Abstract
Because the initial fixation of an uncemented stem may be compromised in patients with osteoporotic bone (Class C, Dorr et al.), many surgeons prefer a cemented stem in this setting. We therefore determined the survival of an uncemented, proximally porous-coated, straight-stemmed, titanium alloy femoral component in patients with Class C bone when compared with Class A and B bone. We implanted proximally plasma-sprayed, straight-stemmed titanium alloy stems in 1994 patients (2321 hips). Of these, 625 hips (27%), 1569 hips (67%), and 127 hips (6%) were classified as Classes A, B, and C, respectively. Minimum followup was 2 years (mean, 5.9 years; range, 2–19.5 years). We identified no differences in Harris hip scores, pain, radiolucencies, or osteolysis among Classes A, B, and C hips. Stem survival at 5, 10, and 15 years for aseptic loosening (failure) was 100% in all patients with Class A bone; 99+% in all patients with Class B bone; and 100% in all patients with Class C bone. Initial stability and durable fixation can be achieved with the use of this uncemented stem in patients in whom a cemented stem traditionally has been preferred as a result of poor bone quality.
Level of Evidence: Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
As classified by Dorr et al. [4], osteoporotic bone (Class C bone) has considerable cortical thinning and is deficient in medial, anterior, and posterior cortices. Because of poor bone quality and its effect on initial fixation of an uncemented femoral stem, several studies suggest using a cemented femoral component in all three Dorr et al. bone classes (A, B, and C) [5, 14, 15]. However, in Class A, the narrow lateral diaphyseal canal isthmus and in Class B the intact funnel shape of the intramedullary canal allow for long-term desired uncemented implant fixation [4]. On the other hand, Class C bone lacks structural integrity, including osteoporotic degradation of the medial and posterior cortices and a wide intramedullary canal, leading to increased risk of loosening of a fixated implant as a result of compromised bone ingrowth between the bone and implant [4].
With recent improvements in uncemented fixation and developmental progress in the prosthesis structure, including porous coating (eg, plasma-sprayed and hydroxyapatite) [6, 10, 11] and titanium alloy composition [11], several recent groups have suggested the use of cementless femoral components in all three classes of bone [1, 3, 9, 11–13]. One straight-stemmed, porous-coated, plasma-sprayed, titanium alloy uncemented femoral component for primary THA reportedly had a 100% survival rate at 10- to 12-year followup for 105 total hip arthroplasties [11]. That study did not, however, examine survival by the class of bone, and in particular whether survival related to the presence or absence of osteoporosis. The use of titanium alloy and a porous coating help prevent femoral resorption and reduce the loss of cortical density, a common concern among cementless prostheses, especially those interfaced with osteoporotic bone [11].
We therefore hypothesized (1) the survival of an uncemented, proximally porous-coated, plasma-sprayed, straight-stemmed, titanium alloy femoral component in patients with Class C bone is similar to those with Class A and Class B bone; (2) hip function and pain scores after clinical assessment will be similar for all three cohorts; and (3) the frequency of formation of osteolysis and the size of radiolucent zones in these cementless components will not differ in deficient bone (Class C) when compared with healthier bone (Classes A and B).
Patients and Methods
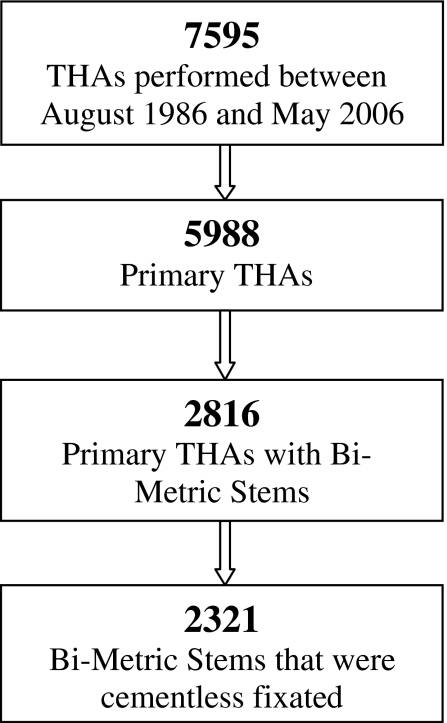
We retrospectively reviewed the records of 1994 patients (2321 hips) in whom we selectively implanted cementless, proximally plasma-sprayed (proximal one-third), porous-coated, straight-stemmed, titanium alloy femoral components (Ti-6Al-4 V) (Bi-Metric; Biomet, Inc, Warsaw, IN) between August 1986 and May 2006. The Bi-Metric stem uses a 3° biplanar taper and is proximally one-third porous plasma spray-coated around titanium alloy (Table 1). The decision to implant the prosthesis uncemented and collared/collarless was by choice of the six participating surgeons (JBM, MAR, RAM, MEB, PFM, EMB). We required a minimum 2-year followup (average, 5.9 years, range, 2–19.5 years). No patients were recalled for the purpose of this study. Four hundred forty-five patients (22%) were lost to followup (Class A, 120 patients; Class B, 299 patients; Class C, 26 patients) (Fig. 1).
