Abstract
Background
The TF (Thomson – Friedenreich) blood group antigen behaves as an onco-foetal carcinoma-associated antigen, showing increased expression in malignancies and its detection and quantification can be used in serologic diagnosis mainly in adenocarcinomas. This study was undertaken to analyze the sera and tissue level detectable mucin-type glycoprotein (TF-antigen) by Peanut agglutinin (PNA) and its diagnostic index in serum as well tissues of human esophageal squamous cell carcinoma as marker.
Results
We examined 100 patients for serological analysis by Enzyme Linked Lectin Assay (ELISA) and demonstrated a sensitivity of 87.5%, specificity of 90% and a positive predictive value of 95%. The immuno-histochemical localization of TF antigen by Fluorescence Antigen Technique (FAT) in 25 specimens of normal esophageal squamous epithelium specimens and 92 specimens with different grades of, allowed a quicker and more precise identification of its increased expression and this did not correlate with gender and tumor size. There was a positive correlation between membrane bound TF antigen expression with different histological progression, from well differentiated to poorly differentiated, determined by PNA binding. Specimens showed morphological changes and a pronounced increase in PNA binding in Golgi apparatus, secretory granules of the cytosol of well differentiated and an increased cell membrane labeling in moderately and poorly differentiated, when compared with ESCC and normal tissues.
Conclusion
The authors propose that the expression of TF-antigen in human may play an important role during tumorigenesis establishing it as a chemically well-defined carcinoma-associated antigen. Identification of the circulating TF-antigen as a reactive form and as a cryptic form in the healthy individuals, using PNA-ELLA and Immunohistochemical analysis of TF antigen by FAT is positively correlated with the different histological grades as a simple and cost-effective method for the early diagnosis of ESCC. The present study reveals that, during tumorigenesis, an aberrant glycosylation takes place in Golgi apparatus leading to over secretion of TF antigen into the cytoplasm along with mucin granules and later into cell membrane. We suggest that the further characterization of TF antigen may unravel pathogenetic aspects of this silent disease.
Keywords: Diagnosis, Thomsen-Friedenreich Antigen, Esophageal Squamous Cell Carcinoma, Peanut Agglutinin.
Background
Synthesis and secretion of mucin are common features of glandular epithelial tissues and the expression of mucin antigens has been investigated mainly in adenocarcinomas [1]. Under certain pathological conditions, particularly during the course of carcinogenesis, their biosynthesis is altered with regard to the rate of production and the degree of glycosylation, also in squamous cell carcinomas [2,3]. Oncogenic transformation is often associated with changes in glycosylation in either glycolipids or glycoproteins in cell membranes. This leads to the incomplete glycosylation of the core carbohydrate structures resulting in the formation of T, Tn and sialyl Tn antigens in a variety of cancers [2,4]. Among them, TF-antigen was found to be a pan-carcinoma marker, i.e., it is expressed by a variety of cancers derived from different tissues [3] and it has been targeted recently for the development of tumor selective vaccines [5].
The circulating levels and cell membrane localization of TF-antigen involves the binding of lectin PNA, a non-toxic TF-antigen binding lectin [6,7], which can covalently bind to the Galβ1–3 GalNAc residue of the TF-antigen. Among various lectins, peanut agglutinin (PNA), is the most widely used to identify new diagnostic and prognostic markers in various squamous cell carcinomas [2,3,8].
Esophageal cancer is the sixth most frequent cause of cancer death worldwide. Invasive esophageal cancer is a multistage progressive process, which involves the conversion of normal epithelium to basal cell hyperplasia (BCH), dysplasia (DYS) or carcinoma in situ (CIS), and then to invasive squamous cell carcinoma. In all these steps there is an interaction between the tumor cell surface and neighboring cells [9], which may be important in the very early stage of the metastatic process of ESCC. Recently, more attention has been paid towards the TF-antigen expression in carcinomas [4,8,10]. The aim of the present study was to determine the TF antigen levels and to define the relationship between circulating levels of TF-antigen with different histological grades of ESCC by using the Galβ1–3 GalNAc-specific lectin, PNA [2,10,11].
Results and Discussion
Blood TF-antigen of patients and its relation to the histological grades
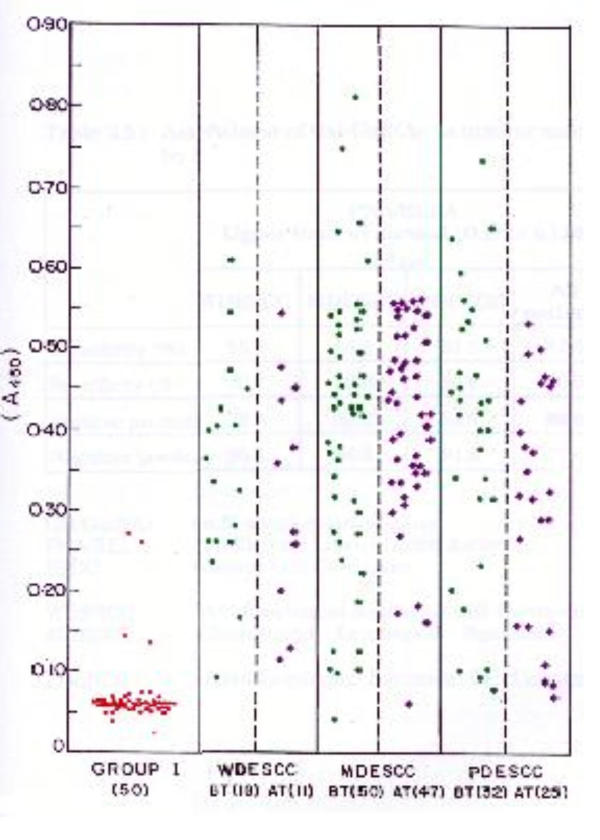
A total of 100 patients consisting of 64 males and 36 females were taken in this study. Their age varied from 31 to 84 years with a mean age of 52 years. Of the hundred patients (ESCC), 18 were classified as well differentiated (WD), 50 as moderately differentiated (MD) and 32 as poorly differentiated (PD) esophageal squamous cell carcinoma (group II). The results confirm the expression of TF-antigen irrespective of the histological differentiation. The level of expression was observed from OD 0.218 to 0.571. Circulating PNA-binding mucin levels were increased in all histological grades of the patients (Table 1 and Figure 1). When OD of 0.159 (Mean OD in normal + 2 standard deviation) was taken as the normal upper limit, PNA/ELLA had a sensitivity of about 87.5% for ESCC patients (Table 2). There was no significant reduction of TF-antigen in group III patients when compared with the group II patients, who received therapy.
