs. T1 | 0.80 | (0.50, 1.30) | 0.369 | 0.66b | (0.46, 0.95) | 0.027 | ||
| + HEI total | 0.99 | (0.97, 1.01) | 0.265 | 0.98b | (0.97, 0.99) | 0.007 | ||
| Adjusted model 3: Model 1 + dietary folate | ||||||||
| Plasma folate,1μg/L | ||||||||
| T2 vs. T1 | 0.98 | (0.63, 1.51) | 0.929 | −7.0% | 0.63b | (0.44, 0.89) | 0.009 | −6.5% |
| T3 vs. T1 | 0.78 | (0.48, 1.27) | 0.328 | 0.62b | (0.43, 0.89) | 0.009 | ||
| + Dietary folate, μg/d | ||||||||
| T2 vs. T1 | 1.00 | (0.63, 1.61) | 0.982 | 0.73a | (0.52, 1.03) | 0.071 | ||
| T3 vs. T1 | 0.91 | (0.57, 1.46) | 0.701 | 0.86 | (0.60, 1.23) | 0.417 | ||
1 ng/mL folate = 2.266 nmol/L.
Change in estimates of plasma exposures when dietary exposures are added into the model. Adjusted model 1 controlled for sociodemographic and lifestyle factors: age, ethnicity, marital status, education, poverty status, smoking status, illicit drug use, and BMI. Adjusted model 2 added HEItotal (Z-score) to adjusted model 1. Adjusted model 3 added dietary folate expressed as tertiles to adjusted model 1. aP < 0.10; bP < 0.05 for null hypothesis that Loge(OR) = 0 for each stratum-specific analysis.
Multivariate linear regression models were conducted to test mediation of the diet-depressive symptoms association through plasma folate (Table 4). Among men, the total effects of HEItotal on CES-D and of dietary folate on CES-D were both borderline significant in models 1a and 1b and indicated an inverse relationship between dietary intake of folate or dietary quality and number of depressive symptoms. When plasma folate was added to the model (model 1b), the effect of X (HEItotal, dietary folate) was markedly attenuated. The percentage of total effect explained by the mediator (M = plasma folate) was 17.4% in the case of HEItotal and 19.8% in the case of dietary folate, and the Sobel-Goodman test indicated significant mediation among men. Among women, however, the total effect of dietary folate on CES-D was not significant, precluding assessment of mediation. In contrast, the association between HEItotal and CES-D based on model 1a was highly significant. Adding plasma folate to that model, however, did not indicate significant mediation according to the Sobel-Goodman test. Thus, significant mediation by M of the effect of X on Y was noted only among men.
TABLE 4.
Associations between CES-D score (continuous) and dietary intake of folate and dietary quality (raw values), stratified by sex: mediating effect of plasma folate (raw values) (HANDLS study)
| Men, n = 734 |
Women, n = 947 |
|||||||
|---|---|---|---|---|---|---|---|---|
| β | ±SE | P | Sobel-Goodman Test (Z); % total effect mediated2 | β | ±SE | P | Sobel-Goodman Test (Z); % total effect mediated2 | |
| MODEL 1: HEItotal | ||||||||
| Adjusted model 1a | ||||||||
| Y = CES-D vs. X = HEItotal | −0.045a | ±0.024 | 0.063 | Z = −2.00c; 17.4% | −0.083b | ±0.023 | <0.001 | Z = −1.64;9.9% |
| Adjusted model 1b: Model 1a + plasma folate,1μg/L | ||||||||
| X = HEItotal | −0.037 | ±0.024 | 0.127 | −0.075b | ±0.024 | 0.002 | ||
| M = plasma folate | −0.085b | ±0.038 | 0.024 | −0.075a | ±0.044 | 0.094 | ||
| Adjusted model 1c | ||||||||
| Y = plasma folate vs. X = HEItotal | 0.092b | ±0.023 | <0.001 | 0.110b | ±0.017 | <0.001 | ||
| MODEL 2: dietary folate, μg/d | ||||||||
| Adjusted model 2a | ||||||||
| Y = CES-D vs. X = dietary folate | −0.002a | ±0.001 | 0.055 | Z = −2.03c; 19.8% | −0.001 | ±0.001 | 0.409 | ___d |
| Adjusted model 2b: Model 2a + plasma folate,1μg/L | ||||||||
| X = dietary folate | −0.001 | ±0.001 | 0.128 | −0.000 | ±0.001 | 0.764 | ||
| M = plasma folate | −0.084b | ±0.038 | 0.028 | −0.101b | ±0.045 | 0.025 | ||
| Adjusted model 2c | ||||||||
| Y = plasma folate vs. X = dietary folate | 0.004b | ±0.001 | <0.001 | 0.007b | ±0.001 | <0.001 | ||
1 ng/mL = 2.266 nmol/L.
