Abstract
Type I interferons (IFN-I) link innate to adaptive immunity in microbial infection, autoimmune disease, and tumor immunity. It is not known whether IFN-I have an equally central role in alloimmunity. Here we tested this possibility by studying skin allograft survival and donor-specific CD8+ T cell responses in mice that lack the IFN-I receptor (IFN-IR−/−). We found that IFN-IR−/−mice reject fully allogeneic wildtype skin grafts at the same rate as wildtype recipients. Similarly, allograft rejection was not delayed if IFN-IR−/− male skin was transplanted to syngeneic IFN-IR−/− female mice. Quantitation of the male (H-Y)-specific CD8+ T cell response in these mice revealed normal generation of donor-specific CD8+ effector T cells but four-fold reduction in CD8+ memory T cells. Memory CD8+ T cells generated in the absence of IFN-IR had normal phenotype and recall function, assessed by ex vivo cytokine production and the ability of IFN-IR−/− mice to mount second set rejection. Finally, these memory T cells were maintained at a constant number despite their inability to respond to IFN-1. Our findings indicate that IFN-I cytokines are not critical for acute allograft rejection or for the expansion and differentiation of donor-specific CD8+ T cells into long-lived, functional memory T cells.
Keywords: rodent, T cells, cytokines, cytokine receptors, transplantation
Introduction
Type 1 interferons (IFN-I) are a multi-member cytokine family with pleiotropic immune functions (1). Their in vivo role has been best characterized in the context of anti-viral immunity (2). Acute viral infection triggers rapid production of large amounts of IFN-I by many cell types, but most potently by plasmacytoid dendritic cells, via both TLR-dependent and independent pathogen sensing pathways. IFN-I released after infection are critical for host survival because they exert direct anti-viral activities, cause the death of virus-infected cells and stimulate both innate and adaptive immune responses (2). One of the more recently recognized effects of IFN-I on adaptive immunity is the enhancement of T cell clonal expansion and memory formation after viral infection (3, 4). Members of the IFN-I family share a ubiquitously expressed heterodimeric receptor, IFN-IR, which is responsible for the biological actions of these cytokines because mice that lack one of the receptor subunits (IFN-IR−/−) have severely impaired anti-viral immunity (5).
IFN-I functions are not restricted to anti-viral defenses but are also implicated in the pathogenesis of autoimmune diseases and generation of anti-tumor immunity (1). IFN-I serum levels are increased in patients with lupus and their magnitude correlates with disease exacerbation. Moreover, IFN-I present in the serum of these patients induce the differentiation of monocytes into antigen-presenting DC that could then sustain the autoimmune response (6). In experimental autoimmunity models, IFN-IR deficiency ameliorates lupus-like disease in the NZB and B6-lpr mice. These findings have led to the hypothesis that IFN-I cytokine family members contribute to the induction and exacerbation of lupus through their effects on both innate (dendritic) and adaptive (T and B) cells of the immune system. IFN-1, however, are protective in other autoimmune disorders such as multiple sclerosis, suggesting that these cytokines have immunoregulatory functions as well. In mouse tumor models, endogenous IFN-I are necessary for the rejection of immunogenic tumors and for preventing the outgrowth of carcinogen-induced neoplasms by enhancing the anti-tumor T cell response. Therefore, IFN-I are innate mediators that shape the adaptive immune response in infection, autoimmunity and tumor immunity.
Several innate mediators are induced shortly after transplanting a solid organ allograft into mice or humans (7). These include cytokines such as IFN-I, IL-1, IL-6, IL-18, and TNFα (8, 9), but it is not known which of these cytokines is critical for ensuring that an optimal adaptive alloimmune response is induced. We hypothesized here that the IFN-I cytokine family could play such a role. This hypothesis is based on several published findings. First, acute renal allograft rejection has been reported in patients receiving IFN-α for the treatment of viral hepatitis (10). Second, maneuvers that abrogate or break transplantation tolerance in mice, for example the injection of TLR agonists, appear to do so via an IFN-IR-dependent pathway (11). Third, allograft rejection is delayed in certain experimental models if antibodies to IFN-I or IFN-IR are administered concomitant with cyclosporin A (12, 13). Despite the supportive evidence provided by these studies, direct assessment of whether and how IFN-I link innate to adaptive immunity in the setting of solid organ transplantation is lacking. In this manuscript, we studied the role of IFN-I in the alloimmune response by performing skin transplantation between wildtype (wt) mice or mice that lack the IFN-IR and analyzing the antigen (donor)-specific CD8+ T cell response. We report that, contrary to our initial hypothesis, IFN-I action is not required for acute allograft rejection or for clonal expansion of donor-specific CD8+ effector T cells and their differentiation into long-lived, functional memory T cells.
