Abstract
Background: Low vitamin B-6 status has been linked to an increased risk of cardiovascular diseases. The cardioprotective effects of vitamin B-6 independent of homocysteine suggest that additional mechanisms may be involved.
Objective: Our objective was to examine the cross-sectional association of vitamin B-6 status with markers of inflammation and oxidative stress.
Design: We measured plasma pyridoxal-5′-phosphate (PLP), C-reactive protein (CRP), and an oxidative DNA damage marker, urinary 8-hydroxydeoxyguanosine (8-OHdG), in Puerto Rican adults who were living in Massachusetts (n = 1205, aged 45–75 y).
Results: There was a strong dose-response relation of plasma PLP concentration with plasma CRP. Increasing quartiles of PLP were significantly associated with lower CRP concentrations (geometric means: 4.7, 3.6, 3.1, and 2.5 mg/L; P for trend < 0.0001) and with lower urinary 8-OHdG concentrations (geometric means: 124, 124, 117, and 108 ng/mg creatinine; P for trend: 0.025) after multivariate adjustment. These negative associations persisted after plasma homocysteine was controlled for. Plasma PLP concentrations were significantly correlated with plasma fasting glucose (r = −0.1, P = 0.0006), glycated hemoglobin (r = −0.08, P = 0.006), and homeostasis model assessment of β cell function (r = 0.082, P = 0.005). Metabolic syndrome, obesity, and diabetes were also significantly associated with low plasma PLP concentrations (P = 0.011, 0.0007, and 0.004, respectively).
Conclusions: Low vitamin B-6 concentrations are associated with inflammation, higher oxidative stress, and metabolic conditions in older Puerto Rican adults. Our data suggest that vitamin B-6 may influence cardiovascular disease risk through mechanisms other than homocysteine and support the notion that nutritional status may influence the health disparities present in this population.
INTRODUCTION
Vitamin B-6 includes pyridoxal, pyridoxine, and pyridoxamine, which function as essential cofactors for enzymes involved in various metabolic activities, which include amino acid, fat, and glucose metabolism (1). The phosphate ester derivative pyridoxal 5′-phosphate (PLP) is the biologically active form of this vitamin (2) and reflects long-term body storage (3). Studies have shown that low plasma PLP concentrations are associated with increased risk of cardiovascular disease (CVD) (4, 5). The potential mechanism has been proposed to act through PLP regulation of homocysteine metabolism, itself an independent risk factor for CVD and stroke (6).
The observation of protective effects of vitamin B-6 on CVD independent of homocysteine (4) suggests that additional mechanisms may be involved. Biochemical studies have revealed some underlying mechanisms of the cardioprotective effect, such as the regulation of cholesterol metabolism (7) and the inhibition of platelet aggregation (8) and endothelial cell proliferation (9). Recent data have shown that plasma PLP was adversely associated with inflammatory markers, which include C-reactive protein (CRP), fibrinogen, and blood cell count (4, 10–12). Additionally, low vitamin B-6 concentrations are commonly present in diseases with a strong inflammatory basis, such as diabetes (13), rheumatoid arthritis (14), and inflammatory bowel disease (15). Current evidence highlights the notion that inflammation may represent another link between vitamin B-6 and CVD. However, the relation of vitamin B-6 status with inflammation and other CVD risk factors has not been investigated extensively in a population at high risk of CVD.
Puerto Ricans who live in the United States represent one of the largest Hispanic ethnic groups. Health disparities have been well documented in this minority population. We have reported previously that Puerto Rican elders who live in Massachusetts have a high prevalence of depressive symptoms, cognitive impairment, type 2 diabetes, obesity, and hypertension compared with non-Hispanic whites and other Hispanic subgroups (16–19). It is therefore important to explore and identify factors that contribute to those disparities. Nutritional status may influence those disadvantageous health outcomes (18). In the present study, we aimed to examine the association of vitamin B-6 status with markers of inflammation and oxidative stress as well as metabolic conditions in older Puerto Rican adults who were living in Massachusetts.