Table 1.
Serum Gal/GalNAc estimation by PNA/ELLA in ESCC (Upper limit of normal = 0.159 A450)
| Parameters | Group | No. Patients | Mean O.D ± S.D. |
| Gal/GalNAc | I Normal | 50 | 0.071 ± 0.044 |
| II WDESCC | 18 | 0.349 ± 0.131* | |
| MDESCC | 50 | 0.402 ± 0.169* | |
| PDESCC | 32 | 0.392 ± 0.171* | |
| III WDESCC | 11 | 0.319 ± 0.142* | |
| MDESCC | 47 | 0.411 ± 0.119* | |
| PDESCC | 25 | 0.319 ± 0.149* | |
Gal/GalNAc – Galactose β-1, 3-α, N-acetyl galactosamine PNA/ELLA – Peanut Agglutinin/Enzyme Linked Lectin Assay ESCC – Esophageal Squamous Cell Carcinoma OD – Optical Density Group I – Healthy volunteers Group II – Before therapy WDESCC – Well Differentiated Esophageal Squamous Cell Carcinoma MDESCC – Moderately Differentiated Esophageal Squamous Cell Carcinoma PDESCC – Poorly Differentiated Esophageal Squamous Cell Carcinoma Group III – After therapy * (P < 0.05) Significant of group II and III against group I
Figure 1.
PNA/ELLA levels in ESCC and normal. 0.187 (A405) is chosen as the upper limit of normal (Normal (red), Before treatment (green), after treatment (violet)). The number of patients is given in parenthesis.
Table 2.
Assessment of usefulness of Gal-GalNAc as tumour marker by PNA/ELLA
| PNA/ELLA Upper limit of normal (O.D) = 0.159 (A450) | ||||
| WDESCC | MDESCC | PDESCC | All patients | |
| Sensitivity (%) | 88.9 | 86.0 | 87.5 | 87.5 |
| Specificity (%) | 90.0 | 90.0 | 90.0 | 90.0 |
| Positive predictive value (%) | 76.2 | 89.6 | 84.9 | 94.6 |
| Negative predictive value (%) | 95.8 | 86.6 | 91.8 | 77.6 |
This study is the first to show the presence of PNA-binding mucin-type glycoprotein in ESCC sera. The infiltrating growth, the mass or the tumor volume and the degree of histological differentiation of tumor may influence serum value of TF-antigen in this study. This suggests that well differentiated ESCC does not contain more circulating TF-antigen. The present results of circulating TF-antigen levels in different histological grades of ESCC patients are similar in terms of intense cytoplasmic and membrane associated TF-antigen expression in in situ carcinomas of colon and ovary. Accordingly, Lin et al [15] and Lalwani et al [16] observed a gradual loss of blood group antigens A, B and H in many of the squamous cell carcinomas of the larynx and this gradual loss parallels morphologic transformations from normal squamous mucosa to various stages of intra-epithelial interactions and finally to overt carcinoma [15]. Shamsudhin et al [17] also reported that malignant transformation of the colon may be accompanied by alterations in carbohydrate production as observed in the present study.
This suggests that in normal cells the TF antigen is cryptic, whereas in malignant tumors, deficient glycosyltransferase activity, may lead to their exposure and during further advancement of the cancer, the O-linked carbohydrates may be drastically altered on the cell membrane [18]. The present result suggests that malignant transformation in ESCC was accompanied by the increased addition of galactose to the α-N-acetyl galactosamine O-linked carbohydrate core on the cell membrane.
Lectin Histochemical Analysis for Membrane bound TF Antigen Expression
The TF antigen was expressed in all patients irrespective of the stage of the development. The degree of expression, classified as mild, moderate and intense, correlated with different histological grades of ESCC. Of the total 92 patients the expression of TF antigen was mild in 21 patients (about 23%), moderate in 32 patients (about 35%) and intense in 39 patients (about 42%) (Table 3). Of the 21 patients who showed mild expression, 10 were graded as WD, 8 were graded as PD and the remaining three were graded as MD. Though, one expects mild antigen expression in WD patients who were in MD and PD also showed mild expression (about 52%). Similarly, one expects moderate and intense expression being associated with MD and PD patients. Even in this instance, 13 patients, who were in WD, showed moderate to intense TF antigen expression.
Table 3.
Fluorescent Antibody Technique (FAT) showing TF-antigen expression in ESCC
| Histological Grade | N | Mild + n (%) | Moderate ++ n (%) | Intense +++ n (%) |
| WDESCC | 23 | 10(43.48) | 11(47.83) | 2(8.70) |
| MDESCC | 25 | 3(12.0) | 9(36.0) | 13(52.0) |
| PDESCC | 44 | 8(18.18) | 12(27.27) | 24(54.55) |
| Total | 92 | 21(22.82) | 32(34.78) | 39(42.39) |
WD: Well differentiated, MD: Moderately differentiated, PD: Poorly differentiated ESCC and N: Number of patients.
