Abstract
Schizophrenia is a devastating psychiatric disorder that affects around 1% of the population worldwide. The disease is characterized by ‘positive symptoms’, ‘negative symptoms’ and cognitive deficits. Over the last 60 years, a large number of family, twin and adoption studies have clearly demonstrated a strong genetic component for schizophrenia, but the mode of inheritance of the disease is complex and, in all likelihood, involves contribution from multiple genes in conjunction with environmental and stochastic factors. Recently, several genome-wide scans have demonstrated that rare alleles contribute significantly to schizophrenia risk. Assessments of rare variants have identified specific and probably causative, disease-associated structural mutations or copy number variants (CNVs, which result from genomic gains or losses). The fact that the effects of such lesions are transparent allows the generation of etiologically valid animal models and the opportunity to explore the molecular, cellular and circuit-level abnormalities underlying the expression of psychopathology. To date, the most common genomic structural rearrangements that are unequivocally associated with the development of schizophrenia, are de novo microdeletions of the 22q11.2 locus. Fortunately, the human 22q11.2 locus is conserved within the syntenic region of mouse chromosome 16, which harbors nearly all orthologues of the human genes. This has made it possible to engineer genetically faithful, and thus etiologically valid, animal models of this schizophrenia susceptibility locus.
Schizophrenia: a clinical overview
Schizophrenia is a severe psychiatric disorder that has a lifetime prevalence of around 1% in most of the populations studied (Karayiorgou and Gogos, 1997), and which has been estimated to be the seventh most costly medical illness to our society (Freedman, 2003). Although patients with schizophrenia can present with several clinical abnormalities, including structural brain pathology (e.g. enlarged third and fourth ventricles; reduced volume of cortical gray matter) and neuropsychological impairments (e.g. deficits in working memory) (Lewis and Lieberman, 2000), none of these are consistently present in all patients who suffer from this disease. Because of the absence of any consistent diagnostic biological markers that exist, for example, for neurodegenerative disorders, such as Alzheimers’s disease or Parkinson’s disease, schizophrenia is defined as a clinical syndrome. The disease is characterized by ‘positive symptoms’ including hallucinations, delusions and thought disorders; ‘negative symptoms’ such as blunted emotions, poverty of speech, avolition and asociality; and cognitive deficits including impairments in attention, executive function and working memory. Although assessment of cognitive symptoms can predict functional outcome, only the positive and negative symptom domains are captured by operational definitions in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR, 2000). However, as a heterogeneous syndrome, schizophrenia lacks any single defining symptom or sign, and no biochemical or physiological test is available for the disorder (Karayiorgou and Gogos, 1997). Furthermore, there is a considerable overlap of diagnostic categories; for example, positive symptoms can be part of at least three different diagnoses – schizophrenia, bipolar disorder and psychotic depression. Although these diagnoses are highly reproducible and clinically useful, they do not necessarily reflect valid, separate constructs on a genetic level. Epidemiological studies indicate a complex pattern of high heritability that interacts with epigenetic, stochastic and environmental factors. Although some genetic variants appear to increase the risk across several diagnostic entities, others only influence the risk for a subset of conditions (i.e. endophenotypes or intermediate phenotypes) (Burmeister et al., 2008). It appears likely that when sufficient, replicated genetic findings have become established, they will reshape the way in which schizophrenia is clinically conceptualized. From a treatment standpoint, antipsychotics target some of the symptoms that patients with schizophrenia suffer from, but often with suboptimal results, and many patients develop side effects that cause them to become non-compliant with their medication (Lieberman et al., 2005). Furthermore, except for possibly clozapine, none of the treatment modalities appear to be effective for the negative symptoms, which to a large extent determine functional outcome for many of the patients (Webber and Marder, 2008). This all points to the imperative need for a better understanding of the pathogenesis of schizophrenia, so that more optimal medications can be developed on the basis of hypothesis-driven research.
