Abstract
Background: We previously reported that supplementation with multivitamins (vitamin B complex, vitamin C, and vitamin E) at multiples of the Recommended Dietary Allowance (RDA) significantly decreased the risk of adverse pregnancy outcomes among HIV-infected women. The minimum dosage of multivitamins necessary for optimal benefits is unknown.
Objective: We investigated the efficacy of multivitamin supplements at single compared with multiple RDAs on decreasing the risk of adverse pregnancy outcomes among HIV-infected women.
Design: We conducted a double-blind, randomized controlled trial among 1129 HIV-infected pregnant women in Tanzania. Eligible women between 12 and 27 gestational weeks were randomly assigned to receive daily oral supplements of either single or multiple RDA multivitamins from enrollment until 6 wk after delivery.
Results: Multivitamins at multiple and single doses of the RDA had similar effects on the risk of low birth weight (11.6% and 10.2%, respectively; P = 0.75). We found no difference between the 2 groups in the risk of preterm birth (19.3% and 18.4%, respectively; P = 0.73) or small-for-gestational-age (14.8% and 12.0%, respectively; P = 0.18). The mean birth weights were similar in the multiple RDA (3045 ± 549 g) and single RDA multivitamins group (3052 ± 534 g; P = 0.83). There were no significant differences between the 2 groups in the risk of fetal death (P = 0.99) or early infant death (P = 0.19).
Conclusion: Multivitamin supplements at a single dose of the RDA may be as efficacious as multiple doses of the RDA in decreasing the risk of adverse pregnancy outcomes among HIV-infected women. This trial was registered at clinicaltrials.gov as NCT00197678.
INTRODUCTION
More than 20 million infants are born with low birth weight every year, the majority of them in developing countries (1). Low birth weight is a strong determinant of infant mortality, morbidity, stunting, and impaired cognitive development (2, 3). In sub-Saharan Africa, HIV infection disproportionately affects women, and HIV-infected pregnant women are at high risk of adverse pregnancy outcomes (4, 5). HIV-infected individuals often suffer from malnutrition, particularly micronutrient deficiencies. Improving the micronutrient status of pregnant women may result in improved fetal growth and survival (6).
We previously reported that supplementation with multivitamins (vitamin B complex, vitamin C, and vitamin E) at multiples of the Recommended Dietary Allowance (RDA) significantly reduced the risks of fetal death, low birth weight, preterm birth, and intrauterine growth retardation among HIV-infected women in Tanzania (7). Beneficial effects may have been mediated through improved maternal immunologic, nutritional, and hematologic status (7–9). High dosages of multivitamin supplements within safe limits were administered in the trial, because HIV-infected individuals may require nutritional intakes greater than the RDA to achieve normal nutritional levels. Earlier studies have found that a high prevalence of vitamin B-6, B-12, and vitamin E deficiencies were observed among HIV-infected individuals who consumed single doses of the RDA for these vitamins (10, 11). Longitudinal studies have shown that nutritional intakes at levels greater than several times the RDA are necessary to slow the progression to AIDS or death (12–15).
Multivitamin supplements at the RDA level are intended to prevent deficiencies among healthy individuals; however, it is unknown whether the single RDA multivitamins would prevent deficiencies and improve birth outcomes among HIV-infected women. We conducted a randomized trial to examine the efficacy of multivitamin supplements at single compared with multiple RDA dosages on reducing the risk of adverse pregnancy outcomes among HIV-infected women.
SUBJECTS AND METHODS
Study design and population
Participants were recruited for a double-blind randomized controlled trial from antenatal clinics in Dar es Salaam, Tanzania. Women were eligible if they were HIV-infected, pregnant between 12 and 27 gestational weeks at enrollment, resided in Dar es Salaam, and intended to stay in the city until delivery and for 1 y thereafter. As part of the standard prenatal care, pregnant women were initially offered consent for HIV-1 testing. HIV-1 serostatus was determined by 2 sequential enzyme-linked immunosorbent assays using Enzygnost anti-HIV-1/2 Plus (Dade Behring, Marburg, Germany) followed by the Wellcozyme HIV-1 recombinant test (Murex Biotech Ltd, Dartford, United Kingdom); discrepant results were resolved by a Western blot test (Bio-Rad Laboratories, Herfordshire, United Kingdom). Women who tested positive for HIV received posttest counseling and were invited to participate in the trial. Consented participants were referred to the study clinic at Muhimbili National Hospital, the main tertiary care hospital in Tanzania. All subsequent visits took place at the study clinic. The study protocol was approved by the Institutional Review Boards at Muhimbili University of Health and Allied Sciences and the Harvard School of Public Health.
