Abstract
The grade of chondrosarcoma relates to the likelihood of local recurrence and metastases. Many Grade I chondrosarcomas behave benignly if aggressively, and the question arises regarding whether wide resection is essential to control the disease. We therefore asked whether intralesional surgery also could be extended to Grade I chondrosarcomas without an increase in recurrence. We retrospectively reviewed 31 patients with Grade I chondrosarcomas of the limbs. The minimum followup was 66 months (mean, 157 months; range, 66–296 months). None of the 16 patients treated by resection had recurrences during the followup and two of the 15 patients with intralesional excision had recurrences, both of which resolved with resection of the site involved by the recurrence without progression of the disease. The Musculoskeletal Tumor Society scores averaged 72% in patients treated with wide resection compared with 89% in the 15 patients treated by intralesional surgery. The two recurrences occurred in patients whose radiographs showed thinning of the cortex combined with bone enlargement and marked endosteal scalloping; histologic examination in these two patients also showed a correlation between radiographic aggressiveness and the presence of myxoid areas and hypercellularity.
Level of Evidence: Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Central chondrosarcoma (CS) is the fourth most common primary malignant tumor of the skeleton, typical of adult age [4]. It grows slowly inside the medullary canal of a long bone for years before producing mild pain or a pathologic fracture [18, 22, 23]. A pathologic fracture usually is caused by a sudden progression of the local disease that is often a sign of transformation into high malignancy (dedifferentiation) [21].
CS is divided histologically into three grades according to the characteristics of its intercellular scaffold: cellularity, characteristics of its nucleus, and the presence of mitotic activity [6]. The benign counterpart of CS is enchondroma, which by its nature grows during infancy and typically has little clinical importance in adulthood [8, 21]. The differential diagnosis between enchondroma and Grades I and II CS is a challenge for the anatomic pathologist and is often the object of studies to determine certain parameters such as permeative infiltration with encapsulation of host bone trabeculae as proposed by Mirra et al. [16] and Schiller [21] or the presence of hypercellularity combined with cellular atypia and myxoid areas [1, 3, 10, 16, 19, 20]. Furthermore, this tumor can have different histologic grades in different areas of the tumor [19]. In fact, a needle biopsy does not always allow a correct diagnosis of grade [23, 24]. For this reason, it is particularly important to combine the radiographic interpretation with the histologic finding. Nevertheless, histologic grade is a good prognostic factor [3, 16, 17, 19].
Grading and staging are fundamental to establish the most appropriate type of surgery for CS [18]. Standard radiography often is sufficient to assess the extent of the tumor in the medullary canal and its relation with the cortex [5, 9, 14, 15]. CT [24] and MRI can show whether there is endosteal involvement or breakthrough in the cortex [4, 11, 17]. The radiographic and histologic elements that allow clear distinction between enchondroma and Grade I central CS remain controversial. Grade I CS, in fact, shows such a harmless clinical pattern that it can be mistaken for an enchondroma [8]. Grade I CS can recur even after 10 to 20 years, whereas Grade II CS recurs within 5 years and Grade III CS often recur within 1 year [4, 11, 19, 23]. Grade II CS can have fewer pulmonary metastases than Grade III; however, the 5-year overall survival for Grades II and III CS ranges from 40% to 50% [4, 11, 12, 19, 23].
Therefore, the question regarding whether to consider Grade I central CS an aggressive benign tumor rather than a low-grade malignancy tumor has led some authors to extend the indication of intralesional surgery to these patients [2, 13]. Although enchondroma can be treated by curettage, or in case of inactive lesions, even nonsurgically [6], Grade I CS usually is treated with en bloc resection with wide margins. The adequacy of intralesional surgery is advocated by some surgeons [2, 13] and opposed by others [17–19, 23].
We therefore asked whether (1) intralesional surgery would lead to greater numbers of local recurrence, late relapses, and subsequent surgical procedures compared with resections performed in patients with Grade I CS; (2) radiographic signs and histologic findings of aggressiveness would predict aggressive behavior; (3) intralesional surgery and resection would provide similar functional scores; and finally, we (4) report the complications of these procedures.
