Abstract
A bone-conserving prosthetic solution, such as hip resurfacing arthroplasty, is desirable for patients with osteonecrosis (ON) of the femoral head because of their young age. However, many surgeons are reluctant to perform hip resurfacing for ON because of large femoral head defects. To ascertain whether this reluctance is warranted, we determined the mid- to long-term effects of ON on the survivorship, radiographic implant fixation, and disease-specific and quality-of-life scores of hip resurfacing. We compared the results of metal-on-metal resurfacing performed for ON of the hip (including large lesions) with those of resurfacing performed for other causes. The ON group had 70 patients (85 hips) and the control group 768 patients (915 hips) including all other etiologies operated on during the same period. The ON group was younger and had a greater incidence of femoral defects, a smaller component size, and a lower body mass index, three variables previously shown to reduce survivorship in hip resurfacing. We observed no difference in survivorship between the ON group and the control group even after adjusting for head size, body mass index, and defect size. Pain relief, walking, and function scores were comparable postoperatively. The activity level was lower in the ON group. Our data suggest ON is not a contraindication for resurfacing even with large femoral head defects.
Level of Evidence: Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The treatment of patients with osteonecrosis (ON) of the hip remains controversial when the natural joint cannot be salvaged. Conventional THA, hemiresurfacing, and now full metal-on-metal hip resurfacing are the current prosthetic options. THA has so far been the treatment of choice for most patients, especially when the progression of the disease has reached Ficat Stage IV. Several reports suggest the clinical scores, radiographic outcome, and survivorship of patients with ON are comparable with those of other etiologies [16, 21, 27], whereas others indicate conventional THA does not perform as well in patients with ON, in particular those younger than 50 years of age [12, 14, 24, 25, 28]. Hemiresurfacing has been previously reported for Ficat Stage II or III as a “time-buying operation” with variable results [1, 11, 15, 18]. Recently, full hip resurfacing has become an alternative to both THA and hemiresurfacing in young patients. Two recent reports suggested good short- to midterm functional scores and survivorship can be achieved with hip resurfacing [23, 26], although one report questioned whether the survivorship was comparable to that of patients with osteoarthritis [22]. Also, the indications for hip resurfacing in these three reports were limited to hips with a lesion smaller than one-third of the femoral head.
Is ON a contraindication for metal-on-metal hip resurfacing? Given the apparent discrepancies in the literature, we compared the (1) survivorship; (2) activity and SF-12 scores; (3) component abduction angles; (4) radiolucencies; and (5) complications in patients with metal-on-metal hybrid resurfacing performed for ON and for other etiologies.
Patients and Methods