Participants enrolled in the trial between November 2002 and June 2004. A list of randomization assignment was generated by a computer program in blocks of 20. Women were randomly assigned to receive a daily oral dose of either single RDA or multiple RDA multivitamins from enrollment until 6 wk after delivery (Table 1). The multiple RDA supplements contained doses at 3 times the RDA for vitamin E, 7 times the RDA for vitamin C, and >10 times the RDA for several B vitamins. Vitamin A and zinc were not included, because previous trials reported harmful effects of vitamin A during pregnancy among HIV-infected women in Tanzania and Zimbabwe (16, 17), and several trials found no beneficial effect of zinc (18, 19). Both experimental tablets were identical in color, taste, and appearance. Study physicians, nurses, and participants were unaware of the treatment groups. Tablets were manufactured by Tishcon Corp (Salisbury, MD), which had no involvement in the study design, implementation, or analyses.
TABLE 1.
Composition of the daily multivitamin supplements1
| Nutrient | Multiple RDA multivitamins | Single RDA multivitamins |
| Thiamine (mg) | 20 | 1.4 |
| Riboflavin (mg) | 20 | 1.4 |
| Niacin (mg) | 100 | 18 |
| Vitamin B-6 (mg) | 25 | 1.9 |
| Vitamin B-12 (μg) | 50 | 2.6 |
| Vitamin C (mg) | 500 | 70 |
| Vitamin E (mg) | 30 | 10 |
| Folic acid (mg) | 0.8 | 0.4 |
All participants received daily doses of iron (60 mg) and folic acid (0.25 mg) as part of standard prenatal care. RDA, Recommended Dietary Allowance.
All participants received the standard prenatal care services in Tanzania. All pregnant women received 60 mg elemental Fe and 0.25 mg folic acid daily. They also received malaria prophylaxis in the form of sulfadoxine-pyrimethamine tablets at 20 and 30 wk of gestation. To prevent mother-to-child transmission of HIV, all women received a single oral dose of 200 mg nevirapine at the onset of labor, and their infants received a dose of 2 mg nevirapine/kg syrup within 72 h of delivery.
Of 1129 participated women, a random subset of 312 women were co-enrolled in a randomized controlled trial examining the effect of daily selenium supplements (200 μg as selenomethionine) during pregnancy on maternal and child outcomes (20). Among them, 155 women were in the multiple RDA multivitamins group and 157 women were in the single RDA multivitamins group. We found no interaction between the effects of selenium and different dosages of multivitamin supplements on primary outcomes.
Study procedures
At the baseline visit, trained research nurses collected information about sociodemographic characteristics and obstetric history. At baseline and every month thereafter, study physicians performed physical examinations, and nurses inquired about health problems and obtained anthropometric measurements. At every monthly visit, participants exchanged a used bottle with a new bottle that contained 45 tablets. Compliance with the study regimens was defined as the number of tablets taken from the returned bottle divided by the total number of tablets the participants should have taken. Women who did not come for their monthly appointments were visited at home and were asked to come to the study clinic if their condition allowed.
A research midwife weighed the infants to the nearest 10 g on a standard beam balance immediately after birth. Placentas were also weighed after removal of blood clots. The midwife measured birth length with a length board and head circumference with a nonstretchable tape, both to the nearest 0.1 cm. Gestational age was based on the date of the last menstrual period.
Blood samples were obtained among a subset of women who co-enrolled in a trial of selenium supplements. The trial found no effect of selenium on maternal CD4, CD8, and CD3 cell counts, viral load, or hemoglobin concentration (20). We analyzed the first measurements taken during the first 10 wk after delivery. In this subset, hemoglobin was measured by using a CBC5 Coulter Counter (Coulter Corp, Miami, FL). Absolute counts of CD4, CD8, and CD3 T lymphocyte subsets were quantified by using the FACScount system (Becton-Dickinson, San Jose, CA). Viral load was measured by using the Roche Ampicor HIV-1 monitor test version 1.5 assay (Roche Diagnostics Corp, Indianapolis, IN).