Genetic architecture of schizophrenia
Schizophrenia, like other common psychiatric and non-psychiatric diseases, is multifactorial in nature with contributions from multiple susceptibility genes in conjunction with epigenetic, stochastic and environmental factors (Karayiorgou and Gogos, 1997). The genetic architecture underlying disease susceptibility is characterized by both the frequency and penetrance of risk alleles. The common disease-common allele (CDCA) hypothesis emphasizes the importance of relatively common alleles, each with a small effect, acting together to increase disease risk. The common disease-rare allele (CDRA) hypothesis instead emphasizes the impact of individually rare, yet highly penetrant, alleles. It is likely that both common and rare alleles contribute to the risk of psychiatric disorders. Although the relative impact of each remains unknown, recent studies have provided strong evidence supporting the importance of rare structural mutations/variants in schizophrenia vulnerability.
Case study.
V.C. is a 24-year-old, previously healthy man, who was brought to the psychiatric emergency room (ER) by his father because he was convinced that he could hear the voice of demons at night, telling him that they were planning to hurt him. During the previous year, V.C. had become increasingly withdrawn and had stopped going out with friends and family. In addition, V.C. started paying less attention to his personal hygiene and his parents noticed that he was becoming increasingly paranoid. His father reported that, 5 months ago, V.C. started talking about being followed in the street and that he felt that people were watching him through hidden cameras in his apartment. During the 3 months leading up to his presentation in the ER, V.C. also noticed that people on television and on magazine covers were looking at him directly and were talking to him. Moreover, V.C. mentioned to his family that he felt that he could read other people’s thoughts. His father reported that V.C.’s maternal uncle also has a history of paranoia and hearing voices. In the ER, V.C. appeared disheveled and was noticeably suspicious towards the medical staff. V.C. endorsed hearing voices of demons and acknowledged that he felt that cameras in the apartment were watching him. Furthermore, he denied any medical history or a history of substance use, and was not receiving any medication. V.C. also denied any symptoms that were indicative of depression, hypomania or mania. Laboratory results all came back negative and a computed tomography (CT) scan of his head revealed no abnormalities. The patient was admitted to an inpatient psychiatric unit, where he was started on 2.5 mg of aripiprazole. His dosage was gradually increased to 10 mg and, over the course of a 4-week period, his symptoms gradually decreased. By the time of discharge, V.C. was no longer hearing voices and was no longer experiencing paranoid ideas. When asked about his previous ideas of being watched and followed, he stated that he still believed that people might be out to hurt him, but no longer appeared preoccupied by this.
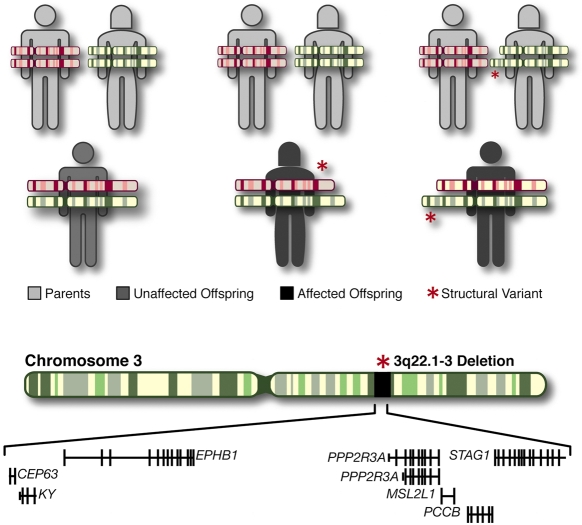
In particular, recent rapid developments in microarray technologies have led to the discovery of extensive copy number variation (CNV) in the human genome (Iafrate et al., 2004; Sebat et al., 2004) and allow for extensive analysis of the association between such structural genetic variants and complex diseases, like schizophrenia. CNVs are segments of DNA of varying size for which copy number differences have been established by a comparison of two or more genomes (Feuk et al., 2006), and which often encompass one or more genes (Redon et al., 2006). CNVs can be either de novo (formed in the parental germline) or inherited from an affected or unaffected parent (Fig. 1). It has been demonstrated that rare structural mutations leading to an altered copy number of dosage-sensitive genes can lead to the development of neuropsychiatric disorders, such as autism and schizophrenia (Lee and Lupski, 2006; Beckmann et al., 2007). Recently, several large-scale genome-wide scans have successfully identified several schizophrenia-associated CNVs, including a number of recurrent variants (Table 1), which appear to be rare but, typically, highly penetrant (Xu et al., 2008; Xu et al., 2009; International Schizophrenia Consortium, 2008; Stefansson et al., 2008; Walsh et al., 2008).