Percent of total effect that is mediated is obtained using Sobel-Goodman test, specifying diet as the independent variable and plasma folate as the potential mediator. All models controlled for sociodemographic and lifestyle factors: age, ethnicity, marital status, education, poverty status, smoking status, illicit drug use, and BMI. aP < 0.10; bP < 0.05 for null hypothesis that β = 0 for each stratum-specific analysis; cP < 0.05 for Sobel-Goodman test (Z-score); dSobel-Goodman test and percent total effect mediated not applicable because total effect in Model 2a was not significant.
A series of SM (Fig. 1), examining the mediation of the association between dietary intake of folate/diet quality (HEItotal and HEI components) and CES-D through plasma level of folate are presented (Table 5). The inverse association of HEItotal with CES-D among men was completely mediated through plasma folate as shown by significant HEItotal-plasma folate and plasma folate-CES-D path coefficients and a nonsignificant direct association of HEI-CES-D (model 1, men). Among women, however, the inverse association between plasma folate and CES-D score appeared to be completely confounded by the HEItotal score, which was significantly associated with both CES-D and folate status (model 1, women). In an alternative pathway in which HEItotal was replaced by dietary folate (model 2), while dietary folate was significantly and positively associated with plasma folate among both men and women, partial mediation of plasma folate in the dietary folate-CES-D association was detected only among men with a borderline significant plasma folate-CES-D association (P < 0.10 based on t-statistic).
TABLE 5.
Structural equations model findings for CES-D score as predicted by plasma folate (Z- score) and dietary intake of folate (Z-score) or diet quality (HEItotal or components of HEI, raw values), stratified by sex (HANDLS study)1
| Men, n = 734 |
Women, n = 947 |
|||||
|---|---|---|---|---|---|---|
| α | ± SE | t-Statistic | α | ± SE | t-Statistic | |
| Model 1: overall diet quality (HEItotal) | ||||||
| Path coefficients from HEItotal | ||||||
| HEItotal → folateplasma (α12) | +0.012 | ±0.003 | 4.234b | +0.014 | ±0.002 | 6.057b |
| HEItotal → CES-D (α13) | −0.035 | ±0.025 | −1.418 | −0.070 | ±0.023 | −3.007b |
| Path coefficient from folateplasma | ||||||
| Folateplasma → CES-D (α23) | −0.606 | ±0.306 | −1.982b | −0.355 | ±0.313 | −1.135 |
| Fit statistics | RMSEA = 0.0373; 90% CI:0.0220–0.0522; AGFI = 0.9586 | RMSEA = 0.0296; 90% CI:0.0131–0.0448; AGFI = 0.9710 | ||||
| Model 2: dietary folate | ||||||
| Path coefficients from dietary folate | ||||||
| Dietary folate → Folateplasma (α12) | +0.157 | ±0.027 | 5.872b | +0.256 | ±0.032 | 7.892b |
| Dietary folate → CES-D (α13) | −0.407 | ±0.224 | −1.813a | −0.203 | ±0.329 | −0.616 |
| Path coefficient from folateplasma | ||||||
| Folateplasma → CES-D (α23) | −0.571 | 0.300 | −1.903a | −0.426 | ±0.318 | −1.339 |
| Fit statistics | RMSEA = 0.0378; 90% I:0.0227–0.0526; AGFI = 0.9590 | RMSEA = 0.0385; 90% CI:0.0256–0.0517; AGFI = 0.9625 | ||||