The primary outcomes of the present trial were low birth weight (<2500 g) and preterm delivery (<37 wk of gestation). Secondary outcomes included low birth weight <2000 g, severe preterm delivery (<34 wk of gestation), small-for-gestational-age (birth weight below the 10th percentile of weight-for-gestational-age by the reference of Brenner et al; 21), fetal death (miscarriage or stillbirths), and infant death during the first 6 wk of life. We also examined the effect of multiple RDA compared with single RDA multivitamins on birth weight, gestational age, length, head circumference, and placental weight, as continuous variables. Maternal outcomes included hemoglobin, anemia (hemoglobin < 11 g/dL), T cell counts, and log10 viral load.
We hypothesized that women receiving multiples of RDA multivitamins would have better pregnancy outcomes than would those receiving single RDA multivitamins. We initially planned to enroll 1300 women to have a >80% power to detect a 60% difference in the incidence of low birth weight and a 35% difference in the incidence of preterm delivery. The sample size calculation was based on the assumption of a 5% type I error and 5% loss to follow-up. We assumed that the incidence of low birth weight would be 8.8% and the incidence of preterm delivery would be 21.2% in the multiples of RDA multivitamin arm. However, we were able to enroll only 1129 women in the trial because of funding constraints.
Data analysis
To verify the validity of randomization, we compared the distribution of baseline characteristics between treatment groups by using the t test for continuous variables and the chi-square test for categorical variables. An intent-to-treat analysis was performed to estimate treatment effects. Participated women gave 1064 singleton pregnancies and 32 pairs of twin pregnancies. We included all pregnancy outcomes in the analyses by using generalized estimating equations with a compound symmetry working correlation matrix, which accounts for correlations due to twin births (22). Binary endpoints were modeled by using the log link and the binomial variance function to estimate risk ratios (RRs), whereas for continuous endpoints were modeled by using the identity link and the Gaussian variance function to estimate mean differences. To assess statistical differences between treatment arms for birth outcomes, we obtained P values from the robust score test. To test treatment effects on women's outcomes, we used a binomial regression model with a log link function for binary endpoints and linear regression model for continuous endpoints. All statistical analyses were conducted by using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
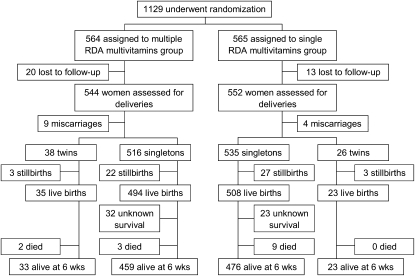
A total of 1129 HIV-infected pregnant women were randomly assigned to receive daily multiples of RDA or single RDA multivitamin supplements (Figure 1). The participants were, on average, 26.5 ± 4.8 y of age and at 21.8 ± 3.4 gestational weeks at randomization. Maternal characteristics at baseline were similar between treatment groups; however, we observed a small difference in the midupper arm circumference (Table 2). The mean duration of follow-up from randomization to delivery was 3.9 ± 1.1 mo, and the mean duration from randomization to 6 wk postpartum was 5.1 ± 1.1 mo. Those periods did not differ between treatment groups (P = 0.22 and P = 0.25, respectively). Mean compliance with study regimen by 6 wk postpartum was 85% (median = 89%) in the multiple RDA multivitamins group and 87% (median = 90%) in the single RDA multivitamins group. The compliance with the study regimen did not differ between treatment groups (P = 0.16).
FIGURE 1.
Trial profile. One of each set of twins died for all cases. RDA, Recommended Dietary Allowance.
TABLE 2.