Table 1.
Breakdown of femoral component use*
| Number of hips | 2321 |
|---|---|
| Bi-Metric | 718 |
| Bi-Metric lateral offset | 92 |
| Bi-Metric collar | 112 |
| Bi-Metric collarless | 1376 |
| Bi-Metric reduced profile | 23 |
* All implanted with cementless fixation.
Fig. 1.
A breakdown is shown of the formation of the entire study population, including total number of THAs performed at the institute, how many were primary THAs, how many were Bi-Metric primary THAs, and those Bi-Metric stems that were cementless.
Two of us (JBM, MAR) retrospectively evaluated the preoperative radiographs of all hips using the structural assessment of bone quality proposed by Dorr et al. [4]. All hips were assigned a class depending on complete bone quality: A (biologically active), B (moderately active), or C (little to no activity). Six hundred twenty-five hips (533 patients), 1569 hips (1349 patients), and 127 hips (112 patients) were classified as Class A, Class B, and Class C, respectively. We observed no major difference in age, body mass index, gender, preoperative hip score, or preoperative diagnosis (Table 2). In all three cohorts, there were more males in the series than females (Table 2).
Table 2.
Patient demographics for the three Dorr classes
| Bone type | A | B | C | Overall |
|---|---|---|---|---|
| Number of hips | 625 | 1569 | 127 | 2321 (1994 patients) |
| Age* | 60 ± 11 years | 62 ± 13 years | 63 ± 14 years | 61 ± 12 years |
| Body mass index* | 31 ± 5.9 kg/m2 | 29.9 ± 6.1 kg/m2 | 28.5 ± 5.9 kg/m2 | 30.1 ± 6.1 kg/m2 |
| Female (%) | 48.2% | 42.5% | 41.1% | 43.5% |
| Preoperative Harris hip score*,† | 51.1 ± 10.8 | 50.5 ± 11.8 | 49.4 ± 12.6 | 50.6 ± 11.6 |
| Preoperative diagnosis (number of hips) | ||||
| Osteoarthritis | 580 | 1407 | 110 | 2097 |
| Rheumatoid arthritis | 4 | 19 | 0 | 23 |
| Osteonecrosis | 41 | 125 | 11 | 177 |
| Femoral fracture | 0 | 12 | 3 | 15 |
| Other‡ | 0 | 6 | 3 | 9 |
* Displayed as mean ± standard deviation; †no significant difference between cohorts; ‡Paget’s disease = 1 (Dorr C), ankylosing spondylitis = 1 (Dorr B), congenital dysplasia of the hip = 6 (Dorr B = 5, C = 1), fused hip = 1 (Dorr C).
The surgical approach was determined preoperatively by choice of the individual surgeon. We used a posterior approach without trochanteric osteotomy in 400 Class A hips along with 225 Class A hips implanted using an anterolateral surgical approach. In the Class B cohort, 957 hips underwent a posterior approach, 611 hips with an anterolateral approach, and one underwent a lateral approach with a trochanteric osteotomy. Seventy-eight Class C hips were performed with a posterior approach, 48 with an anterolateral approach, and one with a trochanteric osteotomy approach. A 28-mm femoral head was used in all cementless femoral component procedures for the duration of this study.
The postoperative rehabilitation followed a standard institutional routine. Starting on postoperative Day 1, the patient exercised twice a day and walked with either a walker or crutches. The distance walked increased each day. At home, the patient was advised to increase exercises up to five times a day and walk at least 1 hour, as tolerated.
We followed patients at 1, 3, 5, 7, 10, 12, and 15 years postoperatively. At each followup visit, all patients were assessed clinically with the Harris hip score rating system [8].
