History and Physical Examination
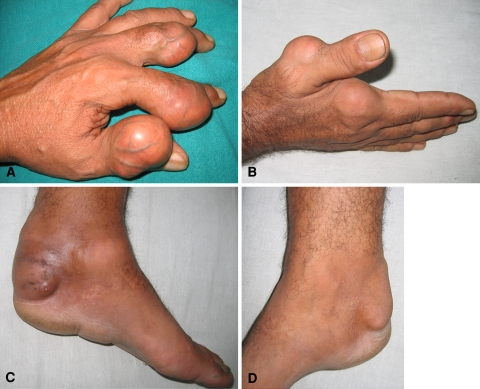
A 60-year-old man from a sub-Himalayan village of India presented with multiple nodular outgrowths on the dorsum of the radial three digits of both hands, both ankles, the left sole, and the fourth toe of the left foot (Fig. 1). The nodules developed over 5 years, progressively increasing in size. They were painless throughout the course until he injured his left ankle 5 months before, after which the nodules over the left ankle and sole became painful on weightbearing.
Fig. 1A–D.
Nodules with stretched-out skin and engorged veins are seen in the digits of the (A) left and (B) right hands, (C) over the Achilles tendon, sole, and dorsomedial aspect of the left ankle, and (D) over the Achilles tendon of the right ankle.
On examination, the nodules were round to oval, measuring approximately 4 × 3 cm in the hands and 10 × 7 cm in the ankles. They were seen mostly over joints, were soft to firm, and nontender except over the left ankle and left sole. They were fixed to the overlying shiny stretched-out skin with visible prominent vascular channels over the nodules. The skin temperature over the lesions was not elevated. Mild erythema was noted over the nodules. There were no scars, sinuses, or ulcers over any of the affected parts. Grip strength in the left hand was limited owing to mechanical obstruction by the nodules, also affecting pinch and grasp. Grip in the right hand was good and the patient could write with a pen in his right hand. There was no neurologic deficit in any of the limbs. Range of motion in the affected joints was painless. The patient had a slight limp attributable to pain in the left ankle and sole. No other musculoskeletal disorder was noted.
Laboratory investigations showed an increased erythrocyte sedimentation rate of 40 mm in the first hour, elevated serum uric acid level (8.8 mg/dL), and negative rheumatoid factor and C-reactive protein.
Digital radiographs of the hands were obtained (Fig. 2). MRI of both ankles and feet also was performed (Fig. 3).
Fig. 2.
A radiograph shows globular soft tissue shadows in both hands and scalloping of the cortex of the phalanges (arrow).
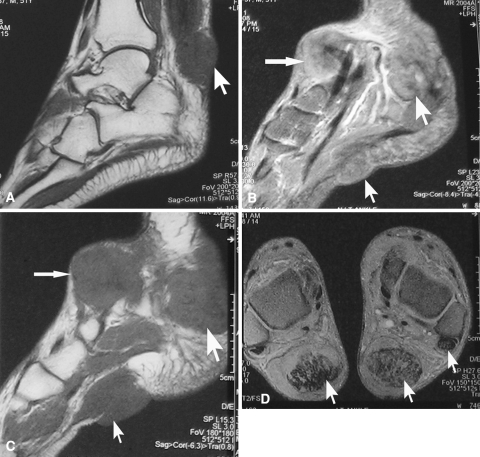
Fig. 3A–D.
(A) A sagittal T1-weighted MR image shows a subcutaneous xanthoma involving the substance of the Achilles tendon of the right ankle (arrow). (B) A sagittal proton density-weighted MR image shows xanthomatous deposits in the left Achilles tendon, left sole, and extensor tendons of the left foot (arrows). (C) A sagittal T1-weighted MR image shows nodules in close proximity to the skin with minimum subcutaneous tissue (arrows) in the left ankle. (D) A transverse section T1-weighted fat suppression MR image of both ankles shows xanthomatous proliferation in the substance of the Achilles tendons and peroneus longus tendon (arrows).
Based on the clinical presentation, physical examination, laboratory values, and imaging studies, what is the differential diagnosis?