Patients and Methods
We reviewed retrospectively all 67 patients with Grade I central CS in the long bones treated surgically from 1977 to 1998. We excluded patients with Ollier’s disease (nine cases), with inadequate radiographic documentation (11 cases), and with less than 60 months followup (five cases); we also excluded patients with CS of the short bones and those seen for consultation only. That left 31 patients with a minimum followup of 66 months (mean, 157 months; range, 66–296 months). There were 13 male and 18 female patients with a mean age of 35 years (median, 33 years; range, 13–67 years). Sixteen patients were treated by wide resection and 15 by intralesional curettage. The location of CS was the femur in 13 patients, nine proximal, and four distal; the tibia in 11 patients, eight proximal, two distal, and one diaphyseal; and the humerus in seven patients, six proximal and one diaphyseal (Fig. 1). The onset symptom was pain in 27 of the 31 patients (87%) with a mean duration of 25 months (range, 2–120 months). Two patients were diagnosed after a pathologic fracture, and in two the disease was discovered as an incidental radiographic finding. We found bone enlargement by palpation of a deep hard mass in 11 of the 31 (35) patients.
Fig. 1.
Distribution of the lesions is seen in these illustrations.
An initial biopsy had been performed elsewhere in 13 patients; we repeated a biopsy in four of these 13 patients. In 17 patients, the initial biopsy (13 incisional, three extemporaneous, and one needle) was performed in our hospital. In one patient, a biopsy was not performed, and diagnosis was based on radiographic evaluation. Of the 31 patients, four had been treated surgically, three with curettage and one by resection, before referral to us. All four had local recurrence and therefore were treated again at our institute, three by wide resection and one by curettage. Fourteen of the other 27 patients were treated by curettage and 13 by resection (Table 1).
Table 1.
Radiographic characteristics of the lesions
| Patient number | Gender | Age (years) | Lesion site | Size (cm2) | Involvement | Enlargement | Scalloping | Type of operation |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 17 | Distal femur | 25 | Cortical interruption, thinning | Yes | Deep | Resection |
| 2 | F | 44 | Proximal humerus | 19 | Irregular surface | No | Slight | Resection |
| 3 | M | 58 | Distal femur | 5.5 | No | No | No | Resection |
| 4 | F | 14 | Proximal femur | 7.4 | Thickening | Yes | No | Resection |
| 5 | M | 47 | Proximal femur | 15.8 | Irregular surface, thinning | Yes | Moderate | Resection |
| 6 | M | 31 | Proximal humerus | 44.5 | Irregular surface, thinning | Yes | Deep | Resection |
| 7 | M | 19 | Tibial diaphragm | 9.4 | No | Yes | Moderate | Curettage |
| 8 | M | 19 | Proximal tibia | 6.4 | Interruption, thinning | Yes | No | Resection |
| 9 | F | 41 | Proximal humerus | 15.8 | No | No | Slight | Curettage |
| 10 | M | 15 | Proximal tibia | 3.2 | Interruption, thinning | Yes | No | Curettage |
| 11 | F | 29 | Distal femur | 4.7 | Interruption | No | No | Resection |
| 12 | M | 64 | Proximal femur | 11 | Irregular surface, thinning | Yes | Moderate | Resection |
| 13 | M | 41 | Proximal tibia | 10 | No | No | No | Curettage |
| 14 | M | 22 | Proximal femur | 57.2 | Irregular surface, thinning | Yes | Moderate | Curettage |
| 15 | F | 33 | Humeral diaphragm | 15.9 | No | Yes | Moderate | Curettage |
| 16 | F | 46 | Proximal tibia | 8.5 | Thinning | No | Moderate | Resection |
| 17 | F | 23 | Distal tibia | 4.7 | Thinning | Yes | Moderate | Curettage |
| 18 | F | 13 | Proximal tibia | 10.5 | No | Yes | Slight | Curettage |
| 19 | M | 27 | Distal tibia | 4.7 | No | Yes | Slight | Curettage |
| 20 | F | 45 | Proximal femur | 23.5 | No | Yes | Deep | Resection |
| 21* | F | 32 | Proximal femur | 12.7 | Irregular surface, thinning | Yes | Moderate | Curettage |
| 22 | F | 62 | Proximal. humerus | 28.2 | Thinning | Yes | Moderate | Resection |
| 23* | F | 34 | Proximal femur | 9.6 | No | No | No | Curettage |
| 24 | F | 45 | Proximal humerus | 35.7 | No | No | Slight | Curettage |
| 25 | F | 42 | Proximal humerus | 8.4 | No | No | Slight | Curettage |
| 26 | F | 49 | Proximal tibia | 23.5 | Thickening | Yes | No | Resection |
| 27 | M | 13 | Proximal tibia | 5.9 | No | No | No | Curettage |
| 28 | F | 67 | Distal femur | 19.6 | Interruption, thinning | Yes | Moderate | Resection |
| 29 | F | 51 | Proximal femur | 44.3 | Interruption | Yes | Deep | Resection |
| 30 | M | 25 | Proximal femur | 13.5 | No | Yes | Slight | Curettage |
| 31 | M | 58 | Proximal tibia | 37.6 | Interruption, thinning | Yes | Deep | Resection |
* Patients with local recurrence; F = female; M = male.