Materials and Methods
Mice
129S6/SvEvTac wt mice were purchased from Taconic Farms (Watertown, NY) and BALB/c mice from Jackson Laboratories (Bar Harbor, ME). IFN-IR-deficient mice (IFN-IR−/− 129S6) were a kind gift of Akiko Iwasaki (Yale University, New Haven, CT) (5). All animals were bred and maintained under SPF conditions and used according to IACUC guidelines.
Surgical procedures
Partial thickness skin transplantation was performed using abdominal skin from BALB/c, male wt 129S6, or male IFN-IR−/− 129S6 donor mice. Subcutaneous layers of donor skin were removed by blunt scraping. A 1 cm2 recipient graft bed was dissected leaving an intact panniculus carnosus with uncompromised vasculature. Donor skin was secured to bed with skin staples and bandages for seven days. Grafts were monitored daily afterwards. Rejection was defined as > 90% graft necrosis. Second set rejection was studied by applying a second skin graft to the same recipient > 4 weeks after first graft was rejected.
Cell harvest
Skin-grafted mice were sacrificed 1, 3, 6, 9, 12, 15, 18, 21, 26, 35, 42, or 60 days after transplantation. Spleen and lymph nodes (mesenteric, iliac, and axillary) were crushed in PBS followed by hypotonic lysis of RBCs. Cells were then washed and resuspended in PBS for staining and analysis.
Flow cytometry and intracellular cytokine staining
Fluorochrome-tagged antibodies against CD4 (RM4-5), CD8α (53-6.7), CD44 (KM201), CD62L (MEL-14), CD127 (A7R34), IFNγ (XMG1.2), and TNFα (MP6-XT22) were purchased from BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA), or SouthernBiotech (Birmingham, AL). Soluble, fluorochrome-tagged MHC class I H-2Db/peptide multimers using the HY peptide Uty (WMHHNMDLI) (14) were a kind gift of the NIAID Tetramer Facility (Atlanta, GA). Tetramer staining was carried out according to NIAID recommendations. Briefly, lymphocytes were stained with anti-CD44-FITC and anti-CD8-PerCP for 10 min at 4 °C followed by staining with tetramers for 30 min at 4 °C. To measure intracellular cytokines (IFNγ and TNFα), lymphocytes were stimulated ex vivo with male splenocytes (1:1) for 5 hrs in the presence of Brefeldin A or Monensin. Cells were washed, stained for surface markers, fixed, permeabilized with 0.25% saponin and incubated with anti-IFNγ or anti-TNFα antibody for 1hr at RT. Flow acquisition was performed on LSRII analyzers (BD Biosciences, San Diego, CA), and data analyzed using Flowjo software (Treestar Corp., Ashland, OR). Absolute CD8+Uty+ cell numbers were determined by counting live cells using a hemocytometer and trypan blue exclusion. Absolute number of CD8+Uty+ cells per tissue (spleen or lymph node) was calculated by multiplying the percentage of CD8+Uty+ cells in the lymphocyte gate by total number of cells counted per tissue. Spleen and lymph nodes were of similar size between groups at any given time point as wt and IFN-IR−/− mice were age-matched.
Statistical Methods
Statistical significance in survival experiments was calculated using the log-rank test (Graphpad Prism v5.0 software). Significance was set at p < 0.05.