Of the total 71 patients whose TF antigen expression was moderate and intense in 13 patients were histologically classified as WD. This works out to about 18% of the patients with moderate and intense expression. These patients constitute about 57% of the WD patients. For example, about 43% of WD patients showed mild expression. In contrast, the proportion of the patients who showed intense expression between MD and PD were 52% and about 55%, respectively (Table 3). It appears that there is no linear dependence of TF antigen expression with the histological grades. In spite of these discrepancies, there is a good correspondence between different histological grades and TF antigen expression in the samples investigated.
In the lectin histochemical analysis for TF-antigen via PNA display many of the properties of an antibody in that they are capable of interacting with a restricted group of polysaccharides [19-21], and this method may become a routine procedure in laboratories concerned with identification and characterization of mucin type antigen. There are no specific and efficient antibodies to perform immuno histochemistry, based on the protein backbone of the mucins. The results of the fluorescent antibody analysis on tissues of ESCC patients suggest that PNA may be used for TF antigen detection, because quantification of gene products by immunohistochemistry and other immunologic means using antibodies is often difficult, owing to the heavy glycosylation of the mucoprotein which precludes effective binding of antibodies [22]. In addition, PNA binding to TF antigen indicates the presence of the disaccharide Gal 1,3-GalNAc. The lectin-binding characteristic of this marker and its high molecular weight suggests that it is a mucin-type glycoprotein.
Ultrastuctural Modifications During TF-antigen formation
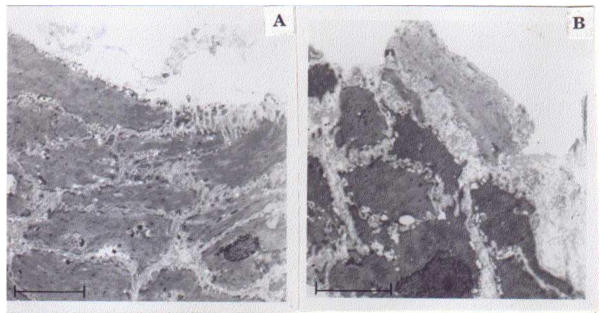
Squamous cells lined the morphology of the normal esophageal mucosa and intercellular bridges could be observed (Figure 2A), whereas in ESCC, the cells were necrotic with serrated cell membrane and enlarged nuclei. The margins of the nuclei were irregular and prominent (Figure 2B).
Figure 2.
Morphological alterations of normal and carcinoma cells of esophagus. A. Electron micrograph showing normal mucosal squamous cells with centrally placed oval nucleus (Uranyl acetate and lead citrate staining. Bar scale = 5 μm). B. Electron micrograph of WD showing neoplastic squamous cells with large irregular nucleus and serrated cell borders (Uranyl acetate and lead citrate staining. Bar scale = 5 μm).
Intracellular Expression
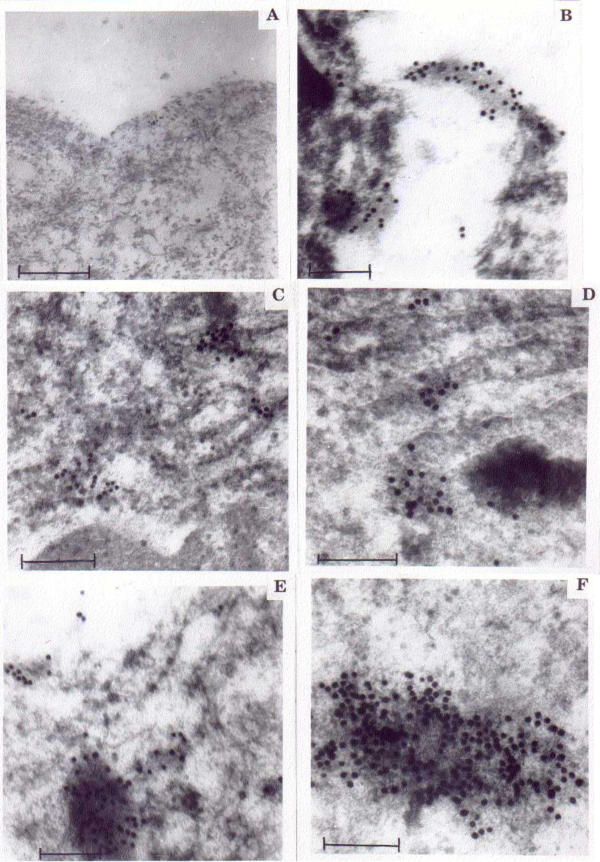
On examination of ESCC for TF antigen expression by immunogold labeling, a pronounced difference in the cell surface labeling was observed when compared to normal squamous epithelium (Figure 3A &3B). Densely labeled PNA-embedded mucin granules were observed in the cytosol of WD (Figure 3C,3D,3E &3F), whereas in moderate and poorly differentiated, abundant labeling was observed in the cell membrane.
Figure 3.
Altered expression of TF antigen in carcinoma cells. A Normal esophagus: Electron micrograph showing lesser expression of TF antigen along the cell membrane (Uranyl acetate and lead citrate staining. Bar scale = 250 nm). B. WD: Electron micrograph showing increased TF antigen expression embedded in the mucinous secretion (Uranyl acetate and lead citrate staining 200 nm). C. WD: Electron micrograph showing cytosol of neoplastic cells with scattered TF antigen expression (Uranyl acetate and lead citrate staining. Bar scale = 250 nm). D. WD:Electron micrograph showing TF antigen positive granules in the near vicinity of mucin in the cytosol (Uranyl acetate and lead citrate staining. Bar scale = 500 nm). E. WD: Electron micrograph showing TF antigen positive granules embedded in the mucin (Uranyl acetate and lead citrate staining. Bar scale = 200 nm). F. WD: Electron micrograph showing TF antigen positive granules abundantly embedded in the mucin (Uranyl acetate and lead citrate staining. Bar scale = 250 nm).