In all 15 patients treated by curettage, the surgical technique involved removal of the newly formed tissue by opening a cortical window and cleaning the cavity using different sized curettes and a high-speed burr. Local adjuvants were used in 12 patients: phenol and/or acrylic cement in nine and liquid nitrogen in three. After curettage, the defects were filled with cement in five patients, allografts in three, and autografts in one. In four patients, the bone was stabilized with fixation devices without the use of other filling material. In the last two patients with metaphyseal location of the proximal femur, the metaphysis was removed together with the epiphysis; therefore, after performing curettage in the operating field, the bone was reused to obtain a cemented composite prosthesis in the resected bone and uncemented in the residual diaphysis.
Of the 16 patients treated by resection, a joint, or part of it, was sacrificed in eight. The joint was reconstructed by a standard prosthesis of the proximal femur in three, HMRS Stryker Howmedica-type modular resection prosthesis (Kiel, Germany) in two, and Wagner-type revision prosthesis (Sulzer, Winterthur, Switzerland) in one, whereas the remaining two were reconstructed by patella procondyle for the distal femur. The other eight patients treated by resection underwent reconstruction owing to an intercalary defect using autologous grafts in four, whereas in the others, one had temporary reconstruction with a plate and cement, which was replaced after 7 months by a homoplastic graft combined with a vascularized fibula, two by a plate and homoplastic graft, and in the last patient, after the resection, an Ilizarov device was applied. Wide margins were achieved in 14 patients treated by resection, a marginal margin was achieved in one, and an intralesional margin was achieved in one. In seven patients, the cortex initially was interrupted; six were treated with surgical resection and one by curettage (extension of the lesion 3.2 cm2). Bone enlargement was observed in 21 patients; 12 were combined with other signs of aggressiveness (interruption of the cortex, invasion of the soft tissues detected by CT, and moderate-deep scalloping) and all were treated by resection; nine with simple enlargement were treated by curettage. Scalloping was present in 22 patients: mild in seven, moderate in 10, and deep in five; in the latter patients, surgical resection was performed.
The patients usually were followed up in our outpatient clinic every 4 to 6 months during the first 5 years and, then yearly for at least 10 years. During each visit, we obtained Musculoskeletal Tumor Society scores [6]. This system assigns numeric scores from 0 to 5 for each of the six considered parameters with a maximum score of 30 points.
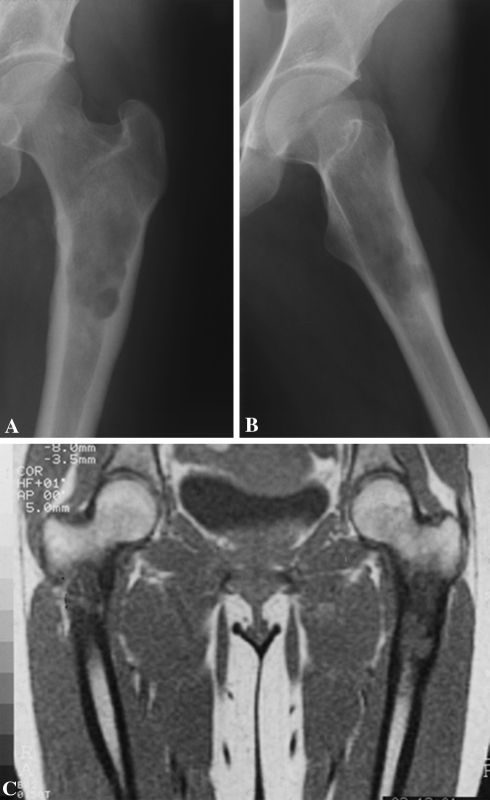
One of the four authors (DD, SC, MC, DBC) determined radiographically the site, shape, and size (measured on the AP projection in centimeters in two dimensions and calculating a 10% mean radiographic enlargement) of the osteolysis, the occurrence of popcorn, ring, or spot-like calcification, and the presence and type of cortical reaction with particular reference to the presence of bone enlargement. We classified endosteal resorption (scalloping) as mild if it involved one-third of the cortical thickness, moderate if it involved two-thirds of the cortex, or deep if there was penetration of the cortex (Fig. 2). On the CT scan, we measured the same parameters as those of the radiographs plus the presence of soft tissue mass, distribution of contrast media, and presence of levels.
Fig. 2A–C.
A CS of the left proximal femur in seen in these radiographs. In the (A) AP and (B) lateral projections, the inner lateral and posterior cortex is invaded (moderate scalloping) by the tumor. The cortical augmentation (enlargement, Grade I) is also evident. (C) The definition of the intramedullary tumor involvement is enhanced by MRI.