SUBJECTS AND METHODS
Subjects
The present study consisted of 1222 self-identified Puerto Ricans aged 45–75 y who were living in Boston, Massachusetts (361 men and 861 women; mean ± SD age: 52 ± 7 y) and were participating in the Boston Puerto Rican Health Study, a longitudinal study on stress, nutrition, health, and aging (18, 20). The design and methodology of the study have been described previously (20). Detailed materials and methods can be found under “Supplemental data” in the online issue. Dietary intake was assessed with the use of a semiquantitative food-frequency questionnaire with 126 items, which was adapted and validated for this population (21). This food-frequency questionnaire has been validated against plasma carotenoids (22), vitamin E (23), and vitamin B-12 (24) in Hispanics aged ≥60 y. A total of 1205 participants with complete data for demographic and biochemical characteristics and dietary intake (600 kcal < energy intake < 4000 kcal) were included in the final analyses. The metabolic syndrome (MetS) was defined in accordance with the 2001 National Cholesterol Education Program Adult Treatment Panel III guidelines modified to reflect glucose recommendations from the American Diabetes Association (25). Type 2 diabetes was determined with the use of American Diabetes Association criteria (fasting glucose ≥ 7 mmol/L or 126 mg/dL) (26) and/or use of diabetes medications. Participants were classified as obese if their body mass index (BMI; in kg/m2) was ≥30. Vitamin B-6 inadequacy was defined as plasma PLP < 20 nmol/L, the concentration used to set the current Recommended Dietary Allowance (RDA) (27).
The protocol for this study was approved by the Human Studies Committee of the Institutional Review Board at Tufts Medical Center. Written informed consent was obtained from all participants.
Biochemical measurements
Plasma PLP was determined enzymatically with the use of tyrosine decarboxylase, based on the principles described by Shin-Buehring et al (28). Serum folate was measured by using Immulite chemiluminescent kits according to the manufacturer's instructions (Diagnostic Products Corporation/Siemens, Los Angeles, CA). Plasma homocysteine was determined by reverse-phase HPLC analysis. Plasma CRP was measured by the Immulite 1000 High Sensitive CRP Kit (LKCRP1) on the Immulite 1000 (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Urinary 8-hydroxydeoxyguanosine (8-OHdG) was measured by a monoclonal antibody enzyme-linked immunosorbent assay kit (EKS-350; Assay Designs, Ann Arbor, MI). Briefly, ≈10 μL of urine collected from each participant after a 12-h overnight period was thawed after storage at −80°C and diluted 20-fold before analysis. Diluted urine samples were measured in duplicate with a standard provided by the vendor in a 96-well plate format. Concentrations of urinary 8-OHdG were calculated by the multiplication of the measured concentration by the total volume of 12-h urine, and then normalized by urinary creatinine concentrations.
Statistical analysis
Statistical analyses were performed with the use of SAS for Windows, version 9.0 (SAS Institute, Cary, NC). A logarithmic transformation was applied to plasma concentrations of CRP, PLP, urinary 8-OHdG, and triglycerides to normalize the distribution of data. Partial Pearson's correlation was applied to examine the relation between plasma PLP and clinical and biochemical measurements. Analysis of covariance was used to compare mean differences across quartiles of plasma PLP with Tukey adjustment for multiple comparisons. Covariates included age, sex, BMI, smoking (current smoker, never smoked, or past smoker), alcohol consumption (current drinker, never drank, or past drinker), medication use (treatment of hypertension, diabetes, hyperlipidemia, and use of hormone therapy by women), physical activity, urinary creatinine, serum homocysteine, vitamin B-6 and folate intake (diet and supplements), protein, and total energy intake. A 2-tailed P value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics for 1205 participants across quartiles of plasma PLP concentrations are presented in Table 1. Because no significant modification by sex was observed, men and women were analyzed together. There were significant associations between plasma PLP concentration and BMI and waist circumference: participants in the highest quartile of PLP had a lower BMI and waist circumference than those in lower quartiles of PLP, after adjustment for age and sex (P < 0.001). Higher plasma PLP concentrations were associated with higher intake of vitamin B-6 (P < 0.001), folate (P < 0.001), and vitamin B-12 (P < 0.001); higher antioxidants, which included vitamin C (P < 0.001), β-carotene (P = 0.006), and vitamin E (P < 0.001); and higher intake of vegetables (P = 0.012). High plasma PLP concentrations were also associated with a higher physical activity score (P < 0.001), higher current drinking status (P = 0.018), and lower current smoking status (P < 0.001).
TABLE 1.