Baseline characteristics by treatment group1
| Characteristic | Multiple RDA multivitamins (n = 564) | Single RDA multivitamins (n = 565) |
| Gestational age at enrollment (wk) | 21.6 ± 3.22 | 21.9 ± 3.5 |
| Maternal age (y) | 26.3 ± 4.7 | 26.6 ± 4.9 |
| Education [n (%)] | ||
| None or adult education | 55 (10.1) | 50 (9.1) |
| Primary | 375 (69.1) | 385 (69.6) |
| Secondary | 82 (15.1) | 88 (15.9) |
| University | 31 (5.7) | 30 (5.4) |
| Money spent on food (TSh)3 | 588 ± 300 | 595 ± 325 |
| Parity [n (%)] | ||
| 0 | 144 (26.5) | 156 (28.2) |
| 1 | 177 (32.6) | 168 (30.4) |
| 2 | 113 (20.8) | 113 (20.4) |
| ≥3 | 109 (20.1) | 116 (21.0) |
| Previous stillbirth or miscarriage [n (%)] | 114 (20.9) | 108 (19.4) |
| BMI (kg/m2) | 25.0 ± 4.0 | 24.7 ± 3.8 |
| Height (cm) | 155.3 ± 6.1 | 155.8 ± 6.1 |
| Midupper arm circumference (cm) | 26.1 ± 3.0 | 26.5 ± 3.2 |
Subject numbers (n) may not add up to 564 or 565 because of missing data. RDA, Recommended Dietary Allowance; TSh, Tanzanian shillings. The chi-square test was used to compare categorical variables, and the t test was used to compare continuous variables. There were no significant differences between treatment groups for any baseline characteristics except for midupper arm circumference (P = 0.03).
Mean ± SD (all such values).
Average household income spent on food per person per day in TSh; 1 US dollar was equivalent to ≈1000 TSh at the time of the study.
Multiples of RDA and single RDA multivitamins had similar effects on the risk of low birth weight (11.6% and 10.2%, respectively; RR = 1.07; 95% CI = 0.70, 1.62; P = 0.75; Table 3). We found no difference between the 2 groups in the risk of preterm birth (RR = 1.04; 95% CI = 0.81, 1.35; P = 0.73) or small-for-gestational-age (RR = 1.30; 95% CI = 0.89, 1.90; P = 0.18). The mean birth weights were similar in the multiple RDA multivitamins group (3045 ± 549 g) and the single RDA multivitamins group (3052 ± 534 g; P = 0.83). No differences were found in duration of gestation (P = 0.85), birth length (P = 0.76), head circumference (P = 0.89), and placental weight (P = 0.61).
TABLE 3.
Birth outcomes in the multiple Recommended Dietary Allowance (RDA) and single RDA multivitamin groups
| Multiple RDA multivitamins |
Single RDA multivitamins |
|||||
| Endpoint | n | Value | n | Value | Mean difference or risk ratio (95% CI)1 | P value |
| Birth weight (g) | 502 | 3045 ± 549 | 498 | 3052 ± 534 | −8 (−75, 60) | 0.83 |
| <2500 g [n (%)] | 502 | 58 (11.6) | 498 | 51 (10.2) | 1.07 (0.70, 1.62) | 0.75 |
| <2000 g [n (%)] | 502 | 23 (4.6) | 498 | 21 (4.2) | 1.07 (0.55, 2.09) | 0.84 |
| Gestational age (wk)2 | 509 | 38.9 ± 3.4 | 521 | 38.8 ± 3.1 | 0 (−0.4, 0.4) | 0.85 |
| Preterm birth [n (%)]2 | ||||||
| <37 wk | 509 | 98 (19.3) | 521 | 96 (18.4) | 1.04 (0.81, 1.35) | 0.73 |
| <34 wk | 509 | 35 (6.9) | 521 | 32 (6.1) | 1.12 (0.70, 1.78) | 0.63 |
| Low birth weight and preterm birth [n (%)]3 | 501 | 33 (6.6) | 498 | 29 (5.8) | 1.03 (0.60, 1.78) | 0.91 |
| Low birth weight and term birth [n (%)]4 | 405 | 25 (6.2) | 405 | 22 (5.4) | 1.12 (0.61, 2.06) | 0.72 |
| Small-for-gestational-age [n (%)]5 | 487 | 72 (14.8) | 485 | 58 (12.0) | 1.30 (0.89, 1.90) | 0.18 |
| Length (cm) | 471 | 48.3 ± 4.2 | 482 | 48.3 ± 4.2 | −0.1 (−0.6, 0.4) | 0.76 |
| Head circumference (cm) | 471 | 34.7 ± 3.0 | 481 | 34.8 ± 3.0 | 0 (−0.4, 0.4) | 0.89 |
| Placental weight (g) | 336 | 503 ± 95 | 346 | 507 ± 101 | −4 (−19, 11) | 0.61 |
For birth outcomes, generalized estimating equations with a compound symmetry working correlation matrix were used to account for correlations due to twin births. For binary endpoints, the log link and binomial variance function were used, whereas the identity link and the Gaussian variance function were used for continuous endpoints.