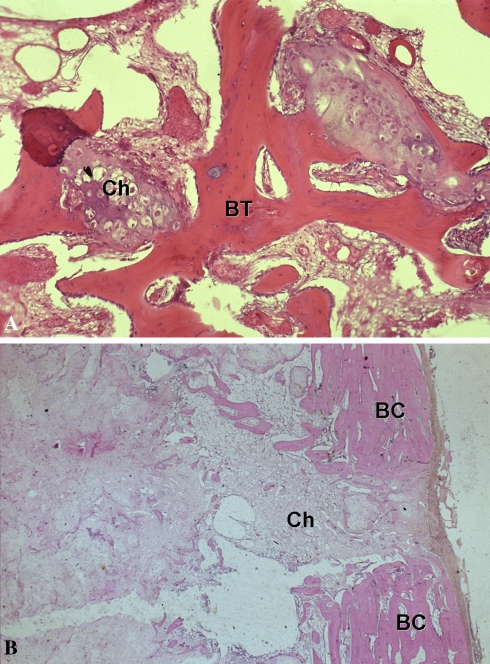
One of the three authors (FB, DD, SC) reviewed each of the histologic slides of the biopsy and slides of the specimen. Histologic analysis was performed by evaluating five parameters characterizing the aggressiveness of the tumor: (1) permeative infiltration with encapsulation of host bone trabeculae as proposed by Mirra et al. [16] and Schiller [21]; (2) presence of host bone trapped in the front of growing cartilage as a sign of permeation versus presence of bone circumferentially surrounding the islands of cartilage as a sign of tissue reaction and therefore of lesion differentiation; (3) cortical erosion combined with the presence of tumor cells in the Haversian canals; (4) hypercellularity combined with cellular atypia; and (5) myxoid areas, bands of perilobular fibrosis, necrosis, and swelling of the nuclei (Fig. 3). Permeative infiltration with the inclusion of host bone trabeculae was present in 25 patients (80.6%) (Table 2). This pattern of aggressiveness differentiates Grade I CS from chondroma. Hypercellularity was scarce (four patients), although it was found in both patients with recurrence. We observed myxoid areas in 10 patients. There were myxoid areas in six of the 12 patients in whom radiographs showed enlargement combined with thinning of the cortex. We observed bands of perilobular fibrosis in four patients and swelling of the nuclei in three patients. Consistent with the low aggressiveness of the lesions, we did not observe signs of necrosis.
Fig. 3A–B.
The photomicrographs show the histologic presentation of Grade I CS. (A) A pattern of permeative infiltration is seen, with encasement of host bone trabeculae (BT) by the progression of the tumor (Ch) at high magnification (Stain, hematoxylin and eosin; original magnification, ×20). (B) The chondrosarcoma (Ch) invaded the host bone cortex (BC) (Stain, hematoxylin and eosin; original magnification, ×10).
Table 2.
Histologic parameters assessed
| Patient number | Biopsy | Operation | Infiltration | Hypercellularity | Myxoid areas | Perilobular fibrosis | Swollen nuclei | Local recurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Resection | Yes | No | No | No | No | No |
| 2 | Yes | Resection | Yes | Yes | No | Yes | Yes | No |
| 3 | Yes | Resection | No | No | No | No | No | No |
| 4 | Yes | Resection | Yes | No | No | No | No | No |
| 5 | Yes | Resection | Yes | No | Yes | No | No | No |
| 6 | Yes | Resection | Yes | No | No | No | No | No |
| 7 | Yes | Curettage | Yes | No | No | No | No | No |
| 8 | Yes | Resection | Yes | No | No | No | No | No |
| 9 | Yes | Curettage | Yes | No | No | No | No | No |
| 10 | Yes | Curettage | Yes | No | Yes | No | No | No |
| 11 | Yes | Resection | Yes | No | Yes | No | No | No |
| 12 | Yes | Resection | Yes | No | Yes | No | No | No |
| 13 | Yes | Curettage | Yes | No | No | No | No | No |
| 14 | Yes | Curettage | Yes | No | Yes | No | No | No |
| 15 | No | Curettage | Yes | Yes | Yes | No | No | No |
| 16 | Yes | Resection | No | No | No | No | No | No |
| 17 | Yes | Curettage | Yes | No | Yes | No | No | No |
| 18 | Yes | Curettage | Yes | No | No | No | No | No |
| 19 | Yes | Curettage | No | No | No | No | No | No |
| 20 | Yes | Resection | Yes | No | Yes | No | No | No |
| 21 | Yes | Curettage | No | Yes | Yes | No | No | Yes |
| 22 | Yes | Resection | Yes | No | No | Yes | No | No |
| 23 | Yes | Curettage | Yes | Yes | No | No | Yes | Yes |
| 24 | Yes | Curettage | Yes | No | No | No | Yes | No |
| 25 | Yes | Curettage | Yes | No | No | No | No | No |
| 26 | Yes | Resection | Yes | No | No | No | No | No |
| 27 | Yes | Curettage | Yes | No | No | No | No | No |
| 28 | Yes | Resection | No | No | No | No | No | No |
| 29 | Yes | Resection | No | No | No | No | No | No |
| 30 | Yes | Curettage | Yes | No | Yes | Yes | No | No |