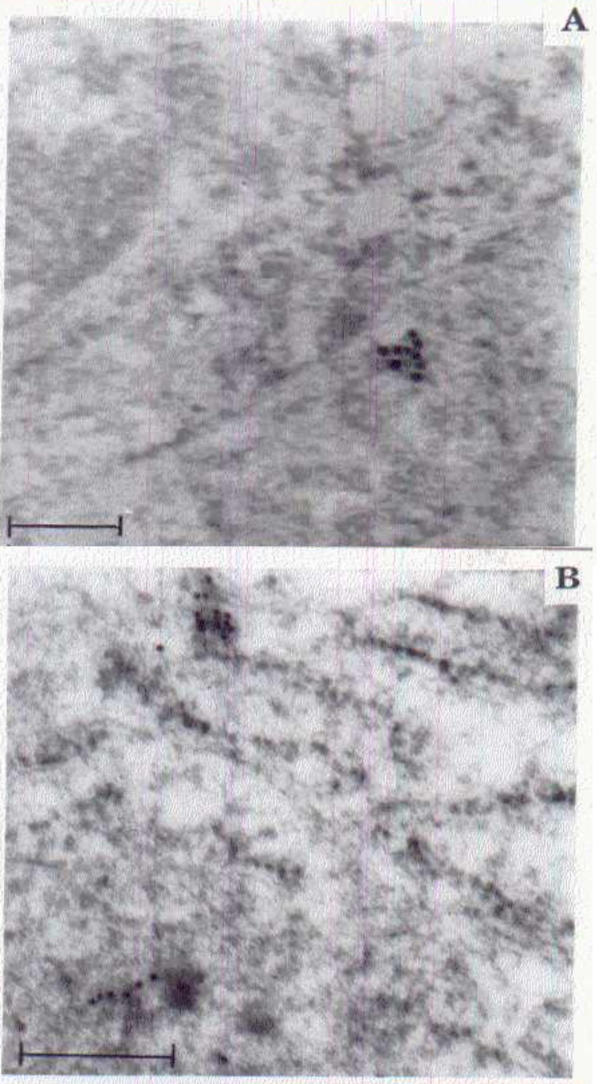
Immunogold labeling was higher in early differentiation of cancer development (WD) and was usually detected over the trans-cisternae trans-Golgi network and vacuoles, in addition to lower labeling in the plasma membrane of WD (Figure 3C, 3D &3E). Whereas, higher intensity of PNA labeling was observed in the plasma membrane of moderately and poorly differentiated (Figure 3B) suggesting that the intensity of PNA labeling from cytosol to plasma membrane increased with disease progression. In normal tissue sections the PNA labeling was very low, when compared to other grades of (Figure 3A &3B). Increased expression of TF antigen (Gal β1–3 GalNac α-) in cytosol of WD (Figure 3C) and on cell membrane of MD and PD (Figure 4) was observed when compared with normal squamous epithelium (Figure 2A &4B).
Figure 4.
Formation of altered TF antigen in ESCC Golgi apparatus. A Electron micrograph of normal squamous cell showing weak TF antigen expression (Uranyl acetate and lead citrate staining. Bar scale = 200 nm). B. Electron micrograph of neoplastic squamous cell of the cisternae of Golgi apparatus showing abundant gold labeling for increased TF antigen (Uranyl acetate and lead citrate staining. Bar scale = 500 nm).
In the present study the ultra structural localization of the TF antigen was observed to be more in WD mostly confined to the golgicisternae, cytosol and secretory mucin granules. In MD and PD the localization was found to be less in the cytosol than in WD whereas it was present mostly in the cell membrane. However, slightly more immune reactivity of TF antigen with PNA was observed in the intercellular spaces of poorly differentiated ESCC. It has recently been shown that the use of PNA against tumor-associated antigens in several cancer patients produced consistent results in the cancer of breast [4], ovary [23,24] and colon [8,19,25]. The synthesis and expression of TF antigen in ESCC patients was not reduced even after therapy. Aberrant glycosylation involves incomplete synthesis resulting in deletion of normally expressed antigens, with or without, exposure of core sugar and peptide structures. Inappropriate expression of antigenic structures, which are not normally present, are O-glycosidically linked through GalNAc to Thr/Ser. These carbohydrate side chains may be divided into core, backbone and peripheral regions. All three regions provide a specific recognition site for antibodies and lectins. Such aberrations in cell surface glycoproteins, especially the carbohydrates are associated frequently with altered adhesion, cell-cell interactions and in signal transduction pathways [22,26-28].
WD expressed higher immunoreactivity in the cytosol (Figure 3D &3E) when compared with the poorly and moderately differentiated esophageal carcinoma where the immune reactivity was higher in the cell membrane for mucin expression. Similar observations were reported for oral cancer [2,20]; breast cancer [29] and cancer of uterine cervix [24,30].