We retrospectively reviewed all 838 patients (1000 hips) treated with a Conserve® Plus metal-on-metal resurfacing device (Wright Medical Technology Inc, Arlington, TN) between 1996 and 2006. Seventy patients (85 hips) underwent resurfacing for arthritis secondary to ON of the femoral head, whereas 768 (915 hips) had the procedure for all other indications. We selected patients with ON Ficat Stage III or greater and older than age 35 years unless cartilage damage was Grade III or greater [11]; we included patients with large lesions as long as the cylindrically reamed bone was intact (Fig. 1). The decision for resurfacing was never changed during surgery to perform a THA, although the defect was often larger than anticipated based on radiographic analysis. During this same time period, we performed 30 hemiresurfacing procedures for 24 patients with ON whose acetabular cartilage was sufficiently preserved and 16 primary THAs in 15 patients with ON who did not receive a resurfacing device for one of the following reasons: (1) femoral neck nonunion after pinning; (2) insurance denial; (3) patient choice of prosthetic device; or (4) femoral head defects too large to perform the surgery. The risk factors for the development of ON were diverse in the study group and included steroids (31 hips [36%]), trauma (19 hips [22%]), alcohol (six hips [7%]), and sickle cell disease (one [1%]). Twenty-eight hips (33%) had no apparent risk factor (idiopathic ON). There were 19 hips rated ON Ficat Stage III and 66 rated Ficat Stage IV. The average age of the patients with ON was 40.1 years (range, 14–61 years). Most of the patients were male (57 of 70 [81.4%]). Thirty-three of the 70 patients (47.1%) had bilateral disease. Twenty-eight hips (32.9%) had undergone at least one previous surgery; 17 had core decompression, three had hemiresurfacing, five had been pinned, two had previous free vascularized fibula graft, and one had a Judet graft. The control group was composed of the remaining 768 patients (915 hips) with the following etiologies for surgery: osteoarthritis (n = 696), developmental dysplasia of the hip (n = 103), posttraumatic arthritis (n = 40), childhood disorders (n = 42), inflammatory (n = 29), and others (n = 5). The minimum followup time for the ON group was 2.2 years (mean, 7.6 years; range, 2.2–12.0 years) and 2.2 years (mean, 6.4 years; range, 2.2–12.0 years) for the control group. Eight patients (two bilateral) died during the followup period of causes not related to the procedure (one in the ON group, seven in the control group). Four patients were lost to followup, one in the ON group and three in the control group. The patients with ON were younger, had a smaller mean component size, and had more often femoral head defects greater than 1 cm compared with the patients of any other etiologies (Table 1). In the ON group, six hips (7.1%) presented no femoral head defects, seven (8.2%) had defects 0 to 1 cm in size, 37 (43.5%) had defects 1 to 2 cm in size, and 35 (41.2%) had defects 2 to 3 cm in size.
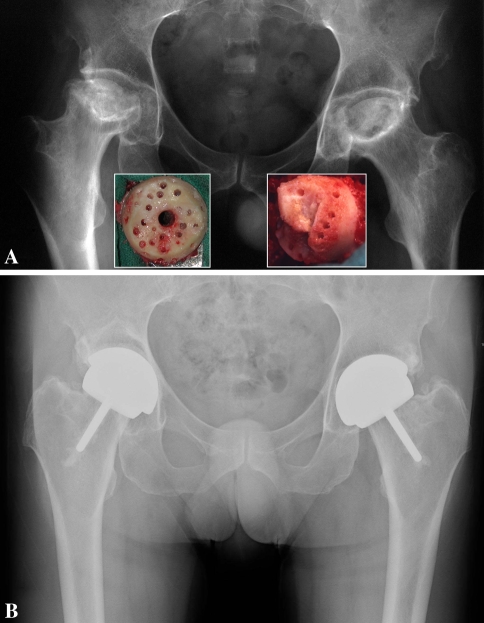
Fig. 1A–B.
(A) Anteroposterior radiograph of a 52-year-old man with bilateral steroid-induced Ficat Stage IV osteonecrosis. The insets show the extent of the femoral defects after removal of the necrotic lesions. In this case, the length of the neck could not be maintained because the femoral defects were too large to preserve a part of the chamfered area. (B) Eight years after resurfacing, the components are securely fixed and the patient’s UCLA hip scores are 10, 10, 10, and 7 for pain, walking, function, and activity, respectively.
Table 1.