Results
Acute allograft rejection is not dependent on IFN-I function
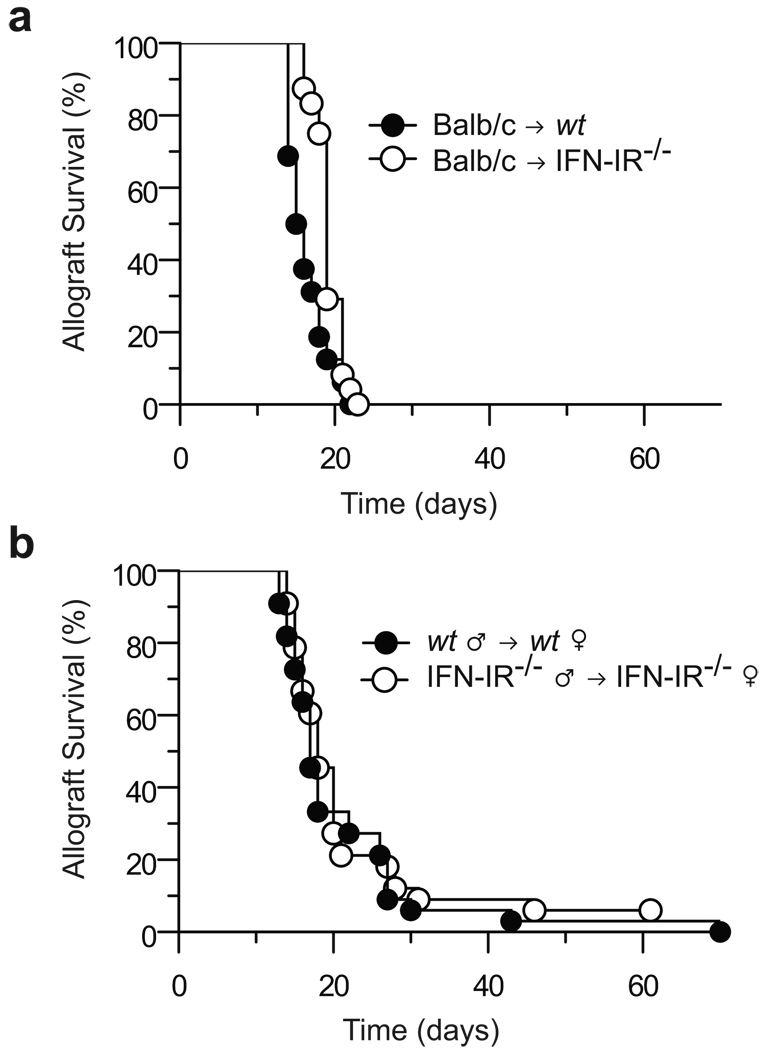
To investigate the role of host IFN-I in acute allograft rejection, we transplanted wt BALB/c (H-2d) skin grafts onto wt or IFN-IR−/− 129S6 (H-2b) mice. IFN-IR−/− mice lack one of the two subunits of the IFN-IR on all cell types and are unresponsive to IFN-I cytokines (5). As shown in Fig. 1a, acute allograft rejection was not significantly delayed in the absence of host IFN-IR (median allograft survival = 18 days vs. 15.5 days in the wt group), indicating that IFN-I function in the recipient is not required for acute rejection of MHC-mismatched grafts.
Figure 1. IFN-I are not required for acute skin allograft rejection.
(a) Skin was harvested from BALB/c (H-2d) mice and grafted onto wt (n = 16) or IFN-1R−/− (n = 24) 129S6 (H-2b) mice. Both recipient groups rejected skin grafts with similar kinetics (MST = 15.5 and 18 days, respectively). Control syngeneic skin grafts were not rejected (data not shown). (b) Skin was harvested from wt or IFN-IR−/− 129S6 male mice and grafted onto wt (n = 33) or IFNI-R−/− 129S6 female mice (n = 33), respectively. Both recipient groups rejected skin grafts with identical kinetics (MST = 17 days).
Since alloimmunity can be initiated not only by recipient APC but also by donor APC that migrate from the graft, we then asked whether allograft rejection would still proceed at a normal rate if IFN-IR was lacking on both donor and recipient cells. To answer this question, we transplanted minor antigen (H-Y)-mismatched skin grafts from IFN-IR−/− male to IFN-IR−/− female 129S6 mice. As shown in Fig. 1b, allograft rejection occurred at the same rate as that observed when skin was transplanted from wt male to wt female 129S6 mice (median survival = 17 days in both groups). Therefore, even in a minor antigen-mismatched transplantation model, complete unresponsiveness to IFN-I in both donor and recipient cells does not delay acute rejection.