Participant characteristics across quartiles (Q) of plasma pyridoxal 5′-phosphate (PLP)1
| PLP |
|||||
| Variables | Q1 (5.5–28.3 nmol/L) | Q2 (28.4–42.4 nmol/L) | Q3 (42.5–65.2 nmol/L) | Q4 (65.3–737 nmol/L) | P for trend |
| n | 301 | 305 | 299 | 300 | — |
| Mean PLP (nmol/L) | 20.4 ± 5.32 | 34.8 ± 4.1 | 52.0 ± 6.4 | 128.2 ± 92.1 | — |
| Age3 (y) | 57.4 ± 7.5 | 56.9 ± 7.3 | 57.1 ± 7.6 | 58.3 ± 7.8 | 0.27 |
| Female4 [n (%)] | 235 (71) | 234 (72) | 231 (72) | 225 (69) | 0.36 |
| BMI (kg/m2) | 32.9 ± 7.7 | 32.3 ± 6.6 | 31.7 ± 6.5 | 30.7 ± 5.6 | <0.001 |
| Waist (cm) | 104 ± 17 | 103 ± 16 | 101 ± 14 | 99 ± 13 | <0.001 |
| Current smoker [n (%)] | 106 (32) | 90 (28) | 73 (23) | 49 (15) | <0.001 |
| Current drinker [n (%)] | 109 (33) | 129 (40) | 136 (42) | 145 (45) | 0.019 |
| Total energy intake (kcal/d) | 2315 ± 1170 | 2380 ± 1218 | 2360 ± 1198 | 2282 ± 1274 | 0.78 |
| Vitamin B-6 intake5 (mg/d) | 2.49 ± 1.31 | 2.54 ± 1.19 | 2.76 ± 1.31 | 2.99 ± 1.59 | <0.001 |
| Folate intake5 (μg/d) | 495 ± 241 | 512 ± 236 | 548 ± 268 | 610 ± 317 | <0.001 |
| Vitamin B-12 intake5 (μg/d) | 9.8 ± 8.9 | 9.8 ± 8.8 | 10.3 ± 8 | 11 ± 10 | 0.006 |
| Vitamin C intake5 (mg/d) | 143 ± 104 | 135 ± 89 | 151 ± 114 | 169 ± 109 | <0.001 |
| β-Carotene intake5 (mg/d) | 3228 ± 3464 | 3026 ± 2824 | 3524 ± 4193 | 3634 ± 3117 | 0.006 |
| Vitamin E intake5 (mg/d) | 13.4 ± 11.3 | 15.4 ± 11.9 | 17.7 ± 14.4 | 22.5 ± 16.2 | <0.001 |
| Fruit intake5 (servings/d) | 2.0 ± 1.9 | 2.0 ± 1.7 | 2.1 ± 1.8 | 2.2 ± 1.9 | 0.13 |
| Vegetable intake5 (servings/d) | 3.6 ± 3.2 | 3.6 ± 2.8 | 3.7 ± 2.7 | 4.3 ± 3.5 | 0.012 |
| Physical activity | 30.6 ± 3.8 | 31.7 ± 5.3 | 31.6 ± 4.5 | 32.1 ± 5.0 | <0.001 |
Values were adjusted for age and sex, except where otherwise indicated, by using a general linear model.
Mean ± SD (all such values).
Adjusted for sex.
Adjusted for age.
Additionally adjusted for energy intake.
There were significant correlations between plasma PLP and plasma fasting glucose (P = 0.0006), glycated hemoglobin (Hb Alc) (P = 0.0058), and homeostasis model assessment (HOMA) of β cell function (P = 0.005), but no significant associations with insulin or HOMA of insulin resistance (Table 2). Participants in the highest quartile of plasma PLP had lower plasma fasting glucose and Hb Alc concentrations than those in the lower quartiles. Higher plasma PLP was significantly correlated with higher HDL cholesterol (P = 0.039). No significant associations were observed for other lipid measures or for blood pressure. Plasma homocysteine was negatively correlated with plasma PLP (P < 0.0001).
TABLE 2.