| Models 3.1–3.12: diet quality components (HEIk) | ||||||
| Path coefficients from HEIk | ||||||
| HEIk → folateplasma (α12k) | ||||||
| K = 1 | +0.052 | ±0.020 | +2.608b | +0.073 | ±0.017 | +4.143b |
| K = 2 | +0.064 | ±0.018 | +3.529b | +0.081 | ±0.016 | +5.120b |
| K = 3 | +0.057 | ±0.025 | +2.278b | +0.014 | ±0.021 | +0.672 |
| K = 4 | +0.057 | ±0.025 | +2.240b | +0.040 | ±0.019 | +2.168b |
| K = 5 | +0.110 | ±0.032 | +3.483b | +0.093 | ±0.027 | +3.469b |
| K = 6 | +0.146 | ±0.028 | +5.307b | +0.100 | ±0.022 | +4.538b |
| K = 7 | +0.068 | ±0.012 | +5.671b | +0.047 | ±0.010 | +4.511b |
| K = 8 | −0.036 | ±0.018 | −1.948a | −0.034 | ±0.015 | −2.331b |
| K = 9 | +0.002 | ±0.011 | +0.211 | +0.017 | ±0.009 | +1.833a |
| K = 10 | −0.001 | ±0.010 | −0.111 | +0.011 | ±0.009 | +1.216 |
| K = 11 | −0.028 | ±0.012 | −2.384b | −0.000 | ±0.010 | −0.160 |
| K = 12 | +0.024 | ±0.005 | +4.431b | +0.024 | ±0.005 | +5.042b |
| HEIk → CES-D (α13k) | ||||||
| K = 1 | −0.322 | ±0.165 | −1.946a | −0.192 | ±0.170 | −1.124 |
| K = 2 | −0.224 | ±0.151 | −1.482 | −0.207 | ±0.156 | −1.327 |
| K = 3 | −0.269 | ±0.204 | +1.231 | −0.816 | ±0.197 | −4.149b |
| K = 4 | −0.396 | ±0.206 | −1.917a | −0.657 | ±0.178 | −3.683b |
| K = 5 | +0.141 | ±0.257 | +0.551 | +0.481 | ±0.258 | +1.860a |
| K = 6 | −0.095 | ±0.235 | −0.404 | +0.317 | ±0.216 | +1.472 |
| K = 7 | −0.041 | ±0.100 | −0.411 | +0.012 | ±0.098 | +0.126 |
| K = 8 | −0.225 | ±0.145 | −1.544 | −0.034 | ±0.015 | −2.331b |
| K = 9 | −0.040 | ±0.087 | −0.457 | −0.094 | ±0.088 | −1.061 |
| K = 10 | +0.079 | ±0.080 | 0.985 | +0.009 | ±0.082 | −0.113 |
| K = 11 | +0.20 | ±0.10 | 2.073b | +0.054 | ±0.096 | +0.570 |
| K = 12 | −0.075 | ±0.046 | −1.637 | −0.106 | ±0.046 | −2.304b |
| Path coefficient from folateplasma | ||||||
| Folateplasma→ CES-D (α23) | ||||||
| K = 1 | −0.621 | ±0.295 | −2.104b | −0.457 | ±0.307 | −1.490 |
| K = 2 | −0.645 | ±0.291 | −2.215b | −0.436 | ±0.308 | −1.413 |
| K = 3 | −0.653 | ±0.296 | −2.208b | −0.587 | ±0.304 | −1.932a |
| K = 4 | −0.647 | ±0.294 | −2.202b | −0.439 | ±0.303 | −1.450 |
| K = 5 | −0.746 | ±0.294 | −2.538b | −0.567 | ±0.306 | −1.853a |
| K = 6 | −0.653 | ±0.300 | −2.175b | −0.565 | ±0.309 | −1.828a |
| K = 7 | −0.664 | ±0.150 | −2.227b | −0.512 | ±0.310 | −1.654a |
| K = 8 | −0.734 | ±0.293 | −2.503b | −0.669 | ±0.302 | −2.211b |
| K = 9 | −0.710 | ±0.292 | −2.430b | −0.483 | ±0.305 | −1.586 |
| K = 10 | −0.710 | ±0.292 | −2.429b | −0.499 | ±0.304 | −1.640 |
| K = 11 | −0.738 | ±0.292 | −2.523b | −0.645 | ±0.298 | −2.167b |
| K = 12 | −0.630 | ±0.298 | −2.112b | −0.390 | ±0.308 | −1.270 |
Full models with all associations (arrows) estimated were reduced using a type I error of 0.10. Exogenous parameters other dietary factors that were nonsignificant were fixed to zero. Reduced models are presented and exogenous variables that were considered as potential confounders in all equations (not shown) included sociodemographic and lifestyle factors (see Table 3 for details). aP < 0.10; bP < 0.05 for null hypothesis that path coefficient α = 0.