Glycoproteins are being used frequently for diagnostic and prognostic purposes in squamous cell carcinoma [4,23]. The carbohydrate moieties of glycoproteins on cell surfaces play an important role in intercellular recognition, adhesion and morphogenesis during oncogenesis, because these glycoproteins function as the major receptors for extra-cellular matrix proteins and as cell adhesion molecules, related to the regulation of numerous cellular biological processes including embryonic development [31]. Aberrant glycosylation of proteins is a common feature found in ESCC patients [28] and aberrations in adhesive interactions can lead to pathological disorders [26]. The positive detection of PNA binding TF-antigen in peripheral blood sample, agrees well with earlier data on the loss of polarity and shedding of mucin into the circulation by abnormal glycosylation of cell surface glycoprotein during tumorigenesis [8,24,26,31]
Recently, it has been reported that a high percentage of ESCC expressed MUC1 and MUC4 genes [32,33]. The expression of mucin genes or proteins in adenocarcinoma is not totally unexpected, although heterogenecity of expression was observed in tumors of [28,33]. Dysregulated expression of both membrane-bound and secreted mucin core protein epitopes, presumably due to both altered mucin mRNA levels and altered mucin glycosylation during neoplastic transformation in [33] leads to the heterogenecity of mucin core protein, which fails to recognize the monoclonal antibodies. There are 7 different anti-TF antibodies, [TF2 (human), TF5 (human), 5A8 (mouse), 8D8 (mouse) and BM22 (mouse), TF1 (human), 49H.9 (mouse), HT-8 (human) and RS1-114 (mouse)] available for the recognition of tandem repeats of mucin core protein as epitopes on TF-antigen [6,10], none of them have shown any correlation between binding pattern and clinico-pathological variables, such as TNM stage, lesions of lymph node and grading for TF-antigen [34]. Whereas, the expression of the reactive form of TF-antigen in ESCC and cryptic form in healthy individuals probed by PNA may provide a distinct advantage over the use of monoclonal antibodies for the detection of TF-antigen as a diagnostic marker.
In conclusion, an enzyme-linked (peanut) lectin binding assay (PNA/ELLA) could be effectively employed in detecting tumor-related glycoproteins present in the sera of patients with ESCC. It is reproducible and easy to perform with a sensitivity of 86 percent and specificity of 90 percent. The study thus establishes the presence of a chemically well-defined carcinoma-associated antigen in the sera of ESCC patients. Therefore, the quantitative estimation of TF-antigen by PNA-ELLA method can be used as a non-invasive technique for rapid screening for early detection of tumorigenesis and tumor progression.
The immunohistochemical method using lectins displays many of the properties of an antibody in that lectins are capable of interacting with a restricted group of polysaccharides [10,14] and this method may become a routine procedure in laboratories concerned with identification and characterisation of mucin type antigen. Identification of mucin-type glycoprotein by antibodies is often difficult, owing to the heavy glycosylation of mucoprotein, which precludes effective binding by the antibodies [14]. The synthesis of TF antigen is based on the composition of ser/thr, where the addition of GalNAc to the predetermined peptide occurs in the cis-golgi, while galactose is added in the trans-golgi network by glycosyltransferases [35]. In the present study, lectin histochemistry by electron microscopy using biotinylated PNA, has also revealed the precise localization of TF-antigen in the trans-golgi apparatus of (Figure 3C).
Thus, during tumorigenesis, changes in the glycosylation at golgi apparatus led to the over secretion of TF antigen into the cytoplasm. Later, it may be transported to the cell membrane and released into the blood stream through secretory granules or embedded in the mucus layer in an aberrantly glycosylated form [24,36]. The present results also suggest that malignant transformation in the squamous cells of esophagus was accompanied by the increased expression of TF antigen when compared to the normal esophageal squamous cells. These findings are similar to those reported earlier for the colon, endothelial metastasis and parietal cells of the stomach [19,37].
The expression of TF-antigen in adenocarcinoma is not totally unexpected [38], because the synthesis and secretion of mucins is a common feature of glandular epithelial tissues [39]. Normal squamous cells do not secrete mucins [37], but they were observed in the ESCC [33] and circulating blood of cervical SCC patients [24].
Conclusion
This study has provided strong evidence for the genesis and expression of TF-antigen in the golgi apparatus, mucin granules in the cytosol of WD and cell membrane of MD and PD, confirmed by PNA in histological classification of ESCC. The above observations indicate that changes occur in intracellular glycoconjugates during, or, after malignant transformation. ELLA and Immuno histochemical analysis of TF antigen by FAT is positively correlated with the different histological grades of ESCC as a simple and cost-effective method for the early diagnosis of human ESCC.
Materials and methods
Patients, Sera and Tumor tissues collections
The study was carried out on human Esophageal Squamous Cell Carcinoma patients [before therapy (group II) and after therapy (group III)], who were receiving treatment from 1996 to 2000 at Barnard Institute of Radiology and Oncology and Institute of Pathology, Chennai Medical College and Research Institute (CMC&RI), Chennai, India. The samples were classified according to the system of the World Health Organization (WHO) [12], after obtaining clearance from the ethical board of the hospital on recommendation of the physicians attached to the above departments. The grades of histological differentiation of the ESCC were classified according to the criteria of the World Health Organization [12] because the TNM classification was not possible for staging most of the unresectable and MD or PD. Serum was also obtained from 50 healthy volunteers (Group I) matched for age for comparative purpose.
Enzyme Linked Lectin Assay (ELLA)
ELLA was performed with PNA (Purchased from Sigma USA), by the method of Zhuang [13]. The diluted serum (1:200) in carbonate-bicarbonate buffer (pH 7) was coated in 96 well flat bottom microtitre Figures, incubated at 4°C for 12 hrs and washed with phosphate buffered saline-Tween20 (PBST). Nonspecific sites were blocked with 0.1% bovine serum albumin (BSA) for 1 hr at 37°C, and washed with PBST. 100 μl of the serially diluted PNA (5 μg/ml) in lectin buffer pH 7.6 containing 50mM Tris; 150 mM NaCl; 3.3 mM MgCl2 and 1.3 mM CaCl2 (pH 7.6) was added to the wells, incubated for 1 hr at 37°C and washed once with PBST. The anti-PNA was diluted (1:5000) in PBS and 100 μl was added to each well, incubated at 37°C. After washing the wells with PBS, 100 μl of goat anti-rabbit IgG horse radish peroxidase conjugate was added and incubated for 1 hr at 37°C. After washing with PBS, freshly prepared substrate (0.75 mg/ml diaminobenzidine in PBS containing 0.03% H2O2) was added for color development. The reaction was arrested by adding 3N H2SO4 and read at 405 nm in a multiscan ELISA reader. Appropriate positive (serum of ESCC patients) and negative (normal serum) controls were maintained. The mean value of normal (N) added with two standard deviation (2SD) of normal was taken as positive (P) for ESCC i.e., N + 2SD = P.