| 31 | Yes | Resection | Yes | No | No | Yes | No | No |
Results
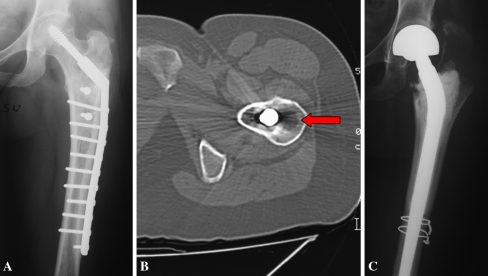
Two of the 15 patients treated by intralesional curettage and none of the 16 patients treated by resection had local recurrences (Figs. 2 and 4). Both patients with recurrences had lesions in the proximal femur and both recurrences occurred 31 months after the operation. One of these patients had been treated at another hospital by curettage. In both cases, histologic examination of the recurrence did not show progression of the grade. Surgical treatment of the local recurrences consisted of resection and reconstruction, one with a modular HMRS (Stryker and Wagner) prosthesis and the other with an allograft-prosthetic composite (Fig. 4). At the last followup (114 and 153 months), there were no additional oncologic relapses in the patients with recurrences. Concerning the radiographic presentation (Table 3), 16 patients treated by surgical resection had a greater mean extension (20.3 cm2) than the patients treated by curettage (14.5 cm2). Calcifications were present in 22 patients but without correlation with other elements of aggressiveness.
Fig. 4A–C.
(A) A local recurrence located in the greater trochanter is evident on this radiograph. (B) Although there were metal artifacts, CT confirmed the radiolucent area close to the bone fixation device (arrow). (C) The proximal end of the femur was resected and substituted with an allograft-prosthetic composite.
Table 3.
Radiographic characteristics of the lesions
| Patient number | Lesion site | Size (cm2) | Cortical involvement | Enlargement | Scalloping | Type of operation |
|---|---|---|---|---|---|---|
| 1 | Distal femur | 25 | Interruption, thinning | Yes | Deep | Resection |
| 2 | Proximal humerus | 19 | Irregular surface | No | Slight | Resection |
| 3 | Distal femur | 5.5 | No | No | No | Resection |
| 4 | Proximal femur | 7.4 | Thickening | Yes | No | Resection |
| 5 | Proximal femur | 15.8 | Irregular surface, thinning | Yes | Moderate | Resection |
| 6 | Proximal humerus | 44.5 | Irregular surface, thinning | Yes | Deep | Resection |
| 7 | Tibial diaphragm | 9.4 | No | Yes | Moderate | Curettage |
| 8 | Proximal tibia | 6.4 | Interruption, thinning | Yes | No | Resection |
| 9 | Proximal humerus | 15.8 | No | No | Slight | Curettage |
| 10 | Proximal tibia | 3.2 | Interruption, thinning | Yes | No | Curettage |
| 11 | Distal femur | 4.7 | Interruption | No | No | Resection |
| 12 | Proximal femur | 11 | Irregular surface, thinning | Yes | Moderate | Resection |
| 13 | Proximal tibia | 10 | No | No | No | Curettage |
| 14 | Proximal femur | 57.2 | Irregular surface, thinning | Yes | Moderate | Curettage |
| 15 | Humerus diaphragm | 15.9 | No | Yes | Moderate | Curettage |
| 16 | Proximal tibia | 8.5 | Thinning | No | Moderate | Resection |
| 17 | Distal tibia | 4.7 | Thinning | Yes | Moderate | Curettage |
| 18 | Proximal tibia | 10.5 | No | Yes | Slight | Curettage |
| 19 | Distal tibia | 4.7 | No | Yes | Slight | Curettage |
| 20 | Proximal femur | 23.5 | No | Yes | Deep | Resection |
| 21* | Proximal femur | 12.7 | Irregular surface, thinning | Yes | Moderate | Curettage |
| 22 | Proximal humerus | 28.2 | Thinning | Yes | Moderate | Resection |
| 23* | Proximal femur | 9.6 | No | No | No | Curettage |
| 24 | Proximal humerus | 35.7 | No | No | Slight | Curettage |
| 25 | Proximal humerus | 8.4 | No | No | Slight | Curettage |
| 26 | Proximal tibia | 23.5 | Thickening | Yes | No | Resection |
| 27 | Proximal tibia | 5.9 | No | No | No | Curettage |
| 28 | Distal femur | 19.6 | Interruption, thinning | Yes | Moderate | Resection |
| 29 | Proximal femur | 44.3 | Interruption | Yes | Deep | Resection |
| 30 | Proximal femur | 13.5 | No | Yes | Slight | Curettage |
| 31 | Proximal tibia | 37.6 | Interruption, thinning | Yes | Deep | Resection |
* Patients with local recurrence.