Two of us (JBM, MAR) analyzed the radiographs (anteroposterior) at each followup for all hips to determine the frequency of femoral osteolysis and the size of the radiolucent zones. Emphasis was applied to the prosthesis-bone interface of Zones 1 and 7 as determined by the seven zones of Gruen [7] as a result of the femoral bone’s potential growth in those regions. Osteolysis was considered present if a subjectively enlarging radiolucent cavity was identified in the periprosthetic bone. Radiographs were evaluated at each followup. We defined aseptic loosening by an increase in the frequency of radiolucency zones (with substantial radiolucency growth greater than 2 mm [3]), widening of the acrylic cement fracture gap, and migration of the component [7].
We determined differences in survivability among the three classes for all patients included in the study using Kaplan-Meier analysis of all patients with failure defined by reoperation for aseptic loosening of the femoral stem. Linear regression testing, including F-tests and individual t-tests, were used to find the differences (F-tests) and comparisons (individual t-tests) of the three classes for Harris hip and pain scores. A Pearson chi square test was run to determine if there was a difference in the frequency of femoral osteolysis among the three bone classes. Statistical analysis was performed with SAS Version 9 (SAS Institute, Inc, Cary, NC).
Results
Survival of the stem was similar for all three classes of bone. For patients with Class A hips (625 hips), after 5-, 10-, and 15-year followup, survivability of the femoral stem, with respect to aseptic loosening, was 100% (135 hips), 100% (37 hips), and 100% (seven hips), respectively. For Class B hips (1569 hips), survivability was 99.9% (308 hips), 99.9% (102 hips), and 99.4% (29 hips), respectively. Two Class B prostheses failed as a result of femoral stem aseptic loosening, both within 7 years postoperatively. Both femoral components were completely revised (revision of both components). One patient experienced failure of the femoral stem as a result of prior radiation. The revision stem was entirely porous-coated leading to ingrowth. The other failure, within 7 years postoperatively, was the result of a grossly undersized implant, which was revised with a larger femoral head and longer stem. No Class C uncemented femoral components (127 hips) were loose at most recent followup, attributing to a survival rating of 100% (32 hips), 100% (12 hips), and 100% (six hips) at 5-, 10-, and 15-year followups (Fig. 2; Table 3). Seven other implants were revised as a result of other surgical and implant problems: two hips for infection (one Class A, one Class B), three for acetabular component loosening (one Class A, two Class B), and three for periprosthetic fracture (two Class A, one Class B). No patients with Class C bone were aseptically loose nor were any revised for implant failure.
Fig. 2.
Fifteen-year survivorship of cementless femoral component in all three classes of hip are shown. Patients with Class C hips had survivorship at 15 years of 100% as did those with Class A bone. Class C bone, with the prescribed implant, maintained long-term survivability at all followup.
Table 3.
Fifteen-year survival probabilities of Dorr classes
| Time | Dorr grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||||
| Probability | 95% confidence interval | Number failed | Number left | Probability | 95% confidence interval | Number failed | Number left | Probability | 95% confidence interval | Number failed | Number left | |
| 1 | 1.0000 | (1.0000, 1.0000) | 0 | 360 | 0.9992 | (0.9942, 0.9999) | 1 | 879 | 1.0000 | (1.0000, 1.0000) | 0 | 78 |
| 3 | 1.0000 | (1.0000, 1.0000) | 0 | 238 | 0.9992 | (0.9942, 0.9999) | 1 | 541 | 1.0000 | (1.0000, 1.0000) | 0 | 53 |
| 5 | 1.0000 | (1.0000, 1.0000) | 0 | 135 | 0.9992 | (0.9942, 0.9999) | 1 | 308 | 1.0000 | (1.0000, 1.0000) | 0 | 32 |
| 7 | 1.0000 | (1.0000, 1.0000) | 0 | 72 | 0.9947 | (0.9716, 0.9990) | 2 | 190 | 1.0000 | (1.0000, 1.0000) | 0 | 20 |
| 10 | 1.0000 | (1.0000, 1.0000) | 0 | 37 | 0.9947 | (0.9716, 0.9990) | 2 | 102 | 1.0000 | (1.0000, 1.0000) | 0 | 12 |
| 12 | 1.0000 | (1.0000, 1.0000) | 0 | 22 | 0.9947 | (0.9716, 0.9990) | 2 | 66 | 1.0000 | (1.0000, 1.0000) | 0 | 11 |
| 15 | 1.0000 | (1.0000, 1.0000) | 0 | 7 | 0.9947 | (0.9716, 0.9990) | 2 | 29 | 1.0000 | (1.0000, 1.0000) | 0 | 6 |
Hip function and pain scores for the three classes were similar. At 1-year postoperatively, the three cohorts had comparably high (A versus B: p = 0.2145, A versus C: p = 0.7124, B versus C: p = 0.7292) Harris hip scores (range, 94.2–94.9) as they also did at final followup (range, 92.8–94.7) (A versus B: p = 0.5366, A versus C: p = 0.9147, B versus C: p = 0.6509) (Table 4). Average pain score (range, 10–44) at final followup was also similar (p = 0.3846) among the three classes (Table 5).