Light microscopical Detection
ESCC specimens were obtained from 92 patients (with different histological grades of ESCC) who underwent treatment at the Department of Medical and Surgical Gastroenterology and Institute of Pathology, CMC & RI. Biopsies were taken from 25 normal areas (200 mm) of squamous esophageal mucosa distant from the ESCC of the same patients. These biopsies were fixed under the same conditions as biopsies of ESCC regions. Paraffin embedded tissues (5 μm thick) were stained with haematoxylin and eosin and periodic acid-Schiff reagent to detect the grade of histological differentiation in the ESCC group and subjected for histological analysis if TF antigen.
Lectin Immunohistochemistry (Florescent Antibody Technique-FAT)
PNA binding analysis was carried out according to the method of Brooks et al [14] with slight modifications. Deparaffinised and rehydrated sections of all groups were transferred to lectin buffer for 10min at room temperature. Then, the sections were exposed to PNA (5 μg/ml) for one h and rinsed thrice in lectin buffer pH 7.6. Subsequently, the sections were incubated with anti-PNA (1:1000) for 30 min. and with FITC labeled Goat anti-rabbit IgG (1:5000) for 30 min and washed 3× for 5 min. each. Finally, the sections were gently rinsed in tap water and observed under a fluorescence microscope at 492 nm. Sections were also processed in the absence of lectin in order to exclude the non-specific interaction of anti-PNA to cells.
Assessment of Immuno histo-chemistry and Statistical analysis
The approximate percentages of fluorescence in normal and neoplastic cells were scored. The intensity of the membrane bound TF antigen for PNA binding was qualitatively expressed in three grades depending upon the proportion of fluorescence on neoplastic cells as follows: 1+, mild (1–25% staining of neoplastic cells) 2+, moderate (25–50% staining of neoplastic cells) and 3+, intense (>50% staining of cells). Qualitative data were analyzed by correlation co-efficient test. P < 0.05 was considered as significant and to predict the clinical progression of the disease.
Electron microscopy
Ten fresh biopsies of each histological grade were received from the Department of Medical Gastroenterology, from patients with ESCC. Biopsies were also taken from normal areas of squamous esophageal mucosa, distant from the tumor region. Transmission electron microscopic (TEM) analysis was carried out on the normal and ESCC specimens for the comparison of morphological changes at the cellular level. Different histological grades of biopsy specimens, determined through haematoxylin and eosin staining to locate the target region, were washed in saline and fixed in, 2.5% glutaraldehyde at room temperature, over night. They were later fixed in 1% osmium tetraoxide for 2 h at 4°C and dehydrated in acetone for 30 min (twice). The dehydrated samples were treated with toluene for 30 min (twice) for clearing and embedded in pure embedding medium using gelatin flat embedding moulds for the orientation of specimen. Embedded blocks were kept at 50°C for polymerization (overnight). Semi thin sections (from normal and ESCC blocks) were stained with methylene blue and tumor regions from the blocks were selected for ultra structural examination. Sections (60 nm thick) were routinely contrasted with uranyl acetate and lead citrate before being observed under the Transmission Electron Microscope (TEM Philips 10) at EM facility AIIMS, New Delhi, India.
Immunoelectron microscopy (IEM)
Desired regions of the normal and ESCC tissues were fixed in 0.3% glutaraldehyde, 2% formaldehyde (v/v) for 15 min, followed by two washes in PBS at room temperature. Dehydration of the fixed tissue was performed in ethanol and infiltrated with 1:1 mixture of 100% acetone and Lowicryl white resin twice (1 h each) and placed in resin filled capsules for block making. Polymerization was performed for 18 h at 60°C. Gold particles as electron dense markers conjugated with streptavidin were used as probe to localize the antigenic site in the specimens.
TF antigen detection by biotin labeled PNA
Ultra thin sections (90 nm) on the grids were treated with blocking buffer for 15–20 min followed by biotinylated PNA for 3 h and washed 3× with lectin buffer and were exposed to Streptavidin – Gold conjugate for 2 h. The grids were then washed 3× in lectin buffer and stained for observation.
Author's contribution
SK carried out all experiments along with the designing and coordination's of HD, ND, ALR & JK. HSA, VV, SC, BR provided patients data, specimens, reviewed the histological and imunohistochemical results. CS provided suggestion for the finalization of this paper. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors thank the Indian Council of Medical Research, India, for the financial support to one of the authors K. Sankaranarayanan, and are grateful to Dr. A. Ramesh, Professor & Head, Department of Genetics, IBMS, University of Madras, Taramani, and Dr. Muralimanohar, Professor & Head, Department of Pathology, Madras Veterinary College, TANUVAS, Chennai, 600 007, India for helping in statistical analysis CS thankfully acknowledges support from the German Research Foundation.
Contributor Information
Sankaranarayanan Kannan, Email: vmsks72@yahoo.com.
Reddi A Lakku, Email: alreddi@yahoo.com.
Devaraj Niranjali, Email: niranjali@yahoo.com.
Kamala Jayakumar, Email: kamalasuji@yahoo.co.in.
Arulraj H Steven, Email: hsarulraj@yahoo.com.
Taralakshmi VV, Email: vtaralakshmi@yahoo.com.
Chandramohan S, Email: scmohan61@yahoo.com.
Ramathilakam Balakrishnan, Email: ramathilakamb@yahoo.com.