None of the patients of the series had metastases and no deaths were caused by the disease. We could discern no differences in radiographic or histologic aggressiveness in patients treated by intralesional surgery versus resection. We observed hypercellularity in both patients with recurrences and one of the 10 patients with myxoid areas had a local recurrence.
In patients treated by intralesional surgery, the mean functional score was 90% of normal function (range, 77%–100%), whereas in patients treated by resection, the mean score was 73% (range, 47%–90%). In this group of patients, four of six scored 50% or less in comparison to zero of 15 in patients treated by curettage.
In the 16 patients treated by curettage, only one had complications: a fracture of the proximal humerus 6 years after surgical treatment, which was treated by bone fixation. Complications occurred in two patients treated by resection: in one, atrophic nonunion occurred after treatment with an Ilizarov’s device and was treated after 9 months by fibula-protibia and the other patient had loosening of the prosthetic stem 9 years after resection and was treated by revision of the implant.
Discussion
The grade of CS reportedly corresponds to the likelihood of local recurrence and metastases. Many Grade I CS behave benignly if locally aggressive. Therefore the question arises regarding whether wide resection is essential to control the disease. We asked whether (1) intralesional surgery would lead to greater numbers of local recurrences, late relapses, and subsequent surgical procedures compared with resections performed in patients with low-grade CS; (2) radiographic signs and histologic findings of aggressiveness would predict subsequent aggressive behavior; (3) intralesional surgery and resection would provide similar functional scores.
There are several limitations to our study. First, because it is a retrospective analysis with patients treated over 21 years, diagnostic approaches and surgical technical skills have changed. Second, a consistent number of patients were referred thereby making the adequacy of the first treatment performed elsewhere difficult to assess. Third, patients treated by curettage had radiographically less aggressive disease than those treated by resection. This should bias patients with recurrences in favor of those with wide resections, yet the recurrences were in patients with intralesional treatment and therefore we do not believe biases the outcomes. However, we included only patients with Grade I central CS of the long bones ultimately treated and followed by the same group of surgeons. We also included patients with more than 5 years of followup. A couple published reports of patients affected by CS include various locations of bone involvement, tumor grades, and followup times [17, 19].
Only two previous studies attempted to address the same issue of whether curettage was reasonable in Grade I CS (Table 4). In 23 patients with Grade I CS treated with curettage, Bauer et al. [2] reported three local recurrences without metastases or progression of the disease. One local recurrence after resection of a phalanx eventually healed (19 years followup), and two recurrences after curettage healed after repeated curettage. Although the reported series are more heterogeneous (any site included, short followups), our findings are consistent with theirs. These authors concluded CS of long bones can be treated by curettage and filling the cavity with either autogenous bone or methylmethacrylate cement. Distal destructive lesions required en bloc resection to prevent local recurrence. The recently published experience of the Mayo Clinic [13] was more controversial; in a group of 13 patients treated by curettage, they had one recurrence followed by death consequent to metastases of the disease. In this patient, the diagnosis of recurrence was dedifferentiated CS. In that series, although there were no details regarding the radiographic and histologic characteristics of the cases, they concluded that in selected patients, less radiographically aggressive Grade I CS could be treated safely by intralesional curettage without compromising the outcome.
Table 4.
Data for oncologic results among three comparable studies
| Study | Number of cases | Resection | Curettage | Local recurrence after resection | Local recurrence after curettage | Metastasis | Mean followup in months (minimum–maximum) |
|---|---|---|---|---|---|---|---|
| Current study | 31 | 16 | 15 | 0 | 2* | 0 | 157 (66–296) |
| Leerapun et al. [13] | 70 | 57 | 13 | 1† | 1‡ | 1 after resection§ 1 after curettage‡ | 102 (2–273) |
| Bauer et al. [2] | 38 | 14 | 24 | 1|| | 2¶ | 0 | 84 (24–300) |
* Both healed after resection and prosthetic substitution, no upgrading in both cases; †healed after new resection, no upgrading; ‡the same patient, 51 years old, had local recurrence and lung metastasis develop 4 months after surgery with a new diagnosis of dedifferentiated chondrosarcoma, he died 9 months after; §patient with lung, abdomen, and deltoid muscle metastasis occurred after 3.5 years, upgraded to Grade II chondrosarcoma; ||foot phalanx chondrosarcoma, soft tissue recurrence excised and eventually healed (followup 19 years); ¶both healed after one or more curettage, no upgrading in both cases.