Comparative demographics of the patients operated on for arthritis secondary to osteonecrosis (ON group) and those operated on for arthritis secondary to other etiologies (control group)
| Demographic variables | ON group (70 patients, 85 hips) | Control group (768 patients, 915 hips) | p |
|---|---|---|---|
| Age (years) | 40.1 (range, 14–61) | 50.9 (range, 15–78) | 0.0001 |
| Weight (kg) | 80.8 (range, 46–114) | 83.5 (range, 42–164) | 0.1586 |
| Height (cm) | 176.3 (range, 148–198) | 175.4 (range, 140–203) | 0.4054 |
| Body mass index (kg/m2) | 25.8 (range, 17–38) | 27.0 (range, 17–46) | 0.0143 |
| Femoral component size (mm) | 46.3 (range, 36–54) | 47.6 (range, 36–56) | 0.0045 |
| Hips with femoral head defects greater than 1 cm | 70 (82.4%) | 281 (30.7%) | 0.0001 |
| Male/female ratio | 57/13 (81.4%/18.6%) | 560/208 (72.9%/27.1%) | 0.1218 |
| Charnley class | |||
| A | 34 (48.6%) | 475 (61.8%) | 0.1724 |
| B | 29 (41.4%) | 250 (32.6.6%) | 0.2179 |
| C | 7 (10.0%) | 43 (5.6%) | 0.1490 |
The fundamentals of the operative technique used for this series have been described in previous publications [2, 4, 10]. Removal of all of the dead, yellowish, friable necrotic bone down to the normal or dense white reactive bone was achieved by alternating burring, irrigation, and drying and, in many cases, led to substantial loss of the head. Improvements in the surgical technique were made over time, which have been previously described [5, 8]. However, by the time Hip 37 was implanted in the ON group and Hip 265 in the control group, the most important modifications had been made (ie, use of dome suction and additional drill holes in the chamfered section). The essence of our current technique includes meticulous removal of all cystic debris with a high-speed burr, multiple small (1/8th-inch) drill holes in the dome, and chamfered areas to maximize surface area for acrylic cementation, cleansing with pulsatile lavage, and thorough drying, now with the aid of a Carbo-Jet® (Kinamed Inc, Camarillo, CA). Because some portion of the head is typically viable up to the chamfered area, the head length is generally preserved. In our opinion, for the osteonecrotic group, the most important change was the pressurization of doughy cement into the defects located in the cylindrical portion of the head before insertion of the component. Fixation was accomplished with a 1-mm cement mantle. The regular viscosity acrylic cement coated the inside of the component and the component was pressed on manually or inserted with light mallet taps, whereas the excess of cement was completely extruded until the component was completely seated. We used bone grafting in eight of the 70 hips (11%) with ON, distal to the head, to fill defects from previous fixation devices or fibular grafts. Defects in the head were grafted in one case. In the ON group, four patients had contralateral hemiresurfacing, 16 had bilateral full metal-on-metal resurfacing (one of them with a device from another manufacturer), three had contralateral conventional THA, and seven had undergone contralateral core decompression. We implanted a higher percent (p = 0.073) of cemented femoral metaphyseal stems in hips with ON than with other indications (44 of 85 hips [52%] versus 356 of 915 [39%]). We currently cement the stem in hips with a femoral head size 46 mm or lower and hips with femoral head defects greater than 1 cm [6], conditions that include nearly all of the ON cases that were routinely cemented since March 2000.
We used the UCLA hip scoring system [9] to evaluate disease-specific patient progress and the SF-12 [29] as an assessment of quality of life. The postoperative Harris hip score was also calculated [19]. Range-of-motion measurements were recorded at each followup visit.
Pre- and postoperative radiographs were available for all patients except one lost to followup. Two of us (MJL, HCA) determined the size of the necrotic lesion from the preoperative anteroposterior radiograph as described by Revell et al. [26]. The size of the lesion was measured on the anteroposterior radiograph as the angle defined from the center of the femoral head to the outer limits of the necrotic lesion. The mean head involvement angle was 121.1° (range, 75°–202°). In this assessment, we did not include any measurements for the three hips that had undergone prior hemiresurfacing. Postoperatively, one of us (MJL) measured the metaphyseal stem-femoral shaft angle and the lateral opening of the socket. Femoral and acetabular radiolucencies were recorded as previously described [3]. We (HCA) used the Brooker et al. [13] grading system to assess heterotopic ossification.
We compared the preoperative and postoperative scores of the two groups using the Mann-Whitney U tests. Time-dependent analyses were made using standard Kaplan-Meier survivorship techniques using the time to revision for any reason and the time to revision for femoral failure only. The Cox proportional hazard model was used to determine the effect of a diagnosis of ON on the risk of revision, adjusting for femoral head size, patient body mass index, and the presence of femoral head defects greater than 1 cm because these variables differed between the two groups (Table 1) and are known to affect prosthetic survival in hip resurfacing [5, 20]. All time-dependent analyses were made using Stata (Stata Corporation, College Station, TX).