Examining each specific component of HEI as an antecedent factor in the pathway between dietary quality and CES-D through plasma folate (model 3), findings among men were consistent with model 1 for most components. In fact, for components k = 1–7 (increased intakes of total fruits, whole fruits, total vegetables, dark green or orange vegetables or legumes, total grains, whole grains, and dairy as percent of energy intake) and component k = 12 (reduced intakes of discretionary energy as fat, alcohol, or added sugars), HEIk was positively and significantly associated with plasma folate, which in turn was inversely related to CES-D score. One exception (component k = 11, reduced sodium intake as percent of energy with higher score) was inversely related to plasma folate, which was in turn inversely related to CES-D. The direct association between HEI11 and CES-D was also positive and significant (i.e. in the same direction as the indirect association) (model 3, men). Among women, however, only 2 components of HEI [k = 4 (increased intakes of dark green or orange vegetables or legumes) and 12 (reduced intake solid fat, added sugars, and alcohol)] exhibited similar association patterns to total HEI score, whereby the plasma folate-CES-D score appeared to be completely confounded by HEIk. In contrast, higher HEI8 (increased meat as percent of energy consumption) had 2 competing associations with CES-D: an inverse direct association and a positive indirect association through a negative relationship with plasma folate, which in turn was inversely related to CES-D score (model 3, women).
Discussion
In this study, we attempted to link folate status to depressive symptoms in an ethnically diverse U.S. population of young and middle-aged adults. In particular, we examined the roles of dietary intakes and diet quality in the folate-depressive symptoms association. Examining CES-D score ≥ 16 as the main outcome, we found an inverse association with plasma folate in women. In fact, compared with the lowest tertile, the middle and uppermost tertiles of plasma folate were associated with 39–40% reduced odds of CES-D ≥ 16 among women [adjusted OR (T3 vs. T1) = 0.60, 95% CI = 0.42–0.86; P = 0.006]. This association was not significant among men, although there was no appreciable effect modification by sex. This inverse plasma folate-CES-D ≥16 association in women was confounded by the HEI total score (∼18% change-in-estimate). In SM, plasma folate mediated the inverse HEI-CES-D association, with complete mediation observed only among men for most HEI components such as dairy intake. Despite the relatively low content of folate in dairy, folate binding protein is a minor whey protein that is crucial to the assimilation, distribution, and retention of folic acid, making folate in milk more bioavailable than other food sources (48). Among men, reduced sodium intake was associated with higher CES-D with partial mediation of this effect through plasma folate. Moreover, the dietary folate-CES-D association among men was partially mediated by plasma folate level, although the plasma folate-CES-D path coefficient in that model was only borderline significant (t = −1.903; d.f. = 1; P < 0.10).
Although sex differences were not significant, men had higher total and naturally occurring intakes of dietary folate than women. Among women, but not men, naturally occurring folate was inversely related to an elevated CES-D score. The percent of total dietary folate that was contributed by natural sources was higher among women (51–55%) than men (44–51%), suggesting that men consume more synthetic folic acid, which is more bioavailable.