We found the most important marker of aggressiveness of the lesion was bone enlargement combined with thinning of the cortex as previously suggested [4]. The only case of recurrence of the disease after initial curettage at our hospital had such a radiographic profile; the other recurrence was in a patient already treated before coming to us for the second curettage. Radiographic signs of aggressiveness (enlargement + cortical thinning) are combined with the histologic observation of myxoid areas and, in patients with recurrence, hypercellularity of the lesion also is associated. Scalloping was not considerably correlated with an increase in histologic aggressiveness, although in the five patients with deep scalloping, resection was always the method of treatment. It has been argued that the size of the lesion is important [7, 20] to differentiate Grade I CS from enchondroma. Owing to the low number of patients in the series, we could not observe correlation between size and radiographic and histologic aggressiveness. The presence, type, and distribution of calcifications also were of secondary importance.
The rationale of using intralesional curettage is strengthened by the better functional results achieved in this group of patients compared with the group treated by resection (Table 5). Despite the small number of patients, based on a series from one center and homogeneous for the type of lesion (Grade I CS of the long bones in all patients) with sufficient followup, our data suggest Grade I CS is a tumor with low potential aggressiveness that can be treated by curettage combined with the use of adjuvant therapies. We found recurrence could be treated by resection without subsequently risking recurrence or metastases. However, we suggest selecting patients on the basis of radiographic appearance and avoiding curettage in patients presenting with bone enlargement associated with thinning of the cortex and deep scalloping.
Table 5.
Functional evaluation with Musculoskeletal Tumor Society score
| Patient number | Gender | Age (years) | Surgery | Score (%) | Result | Followup (months) |
|---|---|---|---|---|---|---|
| 1 | F | 17 | Resection | 76 | Good | 296 |
| 2 | F | 44 | Resection | 46 | Poor | 265 |
| 3 | M | 58 | Resection | 90 | Excellent | 228 |
| 4 | F | 14 | Resection | 80 | Good | 219 |
| 5 | M | 47 | Resection | 50 | Poor | 164 |
| 6 | M | 31 | Resection | 76 | Good | 166 |
| 7 | M | 19 | Curettage | 80 | Good | 251 |
| 8 | M | 19 | Resection | 76 | Good | 194 |
| 9 | F | 41 | Curettage | 76 | Good | 168 |
| 10 | M | 15 | Curettage | 100 | Excellent | 154 |
| 11 | F | 29 | Resection | 76 | Good | 180 |
| 12 | M | 64 | Resection | 76 | Good | 204 |
| 13 | M | 41 | Curettage | 96 | Excellent | 160 |
| 14 | M | 22 | Curettage | 93 | Excellent | 201 |
| 15 | F | 33 | Curettage | 90 | Excellent | 151 |
| 16 | F | 46 | Resection | 76 | Good | 135 |
| 17 | F | 23 | Curettage | 76 | Good | 126 |
| 18 | F | 13 | Curettage | 100 | Excellent | 168 |
| 19 | M | 27 | Curettage | 100 | Excellent | 123 |
| 20 | F | 45 | Resection | 50 | Poor | 179 |
| 21* | F | 32 | Curettage | 76 | Good | 114 |
| 22 | F | 62 | Resection | 76 | Good | 66 |
| 23* | F | 34 | Curettage | 76 | Good | 153 |
| 24 | F | 45 | Curettage | 76 | Good | 94 |
| 25 | F | 42 | Curettage | 100 | Excellent | 139 |
| 26 | F | 49 | Resection | 90 | Excellent | 87 |
| 27 | M | 13 | Curettage | 100 | Excellent | 81 |
| 28 | F | 67 | Resection | 76 | Good | 127 |
| 29 | F | 51 | Resection | 50 | Poor | 72 |
| 30 | M | 25 | Curettage | 100 | Excellent | 87 |
| 31 | M | 58 | Resection | 90 | Excellent | 110 |
* Patients with local recurrence; F = female; M = male.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock, ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ayala G, Liu C, Nicosia R, Horowitz S, Lackman R. Microvasculature and VEGF expression in cartilage tumors. Hum Pathol. 2000;31:341–346. doi: 10.1016/S0046-8177(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 2.Bauer HC, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283–288. doi: 10.3109/17453679508995543. [DOI] [PubMed] [Google Scholar]