Gestational age and preterm birth were considered to be an outcome for women. All other endpoints were considered to be an outcome for infants.
Defined as birth weight <2500 g and gestational age <37 wk.
Defined as birth weight <2500 g and gestational age ≥37 wk.
Defined as birth weight below the 10th percentile of weight-for-gestational-age.
We found no difference in the risk of miscarriage (P = 0.17) or stillbirths (P = 0.52) between treatment groups (Table 4). The risk of fetal death in the multiple RDA multivitamins group was similar to the risk in the single RDA multivitamins group (6.0% compared with 6.0%; RR = 1.00; 95% CI = 0.63, 1.58; P = 0.99). There was no significant difference in the 2 groups in the risk of perinatal death (RR = 0.75; 95% CI = 0.47, 1.21; P = 0.25) or early infant death (RR = 0.46; 95% CI = 0.14, 1.48; P = 0.19); however, power was poor for detecting differences in those outcomes.
TABLE 4.
Fetal and early infant deaths in the multiple Recommended Dietary Allowance (RDA) and the single RDA multivitamins groups
| Endpoint | Time of death | Multiple RDA multivitamins | Single RDA multivitamins | Risk ratio (95% CI)1 | P value |
| n/total n (%) | n/total n (%) | ||||
| Miscarriage2 | Before 28 wk gestation | 9/544 (1.7) | 4/552 (0.7) | 2.28 (0.71, 7.37) | 0.17 |
| Stillbirth | Between 28 wk gestation and delivery | 25/554 (4.5) | 30/561 (5.4) | 0.84 (0.49, 1.44) | 0.52 |
| Fetal loss | Any time before delivery | 34/563 (6.0) | 34/565 (6.0) | 1.00 (0.63, 1.58) | 0.99 |
| Perinatal death | Between 28 wk gestation and 1 wk after delivery | 29/554 (5.2) | 38/561 (6.8) | 0.75 (0.47, 1.21) | 0.25 |
| Early infant death | During the first 6 wk after delivery | 5/497 (1.0) | 9/508 (1.8) | 0.46 (0.14, 1.48) | 0.19 |
| Fetal or early infant death | Any time before delivery or during first 6 wk after delivery | 39/563 (6.9) | 43/565 (7.6) | 0.90 (0.59, 1.37) | 0.63 |
Generalized estimating equations with a compound symmetry working correlation matrix were used to account for correlations due to twin births. The log link and binomial variance function were used in the model.
Miscarriage was considered to be an outcome for women. All other endpoints were considered to be an outcome for infants.
In a subgroup of mothers who also participated in a trial of selenium supplements (20), we assessed hemoglobin, T cell counts, and viral load after delivery and up to 10 wk postpartum. We found no difference in the mean CD4, CD8, or CD3 T cell counts between women who received multiples of RDA multivitamin supplements and those who received single RDA multivitamin supplements (P = 0.34, 0.38, and 0.59, respectively; Table 5). Furthermore, no differences were found in mean hemoglobin concentrations (P = 0.96) and viral load (P = 0.55) between the 2 groups.
TABLE 5.
Maternal hemoglobin concentrations, T cell counts, and viral load among those who received multiple or single Recommended Dietary Allowance (RDA) multivitamins1
| Multiple RDA multivitamins |
Single RDA multivitamins |
|||||
| Endpoint | n | Value | n | Value | Mean difference or risk ratio (95% CI)2 | P value |
| Hemoglobin (g/dL) | 131 | 11.5 ± 1.33 | 141 | 11.5 ± 1.6 | 0 (−0.3, 0.4) | 0.96 |
| <11 g/dL [n (%)] | 131 | 42 (32.1) | 141 | 48 (34.0) | 0.91 (0.55, 1.52) | 0.73 |
| CD4 count (cells/mm3) | 120 | 515 ± 261 | 121 | 549 ± 299 | −35 (−105, 36) | 0.34 |
| CD8 count (cells/mm3) | 120 | 1193 ± 479 | 121 | 1142 ± 422 | 51 (−63, 164) | 0.38 |
| CD3 count (cells/mm3) | 120 | 1839 ± 674 | 121 | 1795 ± 598 | 44 (−114, 207) | 0.59 |
| Viral load (log10) | 81 | 4.05 ± 0.93 | 93 | 4.13 ± 0.94 | −0.09 (−0.36, 0.19) | 0.55 |
Measurements were taken during the first 10 wk after delivery.