More importantly, in both SM and multivariate linear regression models conducted to assess mediation of the diet-depressive symptoms association through plasma folate, there was a consistent indication of mediating effects only among men. It is possible that dietary factors within the HEI components that are not strongly associated with plasma folate may be affecting women's depressive symptoms to a greater extent than men's. Further research is needed to confirm this speculation and examine the nature of those dietary factors.
Among women, dietary quality had a primary role in reducing depressive symptoms independently of plasma folate levels. In particular, significant direct inverse associations between HEIk and CES-D scores were found for components 3 (higher total vegetables), 4 (higher dark green and orange vegetables, beans), 8 (higher meat and beans), and 12 (lower discretionary fat, alcohol, and added sugars). However, a higher meat/bean intake as percent of energy (HEI8) among women had 2 competing roles in affecting depressive symptoms based on SM, with a direct inverse association and an indirect positive relationship through reduced plasma folate levels. It is possible that increased meat intake, which is often associated with reduced intake of folate-rich foods such as fruits, vegetables, and fortified grains, was driving the inverse association with plasma folate.
Several studies had previously examined the associations between folate status (or folate intake) and depressive symptoms (or diagnosis of depression). Among case series and case-control studies, several recorded low plasma and erythrocyte levels of folate in psychiatric patients with major depression (5,6,26,29,33,34,49,50). This inverse association was corroborated by large population-based, cross-sectional studies (17,20,23,24,27,30,51), although an inconsistent effect modification by sex in some of those studies was found (17,24). At least 2 studies did not find a significant association (18,19).
Among the large, population-based cohort studies (21–23,25,31,32,52), the majority found an inverse association between folate status (or folate intake) and depressive symptoms (or depression) (21–23,25), whereas others did not (31,32,52), with inconsistent effect modification by sex found in some (22,23). The lack of association in some of those observational studies (18,19,31,32,52) may be due to the older age group, the instrument under consideration (Geriatric Depression Scale instead of CES-D), and potential inaccuracies in measuring depression.
The term “folate” refers to a group of water-soluble compounds found naturally in foods such as green vegetables, peanuts, legumes, strawberries, and orange juice, predominating in their polyglutamate form (53). In fortified foods, folate is provided as folic acid (pteroylmonoglutamate). When absorbed, folate circulates freely or is bound to albumin as a monoglutamate. Through active transport, it may enter the cerebrospinal fluid, where its concentration is 3 times greater than in plasma. The plasma folate level reflects dietary and supplemental intakes and decreases after diminished intake within 1–6 mo (54).
An essential function of folate is DNA biosynthesis, although its effect on the central nervous system (CNS) differs with several suggested mechanisms that may contribute to neuroprotection. The first pathway involves the synthesis of neurotransmitters through promoting the production of tetrahydro biopterins (BH4), a cofactor in the conversion of phenylalanine to tyrosine as well as the hydroxylation of tyrosine and tryptophan (55). These reactions are limiting steps for synthesizing several neurotransmitters, including dopamine, noradrenalin, and serotonin. In fact, reduced biopterin excretion, a marker of poor BH4 bioavailability, was shown in depressed patients (56,57).
Another mechanism is the series of methylation reactions (MR) within the CNS. These MR function to reduce blood homocysteine (Hcy) levels, a putative metabolite toxic to the vascular system and the CNS. The first reaction (MR1) requires vitamin B-12 to transfer a methyl group from 5-methyltetrahydrofolate (MTHF) to Hcy, producing methionine. An alternative route for the synthesis of methionine (MR2), not requiring vitamin B-12 or MTHF but rather dietary choline, is via the betaine:Hcy methyltransferase reaction. Unlike peripheral tissues, however, the CNS lacks the enzyme betaine:Hcy methyltransferase reaction, making MTHF the only available methyl donor. A 3rd metabolic reaction requires vitamin B-6 for cystationine-β-synthetase, which condenses Hcy with serine, converting it to cystathionine and leading to the formation of glutathione, a major antioxidant (58). Our findings suggest that plasma folate may be a reflection of brain tissue folate, which in turn may play a primary role in neuroprotection, with the BH4 pathway being a possible mechanism.