Christian Schmidt, Email: christian.schmidt@molecular-cancer.org.
Devaraj Halagowder, Email: hdrajum@yahoo.com.
References
- Utsunomyia T, Yonezawa S, Sakamoto H, Kitamwia H, Hokita S, Aiko TT, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC 1 and MUC 2 mucins in gastric carcinomas: its relationship with prognosis of the patients. Clin Cancer Res. 1998;4:2605–2614. [PubMed] [Google Scholar]
- Saussez S, Chant HM, Mazy N, Decausterkor C, Hossid S, Jortay A, Schuring MP, Gabrus HJ, Danguy A, Salmon F, Kiss R. Quantitative lycohistochemistry defines new prognostic markers for cancers of the oral cavity. Cancer. 1998;82:252–260. doi: 10.1002/(SICI)1097-0142(19980115)82:2<252::AID-CNCR2>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kanitakis J, Al-Rifai I, Euvrard S, Faure M, Claudy A. Differential expression of the cancer-associated antigens T (Thomsen-Friedenreich) and Tn in primary and recurring squamous cell carcinomas of the skin. Anticancer Res. 1999;19:619–620. [PubMed] [Google Scholar]
- Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. 2001;21:1327–1334. [PubMed] [Google Scholar]
- Kunz H. Synthetic glycopeptides for the development of tumour-selective vaccines. J Pept Sci. 2003;9:563–73. doi: 10.1002/psc.477. [DOI] [PubMed] [Google Scholar]
- Wang BL, Springer GF, Harwick LC. T (Thomson-Friedenreich) and Tn epitope location and their spatial relation to adhesion plaques on human breast carcinoma cells: Immunogold-silver staining studies at scanning electron microscopic level. J Submicroscop Cytol Pathol. 1998;30:503–509. [PubMed] [Google Scholar]
- Brooks SA, Leathem AJC. Prediction of lymphnode involvement in breast cancer by detection of altered glycosylation in the primary tumor. Lancet. 1991;338:71–74. doi: 10.1016/0140-6736(91)90071-V. [DOI] [PubMed] [Google Scholar]
- Singh R, Campbell BJ, Yu LG, Fernig DG, Milton JD, Goodlad RA, FitzGerald AJ, Rhodes JM. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology. 2001;11:587–592. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- Chu P, Stagias J, West AB, Traube M. Diffuse pagetoid squamous cell carcinoma in situ of the esophagus. Cancer. 1997;79:1865–1870. doi: 10.1002/(SICI)1097-0142(19970515)79:10<1865::AID-CNCR4>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yu G, Tamson B, Fernig DG, Milton JD, Smith JA, Gerasimenko OV, Jones M, Rhodes LM. Stimulation of proliferation in human colon cancer cells by human monoclonal antibodies against the TF-antigen (galactose b1-3 N-acetyl galactosamine) Int J Cancer. 1997;73:424–431. doi: 10.1002/(SICI)1097-0215(19971104)73:3<424::AID-IJC18>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Baldus SE, Hanisch FG, Monaca E, Karsten UR, Zirbes TK, Thiele J, Ienes MP. Immuno reactivity of Thomsen-Friedenreich (TF) antigen in human neoplasms: the importance of carrier-specific glycotype expression on MUC1. Histol Histopathol. 1999;14:1153–1158. doi: 10.14670/HH-14.1153. [DOI] [PubMed] [Google Scholar]
- Springer-Verlag . In proceedings of World Health Organization Geneva. 2. Vol. 1998. International histological classification of tumours; pp. 1969–1981. [Google Scholar]
- Zhuang D, Yousefi S, Dennis JW. In antigen and UDP-Gal: GalNAca-Rb,1–3 galactosyltrasnsferase expression in human breast carcinoma. Cancer Biochem Biophys. 1991;12:185–198. [PubMed] [Google Scholar]
- Brooks SA, Leathem AJC, Schumacher U. In Lectin Histochemistry, a concise practical hand book. 1. Bios Scientific Publishers; 1997. Lectin Histochemistry for light microscope: II. Methods for visualization of lectin binding; pp. 132–130. [Google Scholar]
- Lin F, Liu PI, McGregor DH. Isoantigens A, B, and H in morphologically normal mucosa and in carcinoma of the larynx. Am J Clin Pathol. 1977;68:372–376. doi: 10.1093/ajcp/68.3.372. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Carey TE, Goldstein IJ, Peters BP. Lectin binding characteristics of squamous cell carcinomas of the head and neck. Acta Otolaryngol. 1996;116:125–131. doi: 10.3109/00016489609137725. [DOI] [PubMed] [Google Scholar]
- Shamsuddin SIT, Sherief MA, Talob SG, Araf WF, Kumar D. Comparison of different technique for detection of Gal-GalNAc, an early marker of colonic neoplasia. Histol Hisopathol. 1999;14:351–357. doi: 10.14670/HH-14.351. [DOI] [PubMed] [Google Scholar]
- Kijima H, Chino O, Oshiba G, Tanaka H, Kenmochi T, Kise Y, Shimada H, Abe Y, Tokunaga T, Yamazaki H, Nakamura M, Tanaka M, Makuuchi H, Ueyama Y. Immunohistochemical MUC1 (DF3 antigen) expression of human esophageal squamous cell carcinoma. Anticancer Res. 2001;21:1285–1289. [PubMed] [Google Scholar]
- Campbell BJ, Finnie IA, Hounsell EF, Rhodses JM. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. J Clin Invest. 1995;95:571–576. doi: 10.1172/JCI117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remani P, Bhattathiri VN, Bindu A, Chandralekha B, Vijayakumar T, Nair MK. Correlation of lectin binding with lymph node metastasis in oral cancers. Oral Oncol. 1997;33:19–22. doi: 10.1016/S0964-1955(96)00044-9. [DOI] [PubMed] [Google Scholar]