A linear regression model was used to estimate mean differences, and a binomial regression model with a log link function was used to estimate the risk ratio.
Mean ± SD (all such values).
DISCUSSION
We found no difference in the effects of single compared with multiple RDA multivitamin supplements on the risks of adverse pregnancy outcomes among HIV-infected pregnant women. Similar incidences of low birth weight, preterm birth, and fetal death were found between single and multiple RDA multivitamins groups. Maternal CD4 T cell counts and hemoglobin concentrations were also similar in the early postpartum period. The strengths of our study include the use of randomized double-blind design, high compliance with study regimen, and high follow-up rates. The study may be limited by low statistical power; however, we observed similar incidences between the 2 arms in most outcomes.
In a previous trial among HIV-infected women in Tanzania, multivitamin supplements at multiple doses of RDA with identical doses to those used in the current trial were beneficial as compared with placebo (with all women receiving the same dosage of iron and folate). Women who received multivitamins had a 39% reduction in the risk of fetal death (incidence of 5.9% in the multivitamin group compared with 9.6% in the control group), a 44% reduction in the risk of low birth weight (incidence 8.8% compared with 15.8%), and a 39% reduction in the risk of severe preterm birth (incidence 6.2% compared with 10.2%) (7). Furthermore, multivitamin supplements significantly increased the mean birth weight (3048 g in the multivitamin compared with 2948 g in the control). The incidences of adverse pregnancy outcomes observed in the current study in both multiple RDA and single RDA multivitamin arms were similar to the incidences observed in the multiple RDA multivitamin arm in the previous trial, suggesting that both treatment arms in the current trial had decreased the risks of adverse pregnancy outcomes.
Many clinical trials and observational studies strongly support the use of multivitamin supplements among HIV-infected individuals. Several randomized controlled trials examining the effects of multivitamins on clinical outcomes had found that high-dose micronutrient supplements reduce HIV disease progression or mortality among HIV-infected individuals in Tanzania, Thailand, and Canada (23–25). Furthermore, prospective cohort studies showed that vitamin B-1, B-2, and B-6 intakes at multiples of RDA were associated with improved survival rates among HIV-infected individuals (12). Dietary intakes of vitamin C at 10 times and niacin at 3 times the RDA were associated with a decreased progression to AIDS (13, 14). In a trial in Zimbabwe, micronutrients at single RDA level were associated with a nonsignificant increased birth weight by 101 g, but had no effect on low birth weight (26). Given previous studies and the current study, multivitamin supplements containing at least single RDA doses should be recommended for HIV-infected pregnant women. However, we cannot generalize our findings for clinical outcomes among HIV-infected women who are not pregnant. Whereas multiples of RDA multivitamins have been shown to be beneficial, the long-term benefits of single RDA multivitamins on delaying HIV disease progression among HIV-infected individuals remains unknown.
It is important to consider whether multivitamin supplements should be provided to not only HIV-positive but also HIV-negative pregnant women, because most pregnant women in developing countries do not know their HIV status. In sub-Saharan Africa, HIV testing coverage among pregnant women ranges from 4% in Nigeria to 33% in Tanzania and 64% in South Africa (27). Most of the trials that examined the effect of micronutrient supplements among presumably HIV-negative women used the UNICEF/WHO/UNU international multiple micronutrient preparation (UNIMMAP) supplement, which is composed of 15 vitamins and minerals at the single RDA level. Most, but not all, of these previous trials found improvements in birth weight (26, 28–37). A recent large trial of 31,290 women in Indonesia showed that micronutrient supplements significantly reduced the risk of low birth weight by 14% and significantly decreased the risk of early infant mortality by 18% when compared with iron and folic acid (29). A trial of 8468 HIV-negative women in Tanzania found that multiple RDA multivitamins significantly reduced the risk of low birth weight by 18% (28). A hospital-based trial in India also showed that micronutrient supplements decreased the risk of low birth weight by 70% among 200 undernourished pregnant women (30).