Clinical and biochemical measurements across quartiles (Q) of plasma pyridoxal 5′-phosphate (PLP)1
| PLP |
|||||
| Pearson correlation (r) | Q1 (5.5–28.3 nmol/L) | Q2 (28.4–42.4 nmol/L) | Q3 (42.5–65.2 nmol/L) | Q4 (65.3–737 nmol/L) | |
| n | — | 292 | 290 | 299 | 295 |
| Glucose (mg/dL) | −0.102 | 137 ± 43 | 120 ± 3 | 119 ± 3 | 113 ± 3 |
| Hb Alc (%) | −0.084 | 7.5 ± 0.1 | 7.0 ± 0.1 | 6.9 ± 0.1 | 6.8 ± 0.1 |
| Insulin (uIU/mL) | 0.010 | 21.0 ± 1.7 | 18.2 ± 1.2 | 17.0 ± 0.9 | 16.4 ± 0.9 |
| HOMA-IR | −0.03 | 8.8 ± 1.7 | 6.0 ± 0.6 | 5.5 ± 0.5 | 4.7 ± 0.3 |
| HOMA–β cell function | 0.084 | 141 ± 11 | 158 ± 12 | 153 ± 17 | 166 ± 16 |
| Triglycerides (mg/dL) | 0.02 | 156 ± 6 | 159 ± 7 | 172 ± 9 | 168 ± 6 |
| Total cholesterol (mg/dL) | 0.01 | 177 ± 2 | 185 ± 2 | 186 ± 2 | 187 ± 3 |
| LDL-C (mg/dL) | −0.01 | 103 ± 2 | 109 ± 2 | 109 ± 2 | 108 ± 2 |
| HDL-C (mg/dL) | 0.065 | 43.0 ± 0.7 | 44.4 ± 0.6 | 45.6 ± 0.7 | 46.6 ± 0.8 |
| Homocysteine (μm/L) | −0.112 | 9.9 ± 0.3 | 9.5 ± 0.3 | 8.9 ± 0.3 | 8.3 ± 0.2 |
| Diastolic BP (mm Hg) | −0.04 | 81.2 ± 0.6 | 81.7 ± 0.6 | 81.0 ± 0.6 | 80.0 ± 0.6 |
| Systolic BP (mm Hg) | −0.02 | 137 ± 1.2 | 135 ± 1 | 136 ± 1 | 136 ± 1 |
Hb Alc, glycated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA–β cell function, homeostasis model assessment of β cell function; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; BP, blood pressure. Partial Pearson's correlation coefficient was adjusted for age, sex, BMI, smoking status, alcohol intake, physical activity, and intake of dietary vitamin B-6 and total energy.
P < 0.001.
Mean ± SEM (all such values).
P < 0.01.
P < 0.05.
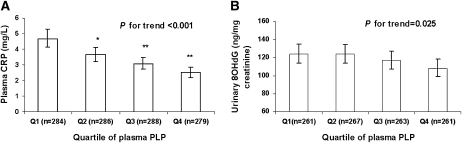
There was a strong dose-response relation of plasma PLP with plasma CRP (Figure 1) after adjustment for age, sex, BMI, smoking status, alcohol intake, physical activity, diabetes status, hormone use among women, and dietary intakes of vitamin B-6, folate, protein, and total energy. Participants in higher quartiles of plasma PLP had lower plasma CRP concentrations than those in lower quartiles, with geometric means of 4.7, 3.6, 3.1, and 2.5 mg/L across quartiles (P for trend < 0.0001). Similarly,urinary 8-OHdG was significantly associated with plasma PLP concentrations with decreasing geometric means of 124, 124, 117, and 108 ng/mg creatinine across quartiles of increasing PLP (P for trend: 0.025) in the multivariate adjusted model (Figure 1). This negative association persisted even after plasma homocysteine was controlled for.
FIGURE 1.
Geometric means (with 95% CIs) of plasma C-reactive protein (CRP) (A) and urinary 8-hydroxydeoxyguanosine (8OHdG) concentrations (B) by quartiles (Q) of plasma pyridoxal 5′-phosphate (PLP). P values for trend, in general linear models, were adjusted for age, sex, smoking status, alcohol intake, physical activity, hormone use among women, dietary vitamin B-6, folate intake, protein and total energy intake, and plasma homocysteine. *, **Significantly different from the lowest quartile: *P < 0.05, **P < 0.001.