Diminished donor-specific CD8+ memory T cell generation in the absence of IFN-I function
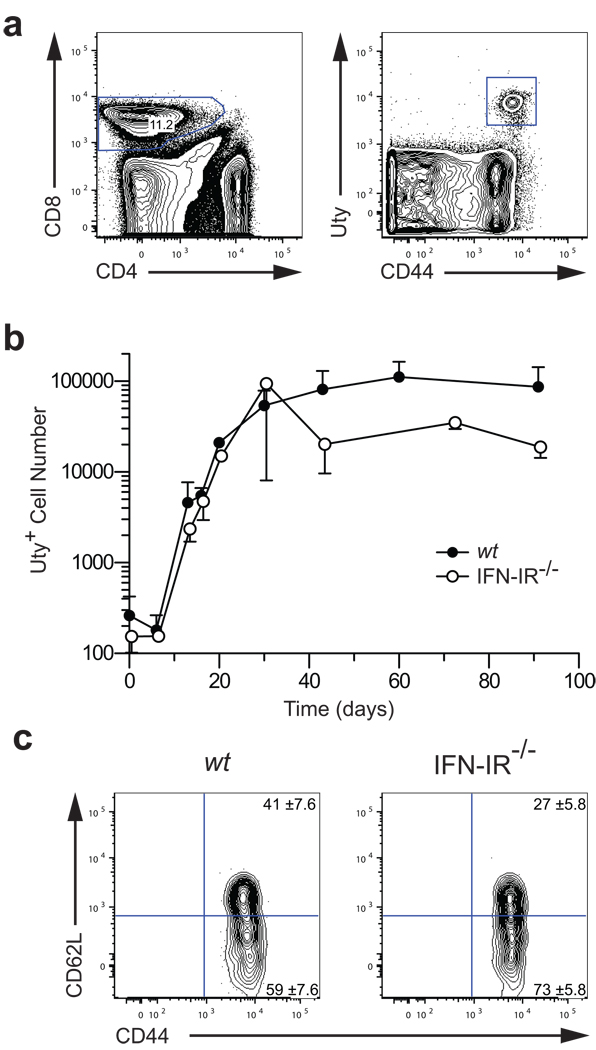
In the absence of IFN-IR, virus-specific T cells expand less and form a diminished memory pool compared to their wt counterparts (3, 4). We therefore investigated whether the same applies to alloimmunity by quantitating H-Y-specific CD8+ T cells in both wt and IFN-IR−/− female recipients of syngeneic male (H-Y-mismatched) skin grafts. H-Y-specific CD8+ T cells were identified in recipient spleen and lymph nodes at multiple time points after transplantation by staining with the MHC-tetramer H-2Db/Uty (Fig. 2a). In the wt group, H-Y-specific CD8+ T cells (CD8+Uty+) increased from < 1,000/mouse at the time of transplantation to approximately 100,000/mouse during the effector phase (1 – 30 days after transplantation) and were maintained at the same level during the memory phase of the response (40 – 90 days after transplantation) (Fig. 2b). The presence of a very small number of tetramer-positive cells on day 0 in Fig. 2b could represent either low frequency of antigen-specific CD8+ T cells in naïve mice or low background noise inherent to flow cytometric methods (14). Although similar expansion of CD8+Uty+ T cells was observed in the IFN-IR−/− group, their number declined by approximately four-fold by day 40 and remained constant thereafter. Similar results were obtained when spleen and lymph nodes were analyzed independently. Phenotypic analysis demonstrated that the CD8+Uty+ population present during the memory phase in either wt or IFN-IR−/− mice contained both central (CD44hiCD62Lhigh) and effector (CD44hiCD62Llow) memory T cells in comparable proportions (Fig. 2c). Although data shown in Fig. 2c represent analysis of the spleen, no differences between wt and IFN-1R−/− groups were observed when lymph nodes were analyzed except for the presence of a higher percentage of central memory CD8+ T cells than in the spleen. These data indicate that, unlike anti-viral immune responses, IFN-I is not required for CD8+ T cell expansion after skin transplantation but is necessary for the generation of maximum number of CD8+ memory T cells. Once generated, these memory T cells are maintained normally in the absence of IFN-I function.
Figure 2. Generation of donor-specific CD8+ memory T cells in the absence of IFN-I function.