Some trials raised concerns that micronutrients may potentially increase perinatal mortality (37–39). Researchers suggested that potential adverse effects may occur from increased risks of birth asphyxia or cephalopelvic disproportion during labor among large-for-gestational-age infants (38). Trials that found potential adverse effects were conducted in the rural settings in Nepal and Burkina Faso (37, 39). Adequate quality of obstetric care may be essential to prevent potential adverse effects. Trials in Tanzania and Indonesia found no effect on perinatal mortality, and they were conducted in the urban and semiurban setting, where adequate prenatal and obstetrical care were provided. A large proportion of perinatal mortality could be prevented by improving care of birth asphyxia, infections, and prematurity as well as improving birth preparedness and access to emergency obstetric care in many developing countries (40).
In conclusion, we found that multivitamin (vitamin B complex, vitamin C, and vitamin E) supplements at a single dose of the RDA may be as efficacious as multiple doses of the RDA in reducing the risk of adverse pregnancy outcomes among HIV-infected women. Multivitamin supplements at a dose of at least a single RDA should be recommended to all HIV-infected pregnant women. However, the long-term benefits of single doses of RDA, rather than multiple doses of RDA, multivitamins on delaying the progression of HIV disease among HIV-infected individuals remain unknown. Multivitamins may also be beneficial among HIV-negative pregnant women in developing countries; however, because of the potential adverse effects on perinatal mortality found in some trials, multivitamin supplements may be considered for pregnant women only where adequate antenatal and obstetrical care are provided. Additional research is warranted to assess the safety and efficacy of supplementation in rural and other settings where the coverage of such services is suboptimal.
Acknowledgments
We thank the mothers and children who participated in this study and the field teams, including physicians, nurses, midwives, supervisors, laboratory staff, and administrative staff. We are very grateful to our colleagues Karim Manji, Gernard Msamanga, and Ruilan Wei.
The authors’ responsibilities were as follows—KK: conducted the data analyses, interpreted the data, and wrote the initial draft of the manuscript; RK: interpreted the data and assisted with data management; FM and SA: participated in the study implementation and field supervision; JO: conducted the data analyses; EV: contributed to the study design and interpreted the data; DS: provided statistical guidance in the study design and data analyses; and WWF (Principal Investigator): contributed to the study design, implemented the study, and interpreted the data. All authors participated in the manuscript preparation. None of the authors had any conflicts of interest.
REFERENCES
- 1.UNICEF/WHO Low birthweight: country, regional and global estimates. New York, NY: UNICEF, 2004 [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? where? why? Lancet 2005;365:891–900 [DOI] [PubMed] [Google Scholar]
- 3.Walker DM, Marlow N. Neurocognitive outcome following fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2008;93:F322–5 [DOI] [PubMed] [Google Scholar]
- 4.Habib NA, Daltveit AK, Bergsjo P, Shao J, Oneko O, Lie RT. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. BJOG 2008;115:616–24 [DOI] [PubMed] [Google Scholar]
- 5.Fawzi W, Msamanga G. Micronutrients and adverse pregnancy outcomes in the context of HIV infection. Nutr Rev 2004;62:269–75 [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 7.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82 [DOI] [PubMed] [Google Scholar]
- 8.Villamor E, Msamanga G, Spiegelman D, et al. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV-1-infected women. Am J Clin Nutr 2002;76:1082–90 [DOI] [PubMed] [Google Scholar]
- 9.Fawzi WW, Msamanga GI, Kupka R, et al. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr 2007;85:1335–43 [DOI] [PubMed] [Google Scholar]
- 10.Baum MK, Shor-Posner G, Bonvehi P, et al. Influence of HIV infection on vitamin status and requirements. Ann N Y Acad Sci 1992;669:165–73, discussion 173–4 [DOI] [PubMed] [Google Scholar]