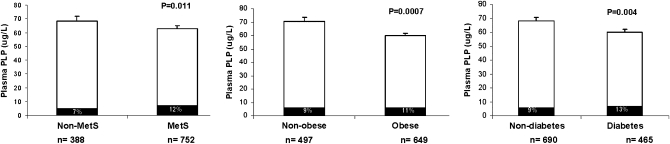
The presence of chronic conditions, such as MetS, obesity, and type 2 diabetes, was strongly associated with lower plasma PLP concentrations (P = 0.011, 0.0007, and 0.004, respectively) (Figure 2). The prevalence of vitamin B-6 inadequacy among participants with MetS was higher than in those without this condition (12% compared with 7%, P = 0.0016). Similarly, 13% of participants with type 2 diabetes were vitamin B-6 inadequate compared with 9% of those without diabetes (P = 0.014). Obese participants tended to show more vitamin B-6 inadequacy compared with nonobese participants, but this association was not statistically significant (11% compared with 9%, P = 0.14).
FIGURE 2.
Mean (±SE) association of plasma pyridoxal 5′-phosphate (PLP) concentration and chronic disease status. P values for mean differences between groups with and without a condition were adjusted for age, sex, smoking status, alcohol intake, physical activity, hormone use among women, dietary vitamin B-6, and folate, protein, and total energy intake in a general linear model. Black bars represent the percentage of vitamin B-6 inadequacy (PLP < 20 nmol/L). MetS, metabolic syndrome.
DISCUSSION
In the present study, we observed strong inverse associations between vitamin B-6 status, measured as plasma PLP concentration, and the systematic inflammatory marker CRP in a cohort of older Puerto Ricans who were living in Massachusetts, a group which has been identified previously to be at higher risk of several age-related diseases (18). Furthermore, chronic inflammatory conditions, such as MetS, diabetes, and obesity, were significantly associated with lower plasma PLP, and participants with those conditions were more likely to have vitamin B-6 inadequacy. Additionally, lower PLP was associated with oxidative stress, as reflected by a higher concentration of the urinary DNA damage marker, 8-OHdG.
Our data confirm previous reports from both healthy subjects and patients with various inflammatory conditions such as CVD (11, 15, 29) that showed that plasma CRP concentrations are associated with lower plasma PLP. CRP is an important downstream inflammatory marker that integrates the action of several activated cytokines. Plasma CRP not only predicts future CVD events (30) but also actively participates in the pathogenesis of atherosclerosis (31). On the other hand, low vitamin B-6 has been shown to increase CVD risk (4, 5). The observed inverse association between plasma CRP and PLP supports the notion that inflammation may represent the common link between low vitamin B-6 status and CVD risk. Although the causal factor remains to be clarified, inflammation has been suggested as facilitating redistribution of PLP from circulation to tissues with high demand. This compartmentalization of PLP could be an important adaptive response under certain circumstances (32). Because of the integral involvement of vitamin B-6 in the synthesis of nucleic acids and consequently in mRNA and protein synthesis, the production of cytokines and inflammatory mediators during the inflammatory response might increase the use of PLP (11).
More than 40% of our study population had type 2 diabetes and more than one-half were obese or had MetS. This confirms the findings of a previous report that Puerto Ricans who live in the United States have a high prevalence of CVD risk factors (18). Furthermore, participants with those metabolic conditions had lower plasma PLP compared with those without the conditions. This observation is in agreement with recent studies that suggested that MetS, obesity, and diabetes are negatively associated with the status of vitamins C, B-6, and E, and carotenoids (13, 33, 34). However, the mechanisms responsible for altered vitamin B-6 among subjects with those conditions are unclear. Given that those disorders were significantly associated with elevated CRP, chronic inflammation could be an underlying cause of low vitamin B-6 status. Although the majority of participants had intakes of vitamin B-6 above the current RDA, with a mean (±SD) intake of 3.0 ± 1.5 mg for men and 2.5 ± 1.3 mg for women, the prevalence of vitamin B-6 inadequacy in participants with MetS, obesity, or diabetes was substantial and significantly higher than that in participants without these conditions. Our results, thus, support the notion that the current RDA for vitamin B-6 may not guarantee adequate vitamin B-6 status in certain subgroups (3). In this regard, improved dietary intake or nutritional supplementation may benefit older Puerto Rican adults and other subgroups with a high prevalence of chronic conditions, by helping them maintain normal function of various metabolic processes and lower risk of future disease, such as CVD.