Imaging Interpretation
Anteroposterior radiographs of the hands showed multiple prominent nodular soft tissue densities without any calcification over the radial three digits of both hands. Scalloping of the cortex was seen over the proximal phalanges of the left index and middle fingers and the base of the distal phalanx of the left thumb (Fig. 2). Mild subluxation of the metacarpophalangeal joints of both thumbs was evident. Multifocal loss of radiographically apparent joint spaces and osteophytosis were seen involving interphalangeal joints, most likely attributable to osteoarthritis.
MRI of the ankles and feet showed nodular enlargement of both Achilles tendons and bilateral extensor hallucis tendons and peroneal tendons of the left side with stippling. The nodules in the Achilles tendons measured 3.5 × 1.5 cm on the right side and 3.5 × 2.5 cm on the left side, whereas the lesion on the left extensor hallucis tendon was 4 × 3.5 cm in size (Fig. 3). In sagittal T1-weighted images, the nodules had a uniformly low signal intensity compared with the surrounding fat. No subcutaneous tissue or fat was seen over the nodules at their prominent parts (Fig. 3A, C). The lesions had an intermediate signal intensity in the proton density-weighted sequences (Fig. 3B). Involvement of the substance of Achilles tendon and peroneus longus tendon was better depicted in the axial T1-weighted fat suppression images (Fig. 3D). The disorder selectively involved the tendons and spared the joints, neurovascular bundles, and bones.
Differential Diagnosis
Multiple gouty tophi
Multiple tendon xanthomata
Rheumatoid nodules
Neurofibromatosis
Giant cell tumor of tendon sheaths
Tubercular tenosynovitis
The patient underwent excisional biopsy of the nodule over the left index finger and the histopathology of the lesion was studied (Figs. 4, 5).
Fig. 4.
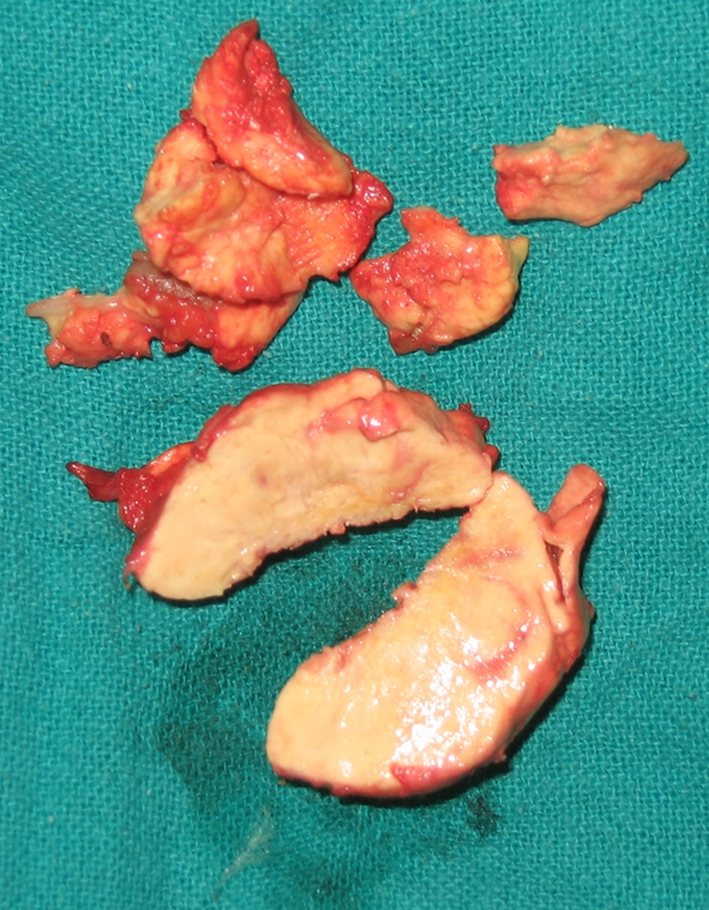
The xanthoma was friable with a yellowish cut surface.
Fig. 5.
A photomicrograph shows large cholesterol deposits in the extracellular tissue with a minimum inflammatory reaction (Stain, hematoxylin and eosin; original magnification, ×40).
Based on the clinical presentation, physical examination, laboratory findings, imaging studies, and histopathologic picture, what is the final diagnosis and how should these lesions be treated?