Our study has several strengths. First, to our knowledge, it is the only large observational study to assess plasma folate-depression association among young and middle-aged White and African-American U.S. population after mandatory folic acid fortification of foods was implemented in the U.S. Second, the study examined complex arrays of associations using SM among other techniques. Third, we used a valid measure of depressive symptoms, the CES-D, and a biomarker of folate status, plasma folate. Fourth, it is among a few large U.S. studies that had 2 24-h dietary recalls instead of one, reducing measurement error and enhancing the value of dietary variables in reflecting usual intake. However, our study is limited by its cross-sectional design, which does not allow for temporality ascertainment, although at least 1 cohort study did show that plasma folate was predictive of incident depression (25), while another did not (32). It is thus important to conduct longitudinal studies in a U.S. community to verify temporality of associations. Second, some findings may be explained by selection bias, given that only about one-half of the original HANDLS sample was included in our present study. Nevertheless, when conducting a 2-stage Heckman selection model (59,60) to account for selectivity of the sample in sociodemographic factors measured on the entire HANDLS sample, we found the results were unaltered (data not shown). Third, measurement errors in dietary exposures are possible, although our study had 2 24-h recalls, an improvement over many large, cross-sectional studies. Those errors are probably random across plasma levels of folate, leading to attenuation of true associations (61). Applying regression calibration (62) to a model in which of the 2 24-h recalls of dietary folate were entered as repeat measurements to account for within-person variability in that covariate, the main association between plasma folate and depressive symptoms was not altered appreciably. Finally, data on supplemental intakes of folate were not available at the time this study was conducted and thus were not accounted for in estimating total intakes.
In conclusion, depressive symptoms in our study may be alleviated by improving overall dietary quality, with plasma folate playing a potential mediating role only among men. Future interventions should take into account overall dietary quality in improving mental health outcomes.
Supplementary Material
Acknowledgments
We thank Drs. Larry Brant and Vonetta Dotson for their careful internal review of this manuscript. M.A.B. wrote and revised the manuscript, planned the analysis, performed data management and statistical analysis, and had primary responsibility for final content. M.T.F.K. wrote and revised parts of the manuscript, participated in planning of the analysis, and provided the dietary database. H.A.B. wrote and revised parts of the manuscript, particularly the literature review. M.R.S. wrote and revised parts of the manuscript and provided support for the statistical analysis. M.A.M. participated in data management and planning the analysis. M.K.E. designed the research, provided databases, and was cosenior author. A.B.Z. designed research, provided databases, wrote and revised parts of the manuscript, and was cosenior author. All authors read and approved the final version of the manuscript.
Supported by the National Institute on Aging, Intramural Research Program (NIA/NIH/IRP).
Author disclosures: M. A. Beydoun, M. T. Fanelli Kuczmarski, H. A. Beydoun, M. R. Shroff, M. A. Mason, M. K. Evans, and A. B. Zonderman, no conflicts of interest.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BH4, tetrahydrobiopterins; BHMT, betaine:homocysteine methyltransferase; CES-D, Center for Epidemiologic Studies-Depression scale; CNS, central nervous system; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Lifespan; Hcy, homocysteine; HEI, Healthy Eating Index; HS, high school; MR, metabolic reactions; MTHF, 5-methyltetrahydrofolate; OR, odds ratio; PIR, poverty income ratio; RMSEA, root mean square error of approximation; SM, structural equations modeling; T, tertile.