Fig. 1.
Structural mutations (CNVs) and their mode of inheritance. Top panel: structural mutations can be either de novo (generated in the parental germline) (middle) or inherited from affected or unaffected parents (right). Bottom panel: a typical CNV (microdeletion) including several genes identified in the Xu et al. study (Xu et al., 2008).
Table 1.
Recurrent CNVs enriched in schizophrenia cohorts
Of all the CNVs, the most extensively studied are the de novo 22q11.2 microdeletions. (Karayiorgou et al., 1995). 22q11.2 microdeletions are relatively common and are associated with a high schizophrenia risk, approximately 25–31 times that of the general population (Pulver et al., 1994; Murphy et al., 1999). Conversely, 22q11.2 microdeletions account for up to 1–2% of nonfamilial (sporadic) cases of schizophrenia (Karayiorgou et al., 1995; Xu et al., 2008). This strong bidirectional association remains to be demonstrated for other potentially pathogenic CNVs (such as the ones shown in Table 1). Thus, to date, microdeletions of 22q11.2 are the only known recurrent CNVs that are unequivocally associated with schizophrenia, and therefore make the mouse model of this CNV an attractive approach to study the disease.
Creating valid models for schizophrenia in mice
Models are approximations of phenomena of interest that are more tractable for experimental investigation. To be useful, animal models of psychiatric disorders need not fully recapitulate the disease, which, given the human uniqueness of these disorders, is actually impossible. In creating mice models of schizophrenia, two main factors must be considered: the genetic and clinical data upon which the model is based, and the methods for characterizing the model (Arguello and Gogos, 2006).
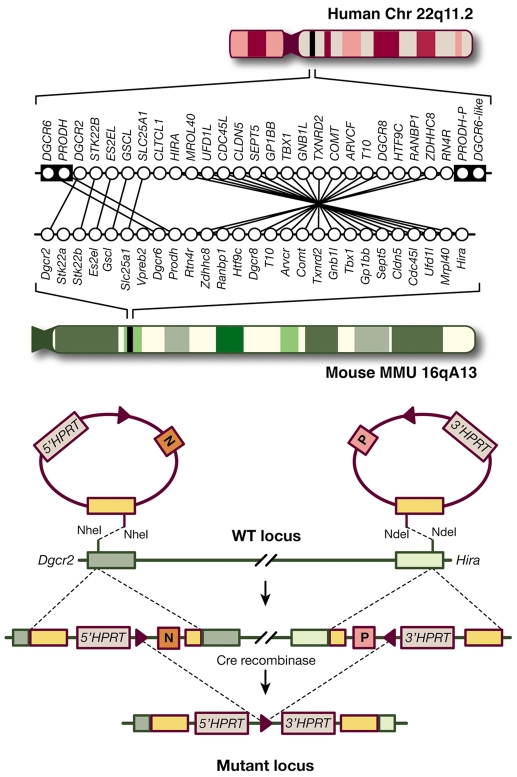
The search for genetic variants that influence complex traits, such as the risk for psychiatric disorders, is often plagued with inconsistent findings and equivocal reports (Shi et al., 2009; Stefansson et al., 2009). In that respect, rare variants offer important advantages in terms of modeling in mice compared with more common and less penetrant variants. Importantly, the potential insight into disease etiology gained from rare variants is greater because their statistical association is more consistent and their functional effects are more transparent. This enables models that faithfully recapitulate the disease-associated allele to be engineered genetically. This genetic fidelity is essential because any given gene product can participate in numerous biological pathways, and modeling the exact risk allele is more likely to affect those pathways that are relevant to disease pathogenesis. Ultimately, a comparison of models of different rare alleles holds the most promise for identifying disease mechanisms. One example of this is the balanced translocation affecting the DISC1 gene that was found in a large Scottish pedigree affected with high rates of psychiatric illness, which was subsequently modeled in mice (Koike et al., 2006). Another case in point is the 22q11.2 microdeletion, which we discuss further below. Nearly all human orthologous genes are located in the syntenic region of mouse chromosome 16, which allowed the generation of a mouse allele with high fidelity to the disease-associated human allele using the Cre/lox technology (Fig. 2).