- 11.Baum M, Cassetti L, Bonvehi P, Shor-Posner G, Lu Y, Sauberlich H. Inadequate dietary intake and altered nutrition status in early HIV-1 infection. Nutrition 1994;10:16–20 [PubMed] [Google Scholar]
- 12.Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol 1996;143:1244–56 [DOI] [PubMed] [Google Scholar]
- 13.Tang AM, Graham NM, Kirby AJ, McCall LD, Willett WC, Saah AJ. Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol 1993;138:937–51 [DOI] [PubMed] [Google Scholar]
- 14.Abrams B, Duncan D, Hertz-Picciotto I. A prospective study of dietary intake and acquired immune deficiency syndrome in HIV-seropositive homosexual men. J Acquir Immune Defic Syndr 1993;6:949–58 [PubMed] [Google Scholar]
- 15.Tang AM, Graham NM, Semba RD, Saah AJ. Association between serum vitamin A and E levels and HIV-1 disease progression. AIDS 1997;11:613–20 [DOI] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 2002;16:1935–44 [DOI] [PubMed] [Google Scholar]
- 17.Humphrey JH, Iliff PJ, Marinda ET, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis 2006;193:860–71 [DOI] [PubMed] [Google Scholar]
- 18.Fawzi WW, Villamor E, Msamanga GI, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. Am J Clin Nutr 2005;81:161–7 [DOI] [PubMed] [Google Scholar]
- 19.Osendarp SJ, West CE, Black RE. The need for maternal zinc supplementation in developing countries: an unresolved issue. J Nutr 2003;133:817S–27S [DOI] [PubMed] [Google Scholar]
- 20.Kupka R, Mugusi F, Aboud S, et al. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. Am J Clin Nutr 2008;87:1802–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol 1976;126:555–64 [DOI] [PubMed] [Google Scholar]
- 22.Fitzmaurice GM, Laid NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons, 2004 [Google Scholar]
- 23.Fawzi WW, Msamanga GI, Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med 2004;351:23–32 [DOI] [PubMed] [Google Scholar]
- 24.Jiamton S, Pepin J, Suttent R, et al. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS 2003;17:2461–9 [DOI] [PubMed] [Google Scholar]
- 25.Austin J, Singhal N, Voigt R, et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur J Clin Nutr 2006;60:1266–76 [DOI] [PubMed] [Google Scholar]
- 26.Friis H, Gomo E, Nyazema N, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr 2004;80:178–84 [DOI] [PubMed] [Google Scholar]
- 27.WHO/UNAIDS/UNICEF Towards universal access: scaling up priority HIV/AIDS in the health sector: progress report, April 2007. Geneva, Switzerland: UNAIDS, 2007 [Google Scholar]
- 28.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med 2007;356:1423–31 [DOI] [PubMed] [Google Scholar]
- 29.Shankar AH, Jahari AB, Sebayang SK, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet 2008;371:215–27 [DOI] [PubMed] [Google Scholar]
- 30.Gupta P, Ray M, Dua T, Radhakrishnan G, Kumar R, Sachdev HP. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: a double-blind, randomized, placebo-controlled trial. Arch Pediatr Adolesc Med 2007;161:58–64 [DOI] [PubMed] [Google Scholar]
- 31.Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: a randomized controlled trial in a semirural community in Mexico. Am J Clin Nutr 2003;77:720–5 [DOI] [PubMed] [Google Scholar]
- 33.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr 2005;59:1081–9 [DOI] [PubMed] [Google Scholar]
- 34.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet 2005;365:955–62 [DOI] [PubMed] [Google Scholar]
- 35.Zagre NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull 2007;28:317–27 [DOI] [PubMed] [Google Scholar]
- 36.Zeng L, Cheng Y, Dang S, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberfroid D, Huybregts L, Lanou H, et al. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr 2008;88:1330–40 [DOI] [PubMed] [Google Scholar]
- 38.Christian P, Osrin D, Manandhar DS, Khatry SK, de L Costello AM, West KP., Jr Antenatal micronutrient supplements in Nepal. Lancet 2005;366:711–2 [DOI] [PubMed] [Google Scholar]
- 39.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr 2003;78:1194–202 [DOI] [PubMed] [Google Scholar]
- 40.Bhutta ZA, Ali S, Cousens S, et al. Alma-Ata: rebirth and revision 6 interventions to address maternal, newborn, and child survival: what difference can integrated primary health care strategies make? Lancet 2008;372:972–89 [DOI] [PubMed] [Google Scholar]