References
- 1.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. [DOI] [PubMed] [Google Scholar]
- 2.Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J. Long-term outcome of episodes of major depression. Clinical and public health significance. JAMA. 1984;252:788–92. [PubMed] [Google Scholar]
- 3.Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–16. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. [DOI] [PubMed] [Google Scholar]
- 5.Carney MW, Chary TK, Laundy M, Bottiglieri T, Chanarin I, Reynolds EH, Toone B. Red cell folate concentrations in psychiatric patients. J Affect Disord. 1990;19:207–13. [DOI] [PubMed] [Google Scholar]
- 6.Carney MW, Sheffield BF. Serum folic acid and B12 in 272 psychiatric in-patients. Psychol Med. 1978;8:139–44. [DOI] [PubMed] [Google Scholar]
- 7.Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. 2008;14:277–85. [DOI] [PubMed] [Google Scholar]
- 8.Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60:121–30. [DOI] [PubMed] [Google Scholar]
- 10.Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309–13. [DOI] [PubMed] [Google Scholar]
- 11.Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto). 2006;46:55–67. [DOI] [PubMed] [Google Scholar]
- 12.Morrison HI, Schaubel D, Desmeules M, Wigle DT. Serum folate and risk of fatal coronary heart disease. JAMA. 1996;275:1893–6. [DOI] [PubMed] [Google Scholar]
- 13.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Vitamin B12 and folate depletion in cognition: a review. Neurol India. 2004;52:310–8. [PubMed] [Google Scholar]
- 14.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. [DOI] [PubMed] [Google Scholar]
- 15.Choumenkovitch SF, Jacques PF, Nadeau MR, Wilson PW, Rosenberg IH, Selhub J. Folic acid fortification increases red blood cell folate concentrations in the Framingham study. J Nutr. 2001;131:3277–80. [DOI] [PubMed] [Google Scholar]
- 16.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–6. [DOI] [PubMed] [Google Scholar]
- 17.Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–8. [DOI] [PubMed] [Google Scholar]
- 18.Lindeman RD, Romero LJ, Koehler KM, Liang HC, LaRue A, Baumgartner RN, Garry PJ. Serum vitamin B12, C and folate concentrations in the New Mexico elder health survey: correlations with cognitive and affective functions. J Am Coll Nutr. 2000;19:68–76. [DOI] [PubMed] [Google Scholar]
- 19.Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B (12) deficiency and depression in physically disabled older women: epidemiologic evidence from the Women's Health and Aging Study. Am J Psychiatry. 2000;157:715–21. [DOI] [PubMed] [Google Scholar]
- 20.Tolmunen T, Voutilainen S, Hintikka J, Rissanen T, Tanskanen A, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133:3233–6. [DOI] [PubMed] [Google Scholar]
- 21.Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and the risk of depression in Finnish middle-aged men. A prospective follow-up study. Psychother Psychosom. 2004;73:334–9. [DOI] [PubMed] [Google Scholar]
- 22.Astorg P, Couthouis A, de Courcy GP, Bertrais S, Arnault N, Meneton P, Galan P, Hercberg S. Association of folate intake with the occurrence of depressive episodes in middle-aged French men and women. Br J Nutr. 2008;100:183–7. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B and vitamin B intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–33. [DOI] [PubMed] [Google Scholar]
- 24.Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, Mishima N. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–7. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–74. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand. 1989;80:78–82. [DOI] [PubMed] [Google Scholar]
- 27.Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–26. [DOI] [PubMed] [Google Scholar]
- 28.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–8. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Wing YK, Fong S. A controlled study of folate levels in Chinese inpatients with major depression in Hong Kong. J Affect Disord. 1998;49:73–7. [DOI] [PubMed] [Google Scholar]
- 30.Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, Christensen H, Jorm AF. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–38. [DOI] [PubMed] [Google Scholar]
- 31.Eussen SJ, Ferry M, Hininger I, Haller J, Matthys C, Dirren H. Five year changes in mental health and associations with vitamin B12/folate status of elderly Europeans. J Nutr Health Aging. 2002;6:43–50. [PubMed] [Google Scholar]
- 32.Kendrick T, Dunn N, Robinson S, Oestmann A, Godfrey K, Cooper C, Inskip H. A longitudinal study of blood folate levels and depressive symptoms among young women in the Southampton Women's Survey. J Epidemiol Community Health. 2008;62:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Gazi F, Papadimitriou A, Lea RW, Kalofoutis A. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem. 2007;40:604–8. [DOI] [PubMed] [Google Scholar]
- 34.Lerner V, Kanevsky M, Dwolatzky T, Rouach T, Kamin R, Miodownik C. Vitamin B12 and folate serum levels in newly admitted psychiatric patients. Clin Nutr. 2006;25:60–7. [DOI] [PubMed] [Google Scholar]
- 35.Lezak M, Lezak M, editors. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004.