Fig. 2.
Modeling structural mutations in mice. Top panel: schematic diagram showing the human chromosome 22q11.2 region and the syntenic mouse region. Almost all of the functional human genes in this segment are represented in the mouse, organized in a slightly different order. The minimal 1.5 Mb deletion is mediated by the low copy repeat sequences LCR-A and LCR-B (illustrated as black boxes in the human chromosome). PRODH-P and DGCR6-like (also known as DGCR6L) indicate pseudogenes. Dgcr2 and Hira are the two end points of the depicted targeted deletion generated in mouse chromosome 16. Bottom panel: Cre-induced recombination between loxP sites in cis leads to the generation of a functional HPRT minigene and a 1.3-Mb deficiency in mice.
Clinical terms.
Endophenotype or intermediate phenotype – a biomarker of an illness that is heritable and found to co-segregate with family members of subjects with the illness
Copy number variation (CNV) – segments of DNA of varying size for which copy number differences have been established by a comparison of two or more genomes
Microdeletion – a CNV where a segment of DNA is deleted
Hypomania – a clinical psychiatric state, where patients experience moderately severe symptoms of decreased need for sleep, increased goal-directed activity, flight of ideas, pressure to keep talking, grandiosity, and increased involvement in high-risk behaviors
Mania – a clinical psychiatric state, where patients experience severe symptoms of decreased need for sleep, increased goal-directed activity, flight of ideas, pressure to keep talking, grandiosity, and increased involvement in high-risk behaviors
Phenotyping animal models
A considerable body of evidence from neuropathological, imaging and genetic studies indicate that schizophrenia pathology exists at a molecular, cellular and neuronal circuit level, and that mouse models should be designed in accordance with this. Thus, despite its complexity, schizophrenia can be deconstructed into these individual phenotypic components that can then be analyzed in animal models (Arguello and Gogos, 2006). Clearly, the psychotic features of schizophrenia, such as the auditory hallucinations and delusions, are challenging, if not impossible, to measure in animal models. Nevertheless, hyperactivity in response to stress or novelty, as well as hypersensitivity to psychostimulants, have been suggested as potentially useful correlates that can be modeled in rodents. However, the basic foundations of perception, attention and memory are present in mice and these can, unlike thought content, be readily measured through objective behavioral tests. In particular, the ability to accurately characterize cognitive function is crucial given that cognitive deficits are more enduring, more predictive of functional outcome, and less responsive to treatment than other symptoms. Furthermore, precise tests of cognitive operations can identify specific underlying neural circuits and processes that can then be more finely characterized at molecular and cellular levels. It is likely that evaluating cognition in animal models will not only lead to a better understanding of disease mechanisms but will also aid in the discovery of novel targets for therapeutic intervention.
Clinical and basic research opportunities.