Skin grafts were transplanted from wt or IFN-IR−/− male 129S6 mice onto wt or IFN-IR−/− female 129S6 mice, respectively. Recipient spleen and lymph nodes were harvested at multiple time points and analyzed for H-Y-specific CD8+ T cells by staining with the Uty tetramer. (a) H-Y-specific T cells (CD44hiUty+) were identified after gating on CD8+ cells. Plot shown is from an IFN-IR−/− recipient spleen at 90 days after transplantation. (b) CD8+CD44hiUty+ T cells were quantitated in recipient spleen and lymph nodes at the indicated time points (n = 2 – 6 mice/time point). H-Y-specific CD8+ T cells expanded similarly in both wt and IFN-IR−/− recipients (~100 fold), but their number declined four-fold after day 40 in the IFN-IR−/− group. No difference in the memory maintenance phase was observed between the two groups. (c) wt and IFN-IR−/− H-Y-specific CD8+ memory T cells displayed both central and effector memory phenotypes. Plot shown is from recipient spleens at 90 days after transplantation. Values shown in gates are percentages ± SD (n = 5, p > 0.05).
IFN-I function is not required for CD8+ memory T cell recall or second set rejection
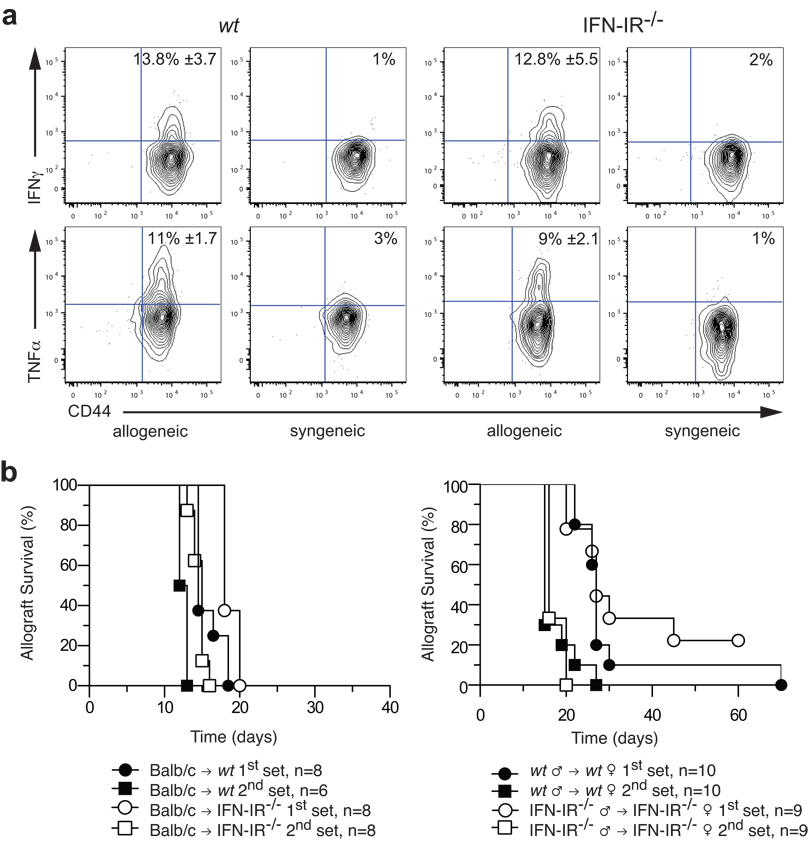
Next we investigated whether allospecific CD8+ memory T cells generated in the absence of IFN-I may have diminished function. Spleen or lymph node cells were harvested from female wt or IFN-IR−/− mice between 50 and 60 days after syngeneic male (H-Y mismatched) skin transplantation and restimulated ex vivo with donor splenocytes for five hours. As shown in Fig. 3a, donor-specific (CD8+Uty+) T cells from either wt or IFN-IR−/− mice rapidly produced IFNγ and TNFα upon ex vivo restimulation. No significant difference in the proportion of cells producing these cytokines was observed between wt and IFN-IR−/− mice, indicating that CD8+ memory T cells generated in the absence of IFN-I function have normal recall capacity as measured by cytokine production.
Figure 3. CD8+ memory T cell recall in the absence of IFN-I function.