Results
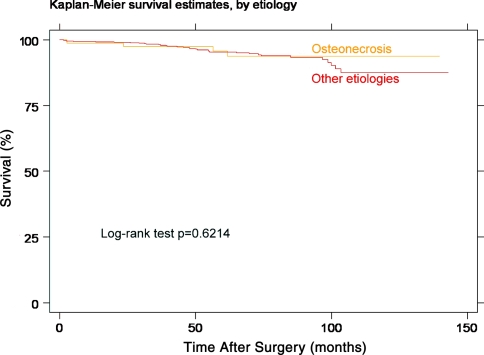
We revised four hips, including three conversions to THA in the ON group. Two were performed for loosening of the femoral component, one at 23 months related to an incomplete seating of the component [7] and the other 61 months after surgery (Fig. 2). In none of these two femoral components had the metaphyseal stem been cemented. Both hips were converted to a THA, leaving the acetabular component in situ. Both of these patients were operated on early in our series (ON Patients 1 and 7). Loosening of the acetabular component caused the third conversion. This patient had some intermittent activity-related symptoms approximately 2 years after surgery. The component was loose when the hip was revised at another institution 56 months after surgery. In addition, extensive bone grafting and revision of the acetabular component were needed after overreaming of the first hip in a patient undergoing a one-stage bilateral procedure. The component had protruded through the acetabular wall. The revision took place 3 days after surgery. There were 35 revisions in the control group. We observed no difference (p = 0.6214) in survivorship between the two groups (Table 2; Fig. 3). Using femoral failure only as end point, the Kaplan-Meier survivorship at 5 years was 98.7% (95% confidence interval [CI], 90.9–99.8) and 96.8% at 8 years (95% CI, 87.4–99.2). We found no difference (p = 0.446) between the ON and control groups after adjusting for femoral head size, femoral defect size, and body mass index (Table 3).
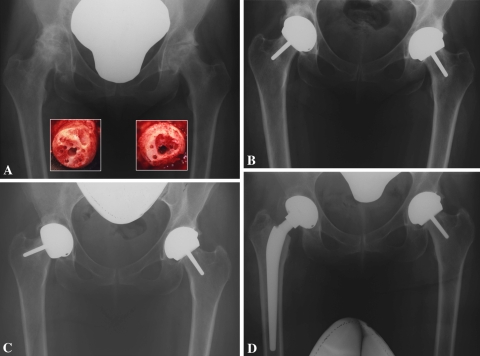
Fig. 2A–D.
(A) Anteroposterior radiograph of a 49-year-old woman with bilateral alcohol-induced osteonecrosis of the hips, Ficat Stage IV. The insets show the femoral heads after preparation for resurfacing. (B) The patient underwent two-stage bilateral resurfacing and is shown 6 months after her right-sided operation (the component was placed in relative varus with the metaphyseal stem left uncemented) and 3 months after the left. (C) Five years after surgery, the femoral component loosened on the right side and tipped into further varus. (D) Conversion to THA; the well-fixed acetabular component was left in situ and the femoral component replaced with an ATH long stem grit-blasted and a 40-mm unipolar head.
Table 2.
Kaplan-Meier survivorship results computed using the time to revision for any reason as the end point
| Followup | Osteonecrosis group | Control group | ||
|---|---|---|---|---|
| Survivorship | 95% confidence interval | Survivorship | 95% confidence interval | |
| 3 years | 97.5% | 90.3%–99.4% | 98.4% | 97.1%–99.1% |
| 5 years | 95.7% | 87.0%–98.6% | 95.4% | 93.1%–96.9% |
| 8 years | 93.9% | 84.1%–97.7% | 93.4% | 90.4%–95.5% |
Fig. 3.
Comparative Kaplan-Meier survivorship curves of the osteonecrosis group and the rest of the cohort. The time to revision for any reason was used as the end point. The two groups of patients had similar survivorship.