Our results suggest that low plasma PLP was associated with higher fasting glucose and Hb Alc, whereas higher plasma PLP was significantly correlated with improved HOMA index for β cell function. Vitamin B-6 deficiency has been shown to cause degenerative changes in β cells in the islets of Langerhans and to decrease both pancreatic and circulating insulin (35, 36). Likewise, pyridoxamine treatment of streptozotocin-induced diabetic hamsters improves glucose tolerance and restores β cell function (37). Moreover, supplementation with pyridoxine lowers blood glucose and decreases Hb Alc in diabetic patients (38, 39). We also observed a positive correlation between plasma PLP and HDL-cholesterol concentrations in this population, which is consistent with a report in European subjects (40).We postulate that, as a coenzyme of δ6-desaturase (7), The effect of PLP on HDL may be mediated through its effect on the metabolism of polyunsaturated fatty acids, which regulates the expression of genes involved in lipid metabolism (41).
In this study, low plasma PLP was significantly associated with higher urinary 8-OHdG, which suggests that low vitamin B-6 status may contribute to oxidative DNA damage. 8-OHdG is a product of the oxidative modification of the DNA base deoxyguanosine, and elevation of 8-OHdG may reflect oxidative damage induced by reactive oxygen species (42). Urinary 8-OHdG has been shown to be associated with atherosclerosis-related risk factors (43) and diabetes (44). A vitamin B-6–deficient diet increases plasma lipid peroxidation and decreases plasma vitamins E and C concentrations in rats (45). Experimental models have further shown that supplementation with vitamin B-6 suppresses the colonic concentrations of 8-OHdG induced by colonic carcinogen (46) and decreases plasma 8-OHdG and malonaldehyde in hyperglycemia-induced oxidative stress (37). Vitamin B-6 compounds can prevent the oxygen radical generation and lipid peroxidation caused by hydrogen peroxide in U937 monocytes as well (47). In this study the association between plasma PLP and the DNA damage marker persisted even after plasma homocysteine was controlled for, which indicates that higher oxidative stress was not mediated through homocysteine. Because PLP serves as a coenzyme for cystathionine β-synthase and cystathionine γ-lyase, both of which are required for the synthesis of cysteine, which is the precursor of glutathione (6, 48), inadequate vitamin B-6 status may decrease the production of glutathione and thus impair the antioxidant defense system (49). Oxidative stress, therefore, may represent a mechanistic pathway through which low vitamin B-6 may lead to CVD.
Several limitations of this study need to be addressed. First, the cross-sectional associations cannot be translated into a clear cause–effect relation. Prospective studies and randomized trials are needed. Second, as with any observational study, there may be unknown residual confounding. Third, despite the fact that CRP and 8-OHdG are widely used markers, results with only one marker of inflammation and one marker of oxidative stress may not satisfactorily reflect the full complexity of these associations. Future studies with multiple measurements may substantiate our findings. Finally, in this particular high-risk population, low plasma PLP concentrations could be due to the redistribution of PLP from circulation to tissues in response to inflammation, and may not necessarily indicate deficiency. Therefore, measurement of PLP concentrations in intracellular depots, such as red blood cells, may be a useful measure of vitamin B-6 status in populations with inflammatory conditions (50).
In conclusion, there was a strong inverse association between plasma PLP and the inflammatory marker CRP in older Puerto Ricans who were living in Massachusetts. Moreover, participants with metabolic conditions—namely MetS, diabetes, or obesity—had a lower vitamin B-6 status and a higher prevalence of vitamin B-6 inadequacy than those without these conditions. Low plasma PLP was also associated with oxidative stress. Our results suggest a potential link between vitamin B-6 and CVD, independent of the homocysteine-mediated pathway. In addition, our findings support the notion that nutritional status, particularly vitamin B-6 status, may influence the association between life stress, physiologic responses, and chronic diseases in this population. This information may help develop more effective dietary recommendations and future dietary interventions to help improve the health of, and decrease health disparities among, Puerto Ricans.
Supplementary Material
Acknowledgments
We thank Laurence D Parnell for manuscript editing and Jimmy W Crott for valuable discussions.
The authors' responsibilities were as follows—JS, JMO, and KLT: study concept and design; CQL and KLT: acquisition of data; JS, JM, JMO, and KLT: analysis and interpretation of data; JS: drafting of the manuscript; JS, CQL, JM, JMO, and KLT: critical revision of the manuscript for intellectual content; JS: statistical analysis; and KLT and JMO: obtaining of funding and supervision. None of the authors had a conflict of interest.