Table 4.
Average Harris hip score at different preoperative and followup periods
| Dorr class | Harris hip score | p Value | ||
|---|---|---|---|---|
| Preoperative | 1-year postoperative | Final followup | ||
| A | 51.1 | 94.9 | 94.3 | < 0.0001 |
| B | 50.5 | 94.2 | 92.8 | |
| C | 49.4 | 94.5 | 94.7 | |
| Overall | 50.6 | 94.4 | 93.2 | |
Table 5.
Average pain score at final followup
| Dorr class | Average pain score (range, 10–44) | p Value |
|---|---|---|
| A | 42.1 ± 5.2 | 0.3846 |
| B | 41.7 ± 6.0 | |
| C | 42.0 ± 6.0 |
We observed a low frequency of osteolysis that was similar (p = 0.8130) among the three bone classes. The overall incidence of osteolysis at last followup of all three classifications was 1.4% (33 of 2321 hips); femoral osteolysis was identified in 10 Class A hips (1.6%), 20 Class B hips (1.3%), and three Class C hips (2.4%). Radiolucent lines were generally small. At most recent followup, two patients (7 years followup) with Class A bone had a nonprogressive radiolucency line measuring less than 1 mm in Zone 1. One patient (3 years followup) had a radiolucency line of less than 1 mm in both Zones 1 and 7, whereas another (1-year followup) had a 3-mm line within Zone 1. In the Class B cohort, two patients (2 and 3 years followup) had a less than 1-mm radiolucency line in Zone 1 and one other patient (2 years followup) had it in Zone 7. One patient (3 years followup) had a less than 1-mm radiolucency line in both zones. Only one Class B bone patient (8 years followup) had a substantial radiolucent progression of 3 mm in Zone 1. One patient with Class C bone had a less than 1-mm radiolucency line in Zone 1 at 3 years followup. We identified no substantial radiolucencies in patients with Class C bone.
Discussion
Aseptic loosening is a major mechanism of failure in TKA with regard to cementless stems [1, 3, 9, 11–13] and depreciated bone [4]. As a result of improved bone ingrowth in new-generation implants, attributed to cementless femoral stems, the fear of aseptic loosening has decreased [11]. Our primary aims were therefore to (1) determine the survivorship of a cementless Bi-Metric femoral component in all three classes of bone, especially those with osteoporotic bone (Dorr et al. [4], Class C), (2) describe the hip function and pain scores, and (3) determine whether the frequency of osteolysis and size of radiolucencies differed among the classes of bone.
The study’s limitations are as follows. First, the study was a retrospective review of a previously collected database. With the study’s intermediate followup of approximately 6 years and a similar Class C case count after 10 years to those reported in literature [1, 2, 12], we were able to attain adequate analysis on implant survivability. Second, we had 22% loss to followup. Third, the surgeries were performed by one group of physicians, all with possible bias in surgical procedure, decision-making, and approaches. We believe with a moderately high number of hips included in the study, the power should overcome potential confounding variables and differences in surgical preference between physicians. Fourth, two surgeons evaluated the patient’s radiographs for bone class, so there may be variation in measurements and observations. The evaluation was by visual observation with Class A hips maintaining a narrow canal isthmus and Class C hips showing substantial osteoporotic degradation of the cortices. This would not alter the conclusions because failures occurred only in Class B hips. These failures, on observation, did not have a narrow canal isthmus (Class A) nor were they depreciated enough to lack any sort of structural integrity (Class B). Despite these limitations, we reviewed a reliable database, medical records, and radiographs to collect a unique set of data that allows us to address the survival of such an uncemented femoral stem in different classifications of femoral bone.