Table 3.
Summary of multivariate analysis using the Cox proportional hazard ratio
| Variable studied | Hazard ratio | p | 95% confidence interval |
|---|---|---|---|
| Etiology (osteonecrosis versus others) | 1.50055 | 0.446 | 0.527873–4.265517 |
| Femoral head size | 0.9019847 | 0.012 | 0.832421–0.9773617 |
| Body mass index | 0.9188939 | 0.063 | 0.84046–1.004648 |
| Femoral defect size | 0.8499815 | 0.308 | 0.6219586–1.161602 |
Preoperatively, the patients from the ON group had lower walking, function, and activity scores than the patients from the control group as well as lower preoperative mental scores of the SF-12 survey (Table 4). At last followup, the mean activity score and mental component of the SF-12 were lower in the ON group compared with the rest of the cohort (7.0 versus 7.5, p = 0.0084 for the former and 49.0 versus 53.7, p = 0.0000 for the latter). In the ON group, two patients had low walking and function scores postoperatively. One was Charnley Class C and the other had lower back pain. The preoperative range of motion of the patients with ON was larger than that of the rest of the cohort in flexion and rotation. Postoperatively, the hip range-of-motion measurements were comparable between the two groups (Table 5).
Table 4.
Mean (range) preoperative (when available) and postoperative values of the clinical scores used in the study
| Outcome scores | Preoperative scores | Postoperative scores | ||||
|---|---|---|---|---|---|---|
| ON group | Control group | p | ON group | Control group | p | |
| UCLA | ||||||
| Pain | 3.5 (1–8) | 3.6 (1–8) | 0.0904 | 9.3 (6–10) | 9.4 (2–10) | 0.6683 |
| Walking | 5.8 (2–10) | 6.5 (2–10) | 0.0000 | 9.6 (4–10) | 9.6 (3–10) | 0.7084 |
| Function | 5.2 (1–9) | 5.9 (1–10) | 0.0002 | 9.4 (4–10) | 9.5 (3–10) | 0.6008 |
| Activity | 4.4 (1–8) | 4.8 (1–10) | 0.0289 | 7.0 (3–10) | 7.5 (2–10) | 0.0099 |
| Harris hip score | — | — | — | 91.3 (42–100) | 93.5 (41–100) | 0.1129 |
| SF-12 | ||||||
| Physical | 31.5 (18.9–56.8) | 33.0 (6.0–56.5) | 0.0784 | 49.2 (22.2–61.4) | 50.9 (17.1–62.1) | 0.1735 |
| Mental | 43.8 (12.9–66.8) | 48.4 (4.0–68.5) | 0.0011 | 49.0 (14.7–64.2) | 53.7 (10.5–66.1) | 0.0004 |
ON = osteonecrosis.
Table 5.
Comparative mean preoperative and postoperative range-of-motion measurements for each group*
| Range of motion measurement | Osteonecrosis group (n = 85) | Control group (n = 915) | p |
|---|---|---|---|
| Preoperative flexion | 111 | 105.1 | 0.0027 |
| Preoperative flexion contracture | 13.9 | 16.4 | 0.1066 |
| Preoperative abduction (in extension) | 23.9 | 23.1 | 0.6383 |
| Preoperative adduction (in extension) | 16.4 | 14.3 | 0.0747 |
| Preoperative rotation arc (in extension) | 29.5 | 23 | 0.004 |
| Postoperative flexion | 127.1 | 126 | 0.4976 |
| Postoperative flexion contracture | 1.3 | 1.7 | 0.4997 |
| Postoperative abduction (in extension) | 46.4 | 45.3 | 0.2752 |
| Postoperative adduction (in extension) | 28.9 | 28 | 0.2731 |
| Postoperative rotation arc (in extension) | 79.4 | 77.1 | 0.2949 |
* Modified with permission from Amstutz H, Le Duff M, Boitano P. Osteonecrosis of the hip. In: Amstutz HC, ed. Hip Resurfacing: Principles, Indications, Technique and Results. Philadelphia: Elsevier; 2008:161–180. © Elsevier 2008.