Identification of consistent biological markers that can be used to diagnose schizophrenia
Determination of the relative contributions of genes, epigenetic, stochastic and environmental factors that influence a person’s susceptibility to schizophrenia
Genetically engineered mouse models that recapitulate the genetic attributes of human disease: mice with rare genetic variant forms of disease that can be used to uncover new potential targets for therapy
There are diverse behavioral paradigms for assessing cognition in models. Memory systems in animals, like in humans, are organized across the temporal domain with both short and long forms. Conditioned fear is a type of long-term memory (LTM) that is critically dependent on the hippocampus and amygdala. Mice with a hemizygous deletion spanning all (Df(16)A+/−) (Stark et al., 2008), or part (Df1/+) (Paylor et al., 2001), of the orthologous genes within the 1.5-Mb region exhibit impaired conditioned fear. Working memory (WM) is a special case of short-term memory and is more labile and transient than LTM. It depends on the orchestration of neural activity across brain regions by the prefrontal cortex. Mice that are hemizygous for the orthologous 22q11.2 deletion seem to have deficits in WM in addition to the LTM deficits described above. These mice are impaired in the acquisition of a task requiring them to alternate between arms in a maze following a brief delay. Interestingly, this deficit arises in part from a deficiency of one gene within the deletion, Dgcr8, a microRNA processor that, when absent, causes changes in microRNA levels and impairs dendritic development; heterozygous deletion of just Dgcr8 impairs acquisition of a similar WM task (Stark et al., 2008). The combined reduction in the levels of two other 22q11.2 genes, cathechol-O-methyltransferase (Comt, encoding an intracellular enzyme that plays a significant role in modulating dopamine clearance in the cortex) and pro-line dehydrogenase (Prodh, encoding an enzyme that metabolizes the neuromodulator L-proline), and their epistatic interaction in modulating cortical dopaminergic transmission, also contributes to the WM deficits that emerge as a result of the 22q11.2 microdeletion (Paterlini et al., 2005). Taken together, this suggests that behavioral deficits associated with the 22q11.2 microdeletion result from the combined effects of genes acting individually and interactively. A promising area of future research is investigating how these cognitive deficits are related to the changes in dendritic complexity, excitatory synapses and dopamine transmission that are found in Df(A) deletion mice (Mukai et al., 2008; Paterlini et al., 2005; Stark et al., 2008).
Other groups have shown that several genes within the 1.5-Mb region differentially affect pre-pulse inhibition (PPI), a measure of pre-attentive processing where a brief, low intensity acoustic stimulus (the ‘pre-pulse’) inhibits the startle reflex caused by a loud stimulus (Powell et al., 2009). PPI is affected in a number of patients with schizophrenia and other psychiatric diseases (Powell et al., 2009). Although PPI is not diagnostically specific or predictive of clinical outcome, it is a relatively robust and widely used assay in phenotyping animal models of schizophrenia, and can be administered to animals in a fashion almost identical to humans. A heterozygous deficiency in a subset of 22q11.2 genes (Gnb1l, Dgcr8 and Tbx1) has been shown to result in reduced PPI levels (Long et al., 2006; Paylor et al., 2006; Stark et al., 2008). Notably, an engineered mouse strain that is deficient for seven other genes spanning around 150 kb of the 1.5-Mb deletion syntenic region (Kimber et al., 1999) has an increased PPI, suggesting that other genes within this region have opposing influences and that PPI deficits emerging as a result of the 22q11.2 microdeletion are the result of interplay between both positive and negative contributions from genes residing in this locus.
Schizophrenia: future directions
Advances in understanding the causes and treatment for schizophrenia require the integration of human genetic findings and animal models. As the resolution and efficiency for identifying structural mutations or other types of rare mutations increases, and as disease phenotypes are better defined, more advanced mouse models will lead to a better understanding of this complex disease.