(a) Spleen cells were harvested 90 days after transplanting wt or IFN-IR−/− 129S6 male skin onto wt or IFN-IR−/− 129S6 female recipients, respectively, and re-stimulated ex vivo with either male (allogeneic) or female (syngeneic) 129S6 splenocytes for 5 hrs to measure memory recall function. IFNγ and TNFα production were assessed by intracellular staining after gating on CD8+Uty+ cells. Values shown in allogeneic gates are percentages ± SD (n = 4 – 5, p > 0.05). (b) Second set rejection was measured > 4 weeks after first set rejection in either fully allogeneic (left panel, n = 6 – 8 mice/group) or H-Y mismatched (right panel, n = 9 – 10 mice/group) donor-recipient pairs. Accelerated second set rejection was observed in both wt and IFN-IR−/− groups.
A stringent measure of memory T cell function is their ability to confer in vivo protection. We therefore tested whether IFN-IR−/− mice, that had rejected a skin graft at least four weeks earlier, were capable of second set rejection; that is, the rapid rejection of a second graft from the same donor. We found that these mice indeed mount an accelerated second set rejection response that is not significantly different from that observed in wt recipients (Fig. 3b). This is true for both fully allogeneic Balb/c and H-Y mismatched transplantation. Prior sensitization with H-Y skin allograft reduced second set graft survival by 11 days in the IFN-1R−/− group and 12 days in the wt group (Fig. 3b). These data confirm that, despite a reduction in the number of donor-specific CD8+ memory T cells, in vivo memory alloresponses remain intact in the absence of both donor and recipient IFN-I function.
Discussion
We investigated in this study whether IFN-I cytokines produced during the innate phase of the immune response to a transplanted organ are critical for the induction of adaptive alloimmunity. We found that absence of IFN-I function in either the recipient alone or in both donor and recipient does not alter the tempo of acute skin allograft rejection, even in a minor histo-incompatibility transplantation model. Analysis of the recipient’s response revealed that IFN-I function is not necessary for the expansion of donor-specific CD8+ T cells and their differentiation into memory cells. CD8+ memory T cells generated in the absence of IFN-I function were maintained long-term and exhibited normal phenotype and recall responses. Therefore, unlike their central role in viral immunity, the IFN-I family of cytokines has subtle but non-critical effects on the generation adaptive alloimmunity.
Divergence in the requirement for IFN-I between viral and transplantation-induced immunity could be the result of differences in the amount of IFN-I produced. Viruses are known potent inducers of IFN-I production, largely via their actions on plasmacytoid dendritic cells (2), and immune responses to viruses with lower capacity to induce IFN-I tend to be less dependent on these cytokines (15). It is quite likely that organ transplantation, in which myeloid rather than plasmacytoid dendritic cells are the principal stimulatory APC population, is a less potent inducer of IFN-I production. Moreover, the large number of cytokine species produced at the time of organ transplantation may render IFN-I functions redundant or dispensable (8). Other cytokines that could influence anti-graft immunity include IL-6 and IL-12. IL-6 produced by the graft and/or host after transplantation enhances adaptive immunity by suppressing the function of regulatory T cells (16). IL-12, on the other hand, provides a direct stimulatory signal (“third signal”) to CD8+ T cells to promote their expansion and differentiation into effector and memory cells (17). IL-12 provides this function either alone or in synergy with IFN-1, depending on the nature of the inciting antigen. For example, generation of T cell memory to vaccinia virus is predominantly supported by IL-12 while both IL-12 and IFN-I contribute to effector and memory T cell formation after Listeria infection (4, 18). In organ transplantation, IL-12 promotes CD8+ alloresponses but inhibits those mediated by CD4+ T cells (19). The effect of combined blockade of IFN-I and IL-12 signaling on graft rejection and the alloimmune response remains to be studied.
The role of IFN-I cytokines in acute allograft rejection may depend on the type of organ transplanted. It is plausible that IFN-I are redundant in the setting of excess inflammation (for example, skin transplantation) but are essential if less inflammation is present (for example, heart transplantation). This possibility is supported by data showing that IFN-1 blockade enhances cardiac allograft survival when combined with low dose cyclosporine in rodents (12). In addition, the extent to which IFN-1 cytokines contribute to T cell expansion and allograft rejection may depend on the degree of mismatching or strain combination used. Although we did not directly test this hypothesis, we did not observe prolongation of either MHC- or H-Y-mismatched allograft survival in recipients that lack IFN-IR expression.