Postoperatively, the mean acetabular component abduction angle was greater (p = 0.0247) in the ON group than the control group (42.3°; range, 24°–60° versus 44.0°; range, 20°–70°, respectively). The mean stem shaft angle was similar (p = 0.2136) in the two groups (137.4°; range, 110°–158° versus 138.5°; range, 111°–163°, respectively).
The percent of metaphyseal stem radiolucencies of Level 7 or greater (visible on all three zones) was similar (p = 0.0595) in the two groups (three hips in the ON group and in 10 in the control group). However, none of these three radiolucencies are associated with clinical symptoms and all appear “stable” at an average of 7.2 years (range, 6.9–7.4 years) after these were first identified. One patient from the ON group formed Brooker et al. Grade III heterotopic ossification. The prevalence of heterotopic ossification Brooker et al. Grade III or IV was similar (p = 0.2313) in the two groups [13].
There were no postoperative neck fractures, dislocations, nerve palsies, or thromboembolic episodes in the ON group. The control group counted nine fractures of the femoral neck (1.0%), 11 dislocations (1.2%), 17 nerve palsies (1.9%), and five thromboembolic adverse events (0.6%).
Discussion
Hip resurfacing is becoming a popular prosthetic solution for young patients, but previous reports at short- to midterm followup disagree on the appropriateness of performing hip resurfacing for patients with ON [22, 23, 26]. There is a need for mid- to long-term clinical data to determine whether ON is a contraindication for hip resurfacing. Given the apparent discrepancies in the literature, we compared the (1) survivorship; (2) activity and SF-12 scores; (3) component abduction angles; (4) radiolucencies; and (5) complications in patients with metal-on-metal hybrid resurfacing performed for ON and for other etiologies.
A limitation of this study is that the study group included hips with posttraumatic ON, which could be considered a different etiology. However, patients with posttraumatic ON present common challenges to those of other ON etiologies to the surgeon performing hip resurfacing, in particular the presence of large femoral head defects. The main difference consisted of the presence of femoral neck defects because of the need to remove internal fixation and the use of bone graft below the head in the posttraumatic group. We otherwise observed no major differences pre- or postoperatively between these hips and those that developed ON for other reasons.
We found the survivorship of metal-on-metal hip resurfacing in patients with ON as good as the survivorship of patients having resurfacing for other causes. This observation is in contrast with the findings of one study reporting a different type of resurfacing device for the treatment of patients with hip ON [22]. In that series, the authors reported a 6.7% failure rate at 4.5 years of average followup and were concerned that failures were the result of advancing necrosis but did not provide supporting data. In addition, the hips included in the present study were not screened based on the size of the osteonecrotic femoral head lesion and our results show proper surgical technique allows the successful resurfacing of femoral heads with large necrotic involvement. We believe our observations relate to proper bone preparation and cementing techniques rather than implant design [22] as the keys to successful hip resurfacing in patients with ON. Our findings complement other shorter-term favorable reports of metal-on-metal resurfacing used for the treatment of hip ON [23, 26] and also compare favorably to those in one report of THA for ON at similar outcome times [24]. We previously reported improvements in our overall survivorship for all etiologies associated with improvements in surgical technique [5, 8]. Even with our “early technique,” there have been no revisions for femoral failure in hips resurfaced for ON in the last 10 years. We wondered why the survivorship has been high given the magnitude of the femoral head defects in this series. We believe pressurization of doughy cement into the defects located in the cylindrically reamed area is certainly important to the success. Also, a review of the intraoperative photographs and operative report indicated our bone preparation always included thorough removal of all of the necrotic bone and cystic material with a high-speed burr before this practice was introduced on a routine basis for other etiologies. Gill et al. recently expressed concern regarding the use of acrylic to fill defects and grout for the femoral component because of the possibility for the heat to trigger bone necrosis [17]. We observed no adverse clinical or radiographic effects that could be attributed to necrosis secondary to the procedure. We earlier reported no loosenings or radiolucencies in 46 hips in which the stem was cemented [6].