Histology Interpretation
The material consisted of multiple, pale white to grey-tan, soft tissue pieces, together measuring 5.5 × 3.5 × 2.2 cm. The cut surface was pale yellowish-white and soft (Fig. 4). Microscopically, there were multiple vaguely circumscribed collections of extracellular cholesterol clefts and sheets of foamy histiocytes and multinucleated giant cells with fibrosis. No crystals were visible under the polarized microscope in the formalin-fixed tissue. No pigment-laden macrophages, hemorrhagic foci, or calcification was seen. No evidence of malignancy was found (Fig. 5).
Diagnosis
Multiple tendon xanthomata
Discussion and Treatment
The clinical presentation was of long-standing, slowly growing, multiple periarticular nodules in the hands and feet with complaints of pain in the foot and ankle nodules and mechanical obstruction in the left hand. Biopsy and histopathologic study of the excised tissue from the nodules confirmed the diagnosis of tendon xanthoma. The photomicrograph showed diffuse collections of extracellular unesterified cholesterol clefts and foamy histiocytes with multinucleated giant cells and fibrosis [1, 3]. Absence of urate crystals and signs of inflammation helped differentiate it from gout.
We initially placed multiple tophaceous gout at the top of our list of possible diagnoses. The high serum uric acid level and increased erythrocyte sedimentation rate in the laboratory reports was consistent with our clinical suspicion. However, there was no inflammation, the patient had minimal joint symptoms, and he had no history of acute exacerbation, along with the fact that the patient did not require regular medications for pain. MRI did not correlate with the initial diagnosis of gouty tophi. The radiographs of the hands showed scalloping of the cortices of the phalanges corresponding to the nodular densities. These findings are not unlike the bony erosions seen in advanced multiple tophaceous gout, which present as punched out lesions in or around joints with overhanging edges. However, in gout, the distribution is usually asymmetric, the lesions have sclerotic margins and may show calcification extending into the nodular tophus, and the joint spaces are characteristically well preserved until late in the disease process [2, 5], very unlike the findings seen in this case. Histologically, monosodium urate crystals are found in the cytoplasm of neutrophils clustering in the synovial tissue. They appear as long, slender, needle-shaped structures and are negatively birefringent under polarized microscopy. Tophi are the hallmark of gout and are formed by aggregation of urate crystals engulfed by foreign-body giant cells, macrophages, and lymphocytes [13]. The MR images ruled out any bone or joint involvement in the lower limbs and showed the lesions to be located in close proximity to the tendons around the ankles.
Rheumatoid nodules seemed an unlikely diagnosis as they appear late in the disease process, usually over pressure regions, such as the elbow, back of the forearm [4], knee, occiput, and metacarpophalangeal joints [9]. They often are accompanied by other late manifestations of systemic rheumatoid disease, such as joint deformities, and almost always associated with a positive serology for rheumatoid factor [9]. Radiographs in rheumatoid arthritis frequently show joint deformities and subluxations, mostly involving the metacarpophalangeal and metatarsophalangeal joints. Histologic study of rheumatoid nodules usually reveals a central core composed of necrotic material, collagen fibrils, and cellular debris. The intermediate zone harbors macrophages expressing HLA-DR antigens and the periphery is made of granulation tissue [9].
Neurofibromatosis was the other possibility, but it rarely involves the hands and feet. It seldom becomes symptomatic and almost never causes mechanical obstruction in joint function. The first presentation of neurofibroma is seen at a much earlier age (in the third decade of life), as compared with the patient in question. MRI in neurofibromatosis may show the typical target pattern lesions [11] involving cutaneous nerves, but in this case the neural structures were uninvolved. On biopsy, neurofibromas typically show neural elements and ectatic blood vessels [11], with disordered proliferation of all elements of peripheral nerve, such as neurites, Schwann cells, and fibroblasts, in a myxoid stromal background.
A giant cell tumor of the tendon sheath appears as a solitary, slow-growing, painless nodule associated with a tendon, mostly in the wrist or hand. Radiographs may reveal erosion of adjacent bony cortices. The pathologic picture shows polyhedral tumor cells more or less resembling synoviocytes forming nodular aggregates with hemosiderin deposits, macrophages, and multinucleated giant cells [13].
Tubercular tenosynovitis may be included in the differential diagnosis of such lesions. They present as progressive swelling and inflammation of tendon sheaths. Clinically, they may appear doughy or fluctuant. The most common site is the flexor tendons of the hand (compound palmar ganglion), although it may involve bursae, extensor tendons of the fingers, and tendons around the ankle. The pathologic picture is of tuberculous granulation tissue replacing the tenosynovial lining. As the disease progresses to the insertion of tendons, rupture may occur [14].