REFERENCES
- 1.Coursin DB. Present status of Vitamin B6 metabolism. Am J Clin Nutr 1961;9:304–14 [DOI] [PubMed] [Google Scholar]
- 2.Tully DB, Allgood VE, Cidlowski JA. Modulation of steroid receptor-mediated gene expression by vitamin B6. FASEB J 1994;8:343–9 [PubMed] [Google Scholar]
- 3.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87:1446–54 [DOI] [PubMed] [Google Scholar]
- 4.Friso S, Girelli D, Martinelli N, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr 2004;79:992–8 [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998;279:359–64 [DOI] [PubMed] [Google Scholar]
- 6.Selhub J. Homocysteine metabolism. Annu Rev Nutr 1999;19:217–46 [DOI] [PubMed] [Google Scholar]
- 7.Tsuge H, Hotta N, Hayakawa T. Effects of vitamin B-6 on (n-3) polyunsaturated fatty acid metabolism. J Nutr 2000;130:333S–4S [DOI] [PubMed] [Google Scholar]
- 8.Chang SJ, Chang CN, Chen CW. Occupancy of glycoprotein IIb/IIIa by B-6 vitamers inhibits human platelet aggregation. J Nutr 2002;132:3603–6 [DOI] [PubMed] [Google Scholar]
- 9.Matsubara K, Matsumoto H, Mizushina Y, Lee JS, Kato N. Inhibitory effect of pyridoxal 5′-phosphate on endothelial cell proliferation, replicative DNA polymerase and DNA topoisomerase. Int J Mol Med 2003;12:51–5 [PubMed] [Google Scholar]
- 10.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B vitamin status and inflammatory markers. Atherosclerosis 2003;169:169–74 [DOI] [PubMed] [Google Scholar]
- 11.Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 2001;103:2788–91 [DOI] [PubMed] [Google Scholar]
- 12.James S, Vorster HH, Venter CS, et al. Nutritional status influences plasma fibrinogen concentration: evidence from the THUSA survey. Thromb Res 2000;98:383–94 [DOI] [PubMed] [Google Scholar]
- 13.Okada M, Shibuya M, Yamamoto E, Murakami Y. Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes Metab 1999;1:221–5 [DOI] [PubMed] [Google Scholar]
- 14.Roubenoff R, Roubenoff RA, Selhub J, et al. Abnormal vitamin B6 status in rheumatoid cachexia. Association with spontaneous tumor necrosis factor alpha production and markers of inflammation. Arthritis Rheum 1995;38:105–9 [DOI] [PubMed] [Google Scholar]
- 15.Saibeni S, Cattaneo M, Vecchi M, et al. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol 2003;98:112–7 [DOI] [PubMed] [Google Scholar]
- 16.Bermudez OI, Tucker KL. Total and central obesity among elderly Hispanics and the association with type 2 diabetes. Obes Res 2001;9:443–51 [DOI] [PubMed] [Google Scholar]
- 17.Lin H, Bermudez OI, Falcon LM, Tucker KL. Hypertension among Hispanic elders of a Caribbean origin in Massachusetts. Ethn Dis 2002;12:499–507 [PubMed] [Google Scholar]
- 18.Tucker KL. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J Med Invest 2005;52(suppl):252–8 [DOI] [PubMed] [Google Scholar]
- 19.Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health 2000;90:1288–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker K, Noel SE, Mattei J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health ( in press ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol 1998;148:507–18 [DOI] [PubMed] [Google Scholar]
- 22.Bermudez OI, Ribaya-Mercado JD, Talegawkar SA, Tucker KL. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. J Nutr 2005;135:1496–502 [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Martin A, Lin H, Bermudez OI, Tucker KL. alpha-Tocopherol intake and plasma concentration of Hispanic and non-Hispanic white elders is associated with dietary intake pattern. J Nutr 2006;136:2574–9 [DOI] [PubMed] [Google Scholar]
- 24.Kwan LL, Bermudez OI, Tucker KL. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J Nutr 2002;132:2059–64 [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes–2008. Diabetes Care 2008;31(suppl 1):S12–54 [DOI] [PubMed] [Google Scholar]
- 27.Food and Nutrition Board IoMVBIDrit, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: Institute of Medicine, National Academy of Sciences, National Academy Press, 2000 [PubMed] [Google Scholar]