We found no differences in postoperative pain, walking, and function scores between our two groups, an observation that supports the findings of Mont et al. [23] who compared patients with ON and those with primary osteoarthritis at a shorter followup time. This suggests patients with ON experience the same benefits as the patients with other etiologies after resurfacing, despite having lower preoperative walking and function scores.
Patients with ON of the hip present specific challenges because of the often present large defects filled initially with extensive yellowish, friable necrotic bone. This necrotic bone must be completely removed down to the underlying white hard reparative bone to ensure proper component fixation and durability. Patients with large defects that have been filled with cement should not, in our opinion, engage in impact activities if optimal durability of the prosthesis is to be achieved. Our results suggest the etiology of ON itself does not constitute a contraindication for resurfacing and the risk factors for the procedure are similar to those of other etiologies provided complete cleaning of the necrotic bone and area for fixation was performed.
Footnotes
One or more of the authors (HCA) received funding from St Vincent Medical Center, Los Angeles, and Wright Medical Technologies Inc.
This study was approved by the Institutional Review Board of St Vincent Medical Center, Los Angeles.
References
- 1.Amstutz H. The future of hip resurfacing. In: Amstutz HC, editor. Hip Resurfacing: Principles, Indications, Technique and Results. Philadelphia, PA: Elsevier; 2008. pp. 253–254. [Google Scholar]
- 2.Amstutz H. Surgical technique. In: Amstutz HC, editor. Hip Resurfacing: Principles, Indications, Technique and Results. Philadelphia, PA: Elsevier; 2008. pp. 77–94. [Google Scholar]
- 3.Amstutz H, Beaulé P, Dorey F, Duff M, Campbell P, Gruen T. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 4.Amstutz H, Beaulé P, Dorey F, Duff M, Campbell P, Gruen T. Metal-on-metal hybrid surface arthroplasty. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1):234–249. doi: 10.2106/JBJS.F.00273. [DOI] [PubMed] [Google Scholar]
- 5.Amstutz H, Duff M. Eleven years of experience with metal-on-metal hybrid hip resurfacing: a review of 1000 conserve plus. J Arthroplasty. 2008;23(Suppl 1):36–43. doi: 10.1016/j.arth.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Amstutz H, Duff M. Cementing the metaphyseal stem in metal-on-metal resurfacing: when and why. Clin Orthop Relat Res. 2009;467:79–83. doi: 10.1007/s11999-008-0570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amstutz H, Duff M, Boitano P. Osteonecrosis of the hip. In: Amstutz HC, editor. Hip Resurfacing: Principles, Indications, Technique and Results. Philadelphia, PA: Elsevier; 2008. pp. 161–180. [Google Scholar]
- 8.Amstutz H, Duff M, Campbell P, Dorey F. The effects of technique changes on aseptic loosening of the femoral component in hip resurfacing. Results of 600 Conserve Plus with a 3–9 year follow-up. J Arthroplasty. 2007;22:481–489. doi: 10.1016/j.arth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Amstutz H, Thomas B, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241. [PubMed] [Google Scholar]
- 10.Beaulé P, Amstutz H. Surface arthroplasty of the hip revisited: current indications and surgical technique. In: Singha R, editor. Hip Replacement: Current Trends and Controversies. New York, NY: Marcel Dekker; 2002. pp. 261–297. [Google Scholar]