Overall, the survivability and performance of cementless proximally plasma-sprayed, porous-coated, straight-stemmed, titanium alloy femoral stems at a minimum of 2 years followup was excellent in all three bone classes. Within the study, there were only two patients who experienced loosening of their cementless femoral stems, which resulted in revision of the primary THA, and both were among those in the Class B cohort. No Class A or C hip implants were reported as being aseptically loose at most recent followup. These low failure rates resulting from overall complications and aseptic loosening, along with low incidence of femoral osteolysis, are similar to those reported in the literature (Table 6).
Table 6.
Comparison of this study to cementless femoral component studies in the current literature
| Author (year) | Cementless femoral component design | Hips (number) | Type C hips (number) | Age (years) | Followup (years) | Harris hip score at last followup | Aseptic loosening failure rate (%) | Overall femoral failure rate (%) | Femoral component survival rate for loosening (%) | Femoral osteolysis rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Burt et al. [2] (1998) | Tri-Lock, proximal porous-coated, tapered Cr | 47 | 62 | 10.0 | 91.5 | 2.2% | 8.5% | 93% at 10 years for radiographic loosening | 11% | |
| Morrey [12] (1989) | 60-mm short-stem with high valgus neck | 20 | 2.2 | 97.8 | 5% | |||||
| Parvizi et al. [13] (2004) | Taperloc tapered | 129 | 60.8 | 11.0 | 92.1 | 0% | 0.7% | 99.1 at 8 years (best-case scenario) | 2.3% | |
| Berend et al. [1] (2004) | Mallory-head, double-tapered | 49 | 10 | 79 | 5.0 | 84.0 | 0% | 2.04% | 98% at 5 years | 0%, distal, 2.3% focal |
| Hozack et al. [9] (1996) | Ti-alloy, plasma-sprayed | 105 | 5 | 61.2 | 6.1 | 88.6 | 0% | 1% | 0% distal, 5% focal | |
| Meding et al. [current study] | Titanium-alloy, straight-stem, porous-coated | 2321 | 127 | 61 | 5.9 | 93.2 | 0.0008% | 0.002% | 99.6 at 15 years for aseptic loosening | 1.4% |
By means of clinical assessment (eg, Harris hip score and pain) and radiographic analysis, all three classes showed high clinical scores and well-intact implant condition with no difference among biologically active (Class A), moderately active (Class B), and poorly active bone (Class C). Kelly et al. [10] reported similarly high clinical scores (median Harris hip score, 94.5) and no femoral fixation failures in 15 Class C bone patients with a minimum of a 9-year followup (average, 11.5 years; range, 9–14 years) and the use of a hydroxyapatite-coated cemented stem. While they had a longer mean followup, our data, with 127 patients with Class C bone confirm their findings.
Although our study’s demographics are similar, especially in regard to age and body mass index, to those reported by Dorr et al. [4], we saw no major difference among the three classifications of bone with consideration to clinical and radiographic success and adequate results with regard to the morphologically depreciated Class C bone. This could be attributed to numerous surgical reasons that not only affect clinical outcome, but also the formation of femoral osteolysis. Our study’s 1.4% incidence of femoral osteolysis is similar to those reported by Parvizi et al. [13] (2.3%) and Berend et al. [1] (0% distal, 2.3% focal). Our institutional standardized procedure of using a proximally coated porous coating along with a titanium alloy component allowed for an improved prosthesis-bone interface, which in turn increased bone ingrowth and promoted implant stability. Several other studies have reported surgical practices that have improved the way cementless femoral components are implemented in THA, including plasma spray (eg, hydroxyapatite) of the femoral stem [10], a straight-stem and/or short-stem component design [11, 12], and the addition of proximal-to-distal coronal and sagittal tapers [1]. The obstacle of finding an adequate prosthesis design to prevent aseptic loosening in osteoporotic bone (Class C) was addressed with the use of a collarless stem, which has been reported to reduce the loss of cortical density of the metadiaphyseal bone [11]. By maintaining the cortical density, the bone is able to maintain initial fixation between it and the prosthesis, creating an interface that promotes implant stability [11].
We observed radiolucencies greater than 2 mm in two hips: one Class A and one Class B. Although relatively low in cohort size (127 hips), the Class C hips had no radiographic evidence of progression of radiolucency in both growth regions (Zones 1 and 7). We observed femoral osteolysis was identified in 33 of the 2321 hips (1.4%) with no difference in the frequency of osteolysis among the three cohorts. As suggested in our previous study [11], we presumed circumferential porous coating obstructs the movement of particles within the intramedullary canal, which in turn prevents the formation of lytic lesions. We believe this “sealing of the femoral canal” allows for cementless fixation of the femoral stem [6] to decrease the chance of osteolysis formation without other means of fixation such as cement, which could compromise function and increase radiolucency.