- 28.Shin-Buehring YS, Rasshofer R, Endres W. A new enzymatic method for pyridoxal-5-phosphate determination. J Inherit Metab Dis 1981;4:123–4 [Google Scholar]
- 29.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med 2003;114:283–7 [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001;103:1813–8 [DOI] [PubMed] [Google Scholar]
- 31.de Maat MP, Trion A. C-reactive protein as a risk factor versus risk marker. Curr Opin Lipidol 2004;15:651–7 [DOI] [PubMed] [Google Scholar]
- 32.Vermaak WJ, Barnard HC, Van Dalen EM, Potgieter GM, Van Jaarsveld H, Myburgh SJ. Compartmentalization of pyridoxal-5′-phosphate during the acute phase of myocardial infarction. Klin Wochenschr 1988;66:428–33 [DOI] [PubMed] [Google Scholar]
- 33.Aasheim ET, Hofso D, Hjelmesaeth J, Birkeland KI, Bohmer T. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr 2008;87:362–9 [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003;52:2346–52 [DOI] [PubMed] [Google Scholar]
- 35.Rogers KS, Mohan C. Vitamin B6 metabolism and diabetes. Biochem Med Metab Biol 1994;52:10–7 [DOI] [PubMed] [Google Scholar]
- 36.Toyota T, Kai Y, Kakizaki M, Ohtsuka H, Shibata Y, Goto Y. The endocrine pancreas in pyridoxine deficient rats. Tohoku J Exp Med 1981;134:331–6 [DOI] [PubMed] [Google Scholar]
- 37.Takatori A, Ishii Y, Itagaki S, Kyuwa S, Yoshikawa Y. Amelioration of the beta-cell dysfunction in diabetic APA hamsters by antioxidants and AGE inhibitor treatments. Diabetes Metab Res Rev 2004;20:211–8 [DOI] [PubMed] [Google Scholar]
- 38.Cohen KL, Gorecki GA, Silverstein SB, Ebersole JS, Solomon LR. Effect of pyridoxine (vitamin B6) on diabetic patients with peripheral neuropathy. J Am Podiatry Assoc 1984;74:394–7 [DOI] [PubMed] [Google Scholar]
- 39.Jain SK. Vitamin B6 (pyridoxamine) supplementation and complications of diabetes. Metabolism 2007;56:168–71 [DOI] [PubMed] [Google Scholar]
- 40.Dierkes J, Weikert C, Klipstein-Grobusch K, et al. Plasma pyridoxal-5-phosphate and future risk of myocardial infarction in the European Prospective Investigation into Cancer and Nutrition Potsdam cohort. Am J Clin Nutr 2007;86:214–20 [DOI] [PubMed] [Google Scholar]
- 41.Jump DB, Clarke SD, Thelen A, Liimatta M, Ren B, Badin M. Dietary polyunsaturated fatty acid regulation of gene transcription. Prog Lipid Res 1996;35:227–41 [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed.New York, NY: Oxford University Press, 1999 [Google Scholar]
- 43.Sakano N, Wang DH, Takahashi N, et al. Oxidative stress biomarkers and lifestyles in Japanese healthy people. J Clin Biochem Nutr 2009;44:185–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron 2002;91:327–9 [DOI] [PubMed] [Google Scholar]
- 45.Cabrini L, Bergami R, Fiorentini D, Marchetti M, Landi L, Tolomelli B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem Mol Biol Int 1998;46:689–97 [DOI] [PubMed] [Google Scholar]
- 46.Komatsu S, Watanabe H, Oka T, Tsuge H, Kat N. Dietary vitamin B6 suppresses colon tumorigenesis, 8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide synthase protein in azoxymethane-treated mice. J Nutr Sci Vitaminol (Tokyo) 2002;48:65–8 [DOI] [PubMed] [Google Scholar]
- 47.Kannan K, Jain SK. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic Biol Med 2004;36:423–8 [DOI] [PubMed] [Google Scholar]
- 48.Finkelstein JD, Chalmers FT. Pyridoxine effects on cystathionine synthase in rat liver. J Nutr 1970;100:467–9 [DOI] [PubMed] [Google Scholar]
- 49.Taysi S. Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin Nutr 2005;24:385–9 [DOI] [PubMed] [Google Scholar]
- 50.Vasilaki AT, McMillan DC, Kinsella J, Duncan A, O'Reilly DS, Talwar D. Relation between pyridoxal and pyridoxal phosphate concentrations in plasma, red cells, and white cells in patients with critical illness. Am J Clin Nutr 2008;88:140–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.