- 3.Brien EW, Mirra JM, Kerr R. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with emphasis on radiology, pathology and clinical biology: I The intramedullary cartilage tumors. Skeletal Radiol. 1997;26:325–353. doi: 10.1007/s002560050246. [DOI] [PubMed] [Google Scholar]
- 4.Campanacci M. Bone and Soft Tissue Tumors. 2. Wien, Germany: Springer-Verlag; 1999. [Google Scholar]
- 5.Beuckeleer LH, Schepper AM, Ramon F, Somville J. Magnetic resonance imaging of cartilaginous tumors: a retrospective study of 79 patients. Eur J Radiol. 1995;21:34–40. doi: 10.1016/0720-048X(96)81067-9. [DOI] [PubMed] [Google Scholar]
- 6.Enneking W, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 7.Eriksson AI, Schiller A, Mankin HJ. The management of chondrosarcoma of bone. Clin Orthop Relat Res. 1980;153:44–66. [PubMed] [Google Scholar]
- 8.Geirnaerdt MJ, Hermans J, Bloem JL, Kroon HM, Pope TL, Taminiau AH, Hogendoorn PC. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol. 1997;169:1097–1104. doi: 10.2214/ajr.169.4.9308471. [DOI] [PubMed] [Google Scholar]
- 9.Helfenstein A, Frahm SO, Krams M, Drescher W, Parwaresch R, Hassenpflug J. Minichromosome maintenance protein (MCM6) in low-grade chondrosarcoma: distinction from enchondroma and identification of progressive tumors. Am J Clin Pathol. 2004;122:912–918. doi: 10.1309/G638TKNNG2CJUXWL. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Taki J, Sumiya H, Kinuya S, Nakajima K, Namura M, Tonami N. Characterization of cartilaginous tumors with 201Tl scintigraphy. Ann Nucl Med. 2005;19:95–99. doi: 10.1007/BF03027387. [DOI] [PubMed] [Google Scholar]
- 11.Jelthi A, Forest M, Tomeno B, Abelanet R. [Bone reabsorption and remodeling in chondrosarcomas of the limbs and pelvis: diagnostic value in 84 cases] [in French] Ann Pathol. 1987;7:198–208. [PubMed] [Google Scholar]
- 12.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338. doi: 10.1302/0301-620X.81B5.9588. [DOI] [PubMed] [Google Scholar]
- 13.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–172. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 14.Littrell LA, Wenger DE, Wold LE, Bertoni F, Unni KK, White LM, Kandel R, Sundaram M. Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics. 2004;24:1397–1409. doi: 10.1148/rg.245045009. [DOI] [PubMed] [Google Scholar]
- 15.Marco RA, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:292–304. doi: 10.5435/00124635-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Mirra JM, Gold R, Downs J, Eckardt JJ. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones: a clinicopathologic analysis of 51 cases. Clin Orthop Relat Res. 1985;201:214–237. [PubMed] [Google Scholar]
- 17.Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics. 1998;18:1213–1237; quiz 1244–1245. [DOI] [PubMed]
- 18.Reith JD, Horodyski MB, Scarborough MT. Grade 2 chondrosarcoma: stage I or stage II tumor? Clin Orthop Relat Res. 2003;415:45–51. doi: 10.1097/01.blo0000093895.12372.c1. [DOI] [PubMed] [Google Scholar]
- 19.Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;391:224–233. doi: 10.1097/00003086-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Sanerkin NG. The diagnosis and grading of chondrosarcoma of bone: a combined cytologic and histologic approach. Cancer. 1980;45:582–594. doi: 10.1002/1097-0142(19800201)45:3<582::AID-CNCR2820450326>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Schiller AL. Diagnosis of borderline cartilage lesions of bone. Semin Diagn Pathol. 1985;2:42–62. [PubMed] [Google Scholar]
- 22.Springfield DS, Gebhardt MC, McGuire MH. Chondrosarcoma: a review. Instr Course Lect. 1996;45:417–424. [PubMed] [Google Scholar]
- 23.Unni KK. Cartilaginous lesions of bone. J Orthop Sci. 2001;6:457–472. doi: 10.1007/s007760170015. [DOI] [PubMed] [Google Scholar]
- 24.Weiner SD. Enchondroma and chondrosarcoma of bone: clinical, radiologic, and histologic differentiation. Instr Course Lect. 2004;53:645–649. [PubMed] [Google Scholar]