Acknowledgments
Work in the authors’ laboratory is supported by grants from the NIMH , McKnight Foundation, March of Dimes, Lieber Center for Schizophrenia Research and Simons Foundation. S.M. is partly supported by a NIMH T32 grant. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Arguello PA, Gogos JA. (2006). Modeling madness in mice: one piece at a time. Neuron 52, 179–196 [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Estivill X, Antonarakis SE. (2007). Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat Rev Genet. 8, 639–646 [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zöllner S. (2008). Psychiatric genetics: progress amid controversy. Nat Rev Genet. 9, 527–540 [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR (2000). Diagnostics and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. Arlington, VA: American Psychiatric Association [Google Scholar]
- Feuk L, Carson AR, Scherer SW. (2006). Structural variation in the human genome. Nat Rev Genet. 7, 85–97 [DOI] [PubMed] [Google Scholar]
- Freedman R. (2003). Schizophrenia. N Engl J Med. 349, 1738–1749 [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C. (2004). Detection of large-scale variation in the human genome. Nat Genet. 36, 949–951 [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium (2008). Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos J. (1997). A turning point in schizophrenia genetics. Neuron 19, 967–979 [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, et al. (1995). Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 92, 7612–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber WL, Hsieh P, Hirotsune S, Yuva-Paylor L, Sutherland HF, Chen A, Ruiz-Lozano P, Hoogstraten-Miller SL, Chien KR, Paylor R, et al. (1999). Deletion of 150 kb in the minimal DiGeorge/velocardiofacial syndrome critical region in mouse. Hum Mol Genet. 8, 2229–2237 [DOI] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, International Schizophrenia Consortium, Wellcome Trust Case Control Consortium. Craddock N, Owen MJ, et al. (2009). Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 18, 1497–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. (2006). Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci USA 103, 3693–3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Lupski JR. (2006). Genomic rearrangements and gene copy-number alterations as a cause of nervous system disorders. Neuron 52, 103–121 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. (2000). Catching up on schizophrenia: natural history and neurobiology. Neuron 28, 325–334 [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 353, 1209–1223 [DOI] [PubMed] [Google Scholar]
- Long JM, LaPorte P, Merscher S, Funke B, Saint-Jore B, Puech A, Kucherlapati R, Morrow BE, Skoultchi AI, Wynshaw-Boris A, et al. (2006). Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics 7, 247–257 [DOI] [PubMed] [Google Scholar]
- McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, et al. (2009). Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 41, 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. (2008). Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 11, 1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. (1999). High rates of schizophrenia in adults with velo-cardiofacial syndrome. Arch Gen Psych. 56, 940–945 [DOI] [PubMed] [Google Scholar]
- Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai J, Westphal KG, Olivier B, Sulzer D, Pavlidis P, et al. (2005). Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 8, 1586–1594 [DOI] [PubMed] [Google Scholar]
- Paylor R, McIlwain KL, McAninch R, Nellis A, Yuva-Paylor LA, Baldini A, Lindsay EA. (2001). Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet. 10, 2645–2650 [DOI] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi CH, Oghalai J, Curran S, et al. (2006). Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA 103, 7729–7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. (2009). Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 204, 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, et al. (1994). Psychotic illness in patients diagnosed with velo-cardiofacial syndrome and their relatives. J Nerv Ment Dis. 182, 476–478 [DOI] [PubMed] [Google Scholar]
- Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, et al. (2006). Global variation in copy number in the human genome. Nature 444, 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, et al. (2009). Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 18, 988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Månér S, Massa H, Walker M, Chi M, et al. (2004). Large-scale copy number polymorphism in the human genome. Science 305, 525–528 [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, et al. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460, 753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai W-S, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, et al. (2008). Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 40, 751–760 [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietiläinen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, et al. (2008). Large recurrent microdeletions associated with schizophrenia. Nature 455, 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors O, Mortensen PB, et al. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, et al. (2008). Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320, 539–543 [DOI] [PubMed] [Google Scholar]
- Webber MA, Marder SR. (2008). Better pharmacotherapy for schizophrenia; what does the future hold? Curr Psychiatry Rep. 10, 352–358 [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. (2008). Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 40, 880–885 [DOI] [PubMed] [Google Scholar]
- Xu B, Woodroffe A, Rodriguez-Murillo L, Roos JL, van Rensburg EJ, Abecasis GR, Gogos JA, Karayiorgou M. (2009). Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proc Natl Acad Sci USA 106, 16746–16751 [DOI] [PMC free article] [PubMed] [Google Scholar]