IFN-I may increase the formation of CD8+ memory after a primary immune response either by early programming of activated lymphocytes or by enhancing their clonal expansion and survival. Evidence for each of these mechanisms has come forth from studies of anti-microbial immunity (3, 18). The mechanism by which IFN-IR deficiency led to a four-fold decline in the number of donor-specific CD8+ memory T cells in our transplantation model is unclear. One possibility is increased apoptosis of activated T cells, as suggested by the exaggerated contraction of the immune response between days 30 and 40 in the IFN-IR−/− but not wt group (Fig. 2a). Of note, the maintenance of CD8+ memory T cells beyond day 40 remained constant, independent of the presence or absence of IFN-I function.
In addition to CD8+ T cells, other lymphocyte subsets, particularly CD4+ T cells, contribute to skin allograft rejection. Here we restricted our analysis to the CD8+ T cell compartment because of the availability of MHC class I tetramers that accurately and reliably detect antigen-specific CD8+ T cells. Other studies have shown that direct action of IFN-I on CD4+ T cells is critical for sustaining clonal T cell expansion in response to viral but not bacterial infection in mice (20). IFN-I also exerts direct actions on NK cells and B cells, especially in the setting of acute viral infection (2).
Our inability to demonstrate a critical function for IFN-I in the primary or secondary immune response to a transplanted organ does not imply that this cytokine family bears no significance to experimental or clinical organ transplantation. IFN-I plays a critical role in ischemia-reperfusion injury that commonly occurs at the time of organ transplantation (9), and endogenous IFN-I (produced during viral infection) or exogenously administered IFN-I may precipitate allograft rejection in clinically stable transplant recipients or in mice in whom transplantation tolerance was presumed to be achieved (10, 11). Moreover, combining IFN-I blockade with conventional immunosuppression has been shown to prolong allograft survival in experimental animals (12, 13). Whether this strategy also prevents chronic rejection and is safe and effective in humans remains to be determined.
Footnotes
This work was supported by NIH grants AI049466 to FL and AI064343 to DR, WS, & FL. MO was supported by an international fellowship from the American Society of Transplantation.
Disclosures
The authors have no financial conflict of interest to disclose.
References
- 1.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312(5775):879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 3.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182(5):2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type 1 and type II interferons in antiviral defense. Science. 1994;(264) doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 6.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 7.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J Immunol. 2007;178(12):7503–7509. doi: 10.4049/jimmunol.178.12.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christopher K, Mueller T, Ma C, Liang Y, Perkins D. Analysis of innate and adaptive phases of allograft rejection by cluster analysis of transcriptional profiles. J Immunol. 2002;169:522–530. doi: 10.4049/jimmunol.169.1.522. [DOI] [PubMed] [Google Scholar]
- 9.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47(1):199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 10.Magnone M, Holley JL, Shapiro R, Scantlebury V, McCauley J, Jordan M, et al. Interferon-alpha-induced acute renal allograft rejection. Transplantation. 1995;59(7):1068–1070. doi: 10.1097/00007890-199504150-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, Markees TG, Bahl K, Brehm MA, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179(10):6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gugenheim J, Tovey M, Gigou M, Crafa F, Fabiani B, Reynes M, et al. Prolongation of heart allograft survival in rats by interferon-specific antibodies and low dose cyclosporin A. Transpl Int. 1992 5 Suppl 1:S460–S461. doi: 10.1007/978-3-642-77423-2_134. [DOI] [PubMed] [Google Scholar]
- 13.Benizri E, Gugenheim J, Lasfar A, Eid P, Blanchard B, Lallemand C, et al. Prolonged allograft survival in cynomolgus monkeys treated with a monoclonal antibody to the human type I interferon receptor and low doses of cyclosporine. J Interferon Cytokine Res. 1998;18(4):273–284. doi: 10.1089/jir.1998.18.273. [DOI] [PubMed] [Google Scholar]
- 14.Millrain M, Chandler P, Dazzi F, Scott D, Simpson E, Dyson PJ. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001;167(7):3756–3764. doi: 10.4049/jimmunol.167.7.3756. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177(3):1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007;84(6):771–777. doi: 10.1097/01.tp.0000281384.24333.0b. [DOI] [PubMed] [Google Scholar]
- 17.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 18.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178(7):4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, et al. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160(3):1132–1138. [PubMed] [Google Scholar]
- 20.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176(6):3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]