- Wang BL, Springer GF, Carlstedt SC. Quantitative computerized image analysis of Tn and T (Thomsen-Friedenreich) epitopes in progrostification of human breast carcinoma. J Histochem Cytochem. 1997;45:1393–1400. doi: 10.1177/002215549704501007. [DOI] [PubMed] [Google Scholar]
- Aeres B, Apostolopouls V, Ballout JM, Wreschner D, Xing PX, Dahlia AH, Bizouarne N, Kiehy MP, McKenzie IFC. MUC 1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–594. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avichezer D, Arno R. Differential reactivities of the Arachis hypogea (peanut) and Vicia villosa B4 lectins with human ovarian carcinoma cells, grown either in vitro or in vivo xerogroff model. FEBS Lett. 1996;395:103–108. doi: 10.1016/0014-5793(96)01010-1. [DOI] [PubMed] [Google Scholar]
- Reddi AL, Sankaranarayanan K, Stephen HA, Niranjali D, Devaraj H. Enzyme linked PNA lectin-binding assay of serum T-antigen in patients with SCC of the uterine cervix. Cancer Lett. 2000;149:207–211. doi: 10.1016/S0304-3835(99)00363-8. [DOI] [PubMed] [Google Scholar]
- Yu LG, Milton JD, Fernig DG, Rhodes JM. Opposite effects on human colon cancer cell proliferation of two dietary Thomsen-Friedenreich antigen-binding lectins. J Cell Physiol. 2001;186:282–287. doi: 10.1002/1097-4652(200102)186:2<282::AID-JCP1028>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. Tumor malignancy defined by aberrant glycosylation and sphyngo (glyco) lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- Labouvie C, Machado JC, Carnerio F, Sarbia M, Vieth M, Porschen R, Seitz G, Blin N. Differential expression of mucins and trefoil peptides in native epithelium, Barrett's metaplasia and squamous cell carcinoma of the oesophages. J Cancer Res Clinic Oncol. 1999;125:71–76. doi: 10.1007/s004320050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher U, Adam E. Lectin histochemical HPA-binding pattern of human breast and colon cancers is associated with metastastis formation in severe combined immunodeficient mice. Histo Chem J. 1997;29:677–684. doi: 10.1023/A:1026404832394. [DOI] [PubMed] [Google Scholar]
- Gahzizadeh M, Ogawa H, Sasaki Y, Araki T, Aihara K. Mucin carbohydrate antigens (T Tn and Sialyl Tn) in human ovarian carcinoma: relationship with histopathology and prognosis. Hum Pathol. 1997;28:960–966. doi: 10.1016/s0046-8177(97)90012-5. [DOI] [PubMed] [Google Scholar]
- Raida M, Sarbia M, Clement JH, Adam S, Gabbere HE, Hoffken K. Expression regulation and clinical significance of bone morphogenetic protein 6 in esophageal squamous cell carcinoma. Int J Cancer. 1999;83:38–44. doi: 10.1002/(SICI)1097-0215(19990924)83:1<38::AID-IJC8>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Guillem P, Billeret V, Buisine MP, Flejou JF, Houck LM, Degand P, Aubert JP, Triboulet JP, Porchet N. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int J Cancer. 2000;88:856–861. doi: 10.1002/1097-0215(20001215)88:6<856::AID-IJC3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sagara M, Yonezawa S, Nagata K, Tezuka Y, Natsugoe S, Xing PX, Mc Kenzige IFC, Aikou T, Sato E. Expression of mucin (MUC1) in esophageal squamous cell carcinoma: its relationship with prognosis. Int J Cancer (Pred Oncol) 1999;84:251–257. doi: 10.1002/(SICI)1097-0215(19990621)84:3<251::AID-IJC9>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Flucke U, Zirbes TK, Schroder W, Monig SP, Koch V, Schmitz K, Thiele J, Dienes HP, Holscher AH, Baldus SE. Expression of mucin-associated carbohydrate core antigens in esophageal squamous cell carcinomas. Anticancer Res. 2001;21:2189–2193. [PubMed] [Google Scholar]
- Campbell BJ, Rowe GE, Leiper K, Rhodes JM. Increasing the intra-Golgi pH of cultured LS174T goblet-differentiated cells mimics the decreased mucin sulfation and increased Thomsen-Friedenreich antigen (Galbeta1-3GalNacalpha-) expression seen in colon cancer. Glycobiology. 2001;11:385–393. doi: 10.1093/glycob/11.5.385. [DOI] [PubMed] [Google Scholar]
- Terasawa K, Furumoto M, Kamada M, Aono T. Expression of Tn and Sialyl -Tn antigens in the neoplastic transformation of uterine cervix epithelial cells. Cancer Res. 1996;56:2229–2232. [PubMed] [Google Scholar]
- Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumor cells. J Physiol. 2003. October 17, 2003 DOI: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed]
- Kumamoto K, Mitsuoka C, Izawa M, Kimura N, Otsubo N, Ishida H, Kiso M, Yamada T, Hirohashi S, Kannagi R. Specific detection of sialyl Lewis X determinant carried on the mucin GlcNAcbeta1–>6GalNAcalpha core structure as a tumor-associated antigen. Biochem Biophys Res Commun. 1998;247:514–517. doi: 10.1006/bbrc.1998.8824. [DOI] [PubMed] [Google Scholar]
- Brockhausen I, Yang J, Lehotay M, Ogata S, Itzkowitz S. Pathways of mucin O-glycosylation in normal and malignant rat colonic epithelial cells reveal a mechanism for cancer-associated Sialyl-Tn antigen expression. Biol Chem. 2001;382:219–232. doi: 10.1515/BC.2001.029. [DOI] [PubMed] [Google Scholar]