- 36.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 37.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res. 2004;126:177–87. [DOI] [PubMed] [Google Scholar]
- 38.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. [DOI] [PubMed] [Google Scholar]
- 39.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. [DOI] [PubMed] [Google Scholar]
- 40.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 41.Bowman SA, Friday JE, Moshfegh A. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003-2004 [Online] Food Surveys Research Group. Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville (MD): 2008. Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg.
- 42.USDA Food and Nutrient Database for Dietary Studies, 3.0. Beltsville (MD): 2008. Agricultural Research Service, Food Surveys Research Group. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=17031.
- 43.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–901. [DOI] [PubMed] [Google Scholar]
- 44.STATA. Statistics/Data Analysis: release 10.0. College Station (TX): STATA Corporation; 2007.
- 45.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 46.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–58. [Google Scholar]
- 47.Hatcher L. A step-by-step approach to using the SAS system for factor analysis ad structural equation modeling. Cary (NC): SAS; 1994.
- 48.Verwei M, Arkbage K, Havenaar R, van den Berg H, Witthoft C, Schaafsma G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J Nutr. 2003;133:2377–83. [DOI] [PubMed] [Google Scholar]
- 49.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry. 2002;159:2099–101. [DOI] [PubMed] [Google Scholar]
- 50.Tiemeier H, Fekkes D, Hofman A, van Tuijl HR, Kiliaan AJ, Breteler MM. Plasma pterins and folate in late life depression: the Rotterdam Study. Psychiatry Res. 2006;145:199–206. [DOI] [PubMed] [Google Scholar]
- 51.Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US population. Psychother Psychosom. 2003;72:80–7. [DOI] [PubMed] [Google Scholar]
- 52.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B (6–9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–45. [DOI] [PubMed] [Google Scholar]
- 53.Chanarin I, editor. The megaloblastic anaemias. 2nd ed. Oxford: Blackwell Scientific; 1979.
- 54.Paul RT, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19:477–88. [DOI] [PubMed] [Google Scholar]
- 55.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 56.Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: the folate connection. J Affect Disord. 1989;16:103–7. [DOI] [PubMed] [Google Scholar]
- 57.Anderson DN, Abou-Saleh MT, Collins J, Hughes K, Cattell RJ, Hamon CG, Blair JA, Dewey ME. Pterin metabolism in depression: an extension of the amine hypothesis and possible marker of response to ECT. Psychol Med. 1992;22:863–9. [DOI] [PubMed] [Google Scholar]
- 58.Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1103–12. [DOI] [PubMed] [Google Scholar]
- 59.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- 60.Beydoun MA, Kuczmarski MT, Mason MA, Ling SM, Evans MK, Zonderman AB. Role of depressive symptoms in explaining socioeconomic status disparities in dietary quality and central adiposity among US adults: a structural equation modeling approach. Am J Clin Nutr. 2009;90:1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thiebaut AC, Kipnis V, Schatzkin A, Freedman LS. The role of dietary measurement error in investigating the hypothesized link between dietary fat intake and breast cancer: a story with twists and turns. Cancer Invest. 2008;26:68–73. [DOI] [PubMed] [Google Scholar]
- 62.Carroll RJ, Ruppert D, Stefanski LA. Measurement error in nonlinear models. Boca Raton (FL): CRC Press; 1995.
- 63.USDA. Healthy Eating Index 2005. [cited 2007 Mar 20]. Available from: http://www.cnpp.usda.gov/Publications/HEI/healthyeatingindex2005factsheet.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.