With recent alterations in implant composition, cementless stem fixation, and porous coating to enhance bone ingrowth, the use of cementless stems, including those with osteoporotic bone (Class C), has greatly increased [1, 3, 6, 9–13]. We reviewed a large population of a cementless proximally porous-coated, straight-stemmed, titanium alloy component in patients with Class A, B, and C bone showing secure long-term fixation along with adequate clinical and radiographic assessment.
Acknowledgments
We thank Dr Michael E. Berend, Dr Robert A. Malinzak, Dr E. Michael Keating, Dr Philip M. Faris, and Matthew J. Brunsman, MS, for their assistance.
Footnotes
The foundation of the authors (Joint Replacement Surgeons of Indiana) has received funding from Biomet, Inc (Warsaw, IN).
Each author certifies that his institution has approved human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Berend KR, Lombardi AV, Mallory TH, Dodds KL, Adams JB. Cementless double-tapered total hip arthroplasty in patients 75 years of age and older. J Arthroplasty. 2004;19:288–295. doi: 10.1016/j.arth.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Burt CF, Garvin KL, Otterberg ET, Jardon OM. A femoral component inserted without cement in total hip arthroplasty. A study of the Tri-Lock component with an average ten-year duration of follow-up. J Bone Joint Surg Am. 1998;80:952–960. doi: 10.2106/00004623-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Clohisy JC, Harris WH. The Harris-Galante uncemented femoral component in primary total hip replacement at 10 years. J Arthroplasty. 1999;14:915–917. doi: 10.1016/S0883-5403(99)90003-7. [DOI] [PubMed] [Google Scholar]
- 4.Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242. doi: 10.1016/8756-3282(93)90146-2. [DOI] [PubMed] [Google Scholar]
- 5.Edidin AA, Merritt PO, Hack BH, Manley MT. A ported, proximally-cemented femoral stem for total hip arthroplasty. Development and clinical application. J Bone Joint Surg Br. 1998;80:869–875. doi: 10.1302/0301-620X.80B5.8670. [DOI] [PubMed] [Google Scholar]
- 6.Emerson RH, Sanders SB, Head WC, Higgins L. Effect of circumferential plasma-spray porous coating on the rate of femoral osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1291–1298. doi: 10.2106/00004623-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 8.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 9.Hozack WJ, Rothman RH, Eng K, Mesa J. Primary cementless hip arthroplasty with a titanium plasma sprayed prosthesis. Clin Orthop Relat Res. 1996;333:217–225. doi: 10.1097/00003086-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Kelly SJ, Robbins CE, Bierbaum BE, Bono JV, Ward DM. Use of a hydroxyapatite-coated stem in patients with Class C femoral bone. Clin Orthop Relat Res. 2007;465:112–116. doi: 10.1097/BLO.0b013e318156bf96. [DOI] [PubMed] [Google Scholar]
- 11.Meding JB, Keating EM, Ritter MA, Faris PM, Berend ME. Minimum ten-year follow-up of a straight-stemmed, plasma-sprayed, titanium-alloy, uncemented femoral component in primary total hip arthroplasty. J Bone Joint Surg Am. 2004;86:92–97. doi: 10.2106/00004623-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Morrey BF. Short-stemmed uncemented femoral component for primary hip arthroplasty. Clin Orthop Relat Res. 1989;249:169–175. [PubMed] [Google Scholar]
- 13.Parvizi J, Keisu KS, Hozack WJ, Sharkey PF, Rothman RH. Primary total hip arthroplasty with an uncemented femoral component: a long-term study of the Taperloc stem. J Arthroplasty. 2004;19:151–156. doi: 10.1016/j.arth.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rasquinha VJ, Ranawat CS. Durability of the cemented femoral stem in patients 60 to 80 years old. Clin Orthop Relat Res. 2004;419:115–223. doi: 10.1097/00003086-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Williams HD, Browne G, Gie GA, Ling RS, Timperley AJ, Wendover NA. The Exeter universal cemented femoral component at 8 to 12 years. A study of the first 325 hips. J Bone Joint Surg Br. 2002;84:324–334. doi: 10.1302/0301-620X.84B3.12261. [DOI] [PubMed] [Google Scholar]