- 11.Beaulé P, Schmalzried T, Campbell P, Dorey F, Amstutz H. Duration of symptoms and outcome of hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res. 2001;385:104–117. doi: 10.1097/00003086-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Brinker M, Rosenberg A, Kull L, Galante J. Primary total hip arthroplasty using noncemented porous-coated femoral components in patients with osteonecrosis of the femoral head. J Arthroplasty. 1994;9:457–468. doi: 10.1016/0883-5403(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 13.Brooker A, Bowerman J, Robinson R, Riley L. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 14.Chiu KH, Shen WY, Ko CK, Chan KM. Osteonecrosis of the femoral head treated with cementless total hip arthroplasty. A comparison with other diagnoses. J Arthroplasty. 1997;12:683–688. doi: 10.1016/S0883-5403(97)90142-X. [DOI] [PubMed] [Google Scholar]
- 15.Cuckler J, Moore K, Estrada L. Outcome of hemiresurfacing in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2004;429:146–150. doi: 10.1097/01.blo.0000150121.88033.50. [DOI] [PubMed] [Google Scholar]
- 16.Dastane M, Long W, Wan Z, Chao L, Dorr L. Metal-on-metal hip arthroplasty does equally well in osteonecrosis and osteoarthritis. Clin Orthop Relat Res. 2008;466:1148–1153. doi: 10.1007/s11999-008-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill H, Campbell P, Murray D, Smet K. Reduction of the potential for thermal damage during hip resurfacing. J Bone Joint Surg Br. 2007;89:16–20. doi: 10.1302/0301-620X.89B1.18369. [DOI] [PubMed] [Google Scholar]
- 18.Grecula M, Thomas J, Kreuzer S. Impact of implant design on femoral head hemiresurfacing arthroplasty. Clin Orthop Relat Res. 2004;418:41–47. doi: 10.1097/00003086-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 20.Duff M, Amstutz H, Dorey F. Metal-on-metal hip resurfacing for obese patients. J Bone Joint Surg Am. 2007;89:2705–2711. doi: 10.2106/JBJS.F.01563. [DOI] [PubMed] [Google Scholar]
- 21.Letson GD, D’Ambrosia RD, Aguilar EA, Wagespack A. Activity relationships of total hip arthroplasty in patients with osteonecrosis and osteoarthritis. Orthopedics. 1996;19:665–668. doi: 10.3928/0147-7447-19960801-10. [DOI] [PubMed] [Google Scholar]
- 22.McMinn D, Daniel J, Pradhan C, Ziaee H. Avascular necrosis in the young patient: a trilogy of arthroplasty options. Orthopedics. 2005;28:945–947. doi: 10.3928/0147-7447-20050901-19. [DOI] [PubMed] [Google Scholar]
- 23.Mont M, Seyler T, Marker D, Marulanda G, Delanois R. Use of metal-on-metal total hip resurfacing for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):90–97. doi: 10.2106/JBJS.F.00543. [DOI] [PubMed] [Google Scholar]
- 24.Ortiguera C, Pulliam I, Cabanela M. Total hip arthroplasty for osteonecrosis. Matched-pair analysis of 188 hips with long-term follow-up. J Arthroplasty. 1999;14:21–28. doi: 10.1016/S0883-5403(99)90197-3. [DOI] [PubMed] [Google Scholar]
- 25.Radl R, Hungerford M, Materna W, Rehak P, Windhager R. Higher failure rate and stem migration of an uncemented femoral component in patients with femoral head osteonecrosis than in patients with osteoarthrosis. Acta Orthop. 2005;76:49–55. doi: 10.1080/00016470510030319. [DOI] [PubMed] [Google Scholar]
- 26.Revell M, McBryde C, Bhatnagar S, Pynsent P, Treacy R. Metal-on-metal hip resurfacing in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):98–103. doi: 10.2106/JBJS.F.01070. [DOI] [PubMed] [Google Scholar]
- 27.Ritter M, Meding J. A comparison of osteonecrosis and osteoarthritis patients following total hip arthroplasty: a long-term follow-up study. Clin Orthop Relat Res. 1986;206:139–146. [PubMed] [Google Scholar]
- 28.Saito S, Saito M, Tetsuhiko N, Kenji O, Keiro O. Long-term results of total hip arthroplasty for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989;244:198–207. [PubMed] [Google Scholar]
- 29.Ware J, Kosinski M, Keller S. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]