Tendon xanthomata are associated with disorders of lipid metabolism, such as familial hypercholesterolemia (FH) and familial defective apolipoprotein B100 (FD apo-B100). The clinical appearance in both diseases is identical and their presence in the Achilles tendons, extensor tendons of the hands and feet, knees, palms, and elbows is pathognomonic of these conditions [1, 6, 10, 15]. MRI and ultrasonography are useful adjuncts to diagnosis, along with conventional radiographs [3]. Axial-view MR images of xanthomatous tendons characteristically show heterogeneous signal, which gives a speckled or reticulated appearance in all image sequences (T1, T2, proton density weighted). This appearance is more appreciated in fat suppression images (Fig. 3D). The heterogeneity of signal decreases in sagittal images, in which the tendons look more nodular and enlarged than speckled [3]. On ultrasonography, the xanthomas appear as diffuse or focal hypoechoic lesions. The nodular enlargements are well seen and lack the characteristic fibrillar architecture of normal tendons [3]. Ultrasonography can be used as a screening tool in patients with FH to diagnose subclinical Achilles tendon xanthomas.
FH is an autosomal dominant disorder caused by mutations in the low-density lipoproteins (LDL) receptor gene in chromosome 19. Defects such as lack of LDL receptors or abnormally functioning receptors lead to impaired clearance of the lipoproteins from the circulation [8, 10, 12]. In the heterozygous state, this results in a two- to threefold increase in plasma cholesterol concentration and atherosclerotic heart disease by the fourth decade of life, whereas in the homozygous state, LDL cholesterol increases by as much as sixfold and the manifestations start as early as the first year of life [7]. Plasma LDL is taken up by scavenging macrophages in a nonsaturable oxidized form [10]. These cholesterol-laden macrophages accumulate in tissues, such as the arterial walls, tendons, and skin, producing xanthomata. The diagnosis of a homozygous state can be confirmed by culture of skin fibroblasts to see LDL receptor activity [10, 11]. FD apo-B100 is a common disorder caused by a single mutation in the apo-B100 gene (glutamine for arginine at amino acid 3500), which results in inability of this ligand to bind with LDL. Serum triglyceride level usually remains normal in both these conditions [11].
Cholesterol deposits in heterozygous FH lead to xanthoma formation most commonly in the Achilles tendon and the tendons of hand. Other cholesterol deposits, such as xanthelasma, eruptive xanthoma of the skin, planar xanthoma, arcus cornea, and palmar or tuberous xanthoma, may be seen in the homozygous state of FH and other disorders of lipid metabolism [12].
Coronary heart disease (CHD) frequently accompanies FH and FD apo-B100. Any individual with premature heart disease should be suspected of having one of the above two hereditary conditions. The diagnosis is not only important for the affected patient but also for the family members who are at high risk of having CHD develop [10, 12]. The average age of manifestation of CHD is 45 years in men and 55 years in women. Genetic analysis for differentiating between these two conditions is unnecessary, because the treatment modalities for both are the same and so is the prognosis.
Surgery for tendon xanthomata is required only in cases of mechanical obstruction of the joints resulting in disability or when painful. Dietary modifications and drug therapy for FH and FD apo-B100 usually lead to regression of the nodules. Serum lipid profile guides the management of these conditions of hyperlipidemia. Lifestyle modifications with low intake of saturated fat, consumption of a fiber-rich diet, and exercise are recommended for the affected individuals and all first-degree relatives. Antilipidemic drug therapy should be instituted for patients with plasma LDL-C levels greater than 4.9 mmol/ L (190 mg/dL) and serum triglyceride levels greater than 11.3 mmol/L (1000 mg/dL). Patients with low plasma levels of HDL-C (< 1.0 mmol/L or 40 mg/dL) are also candidates for drug treatment. The different lipid-lowering agents are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), bile acid sequestrants (cholestyramine), nicotinic acid and fibric acid derivatives (fenofibrate) [12].
Our patient underwent excision of the xanthomata on the radial three digits of the left hand, as he was having difficulty in grip and pinch. The yellowish cholesterol deposits were large, subcutaneously placed over interphalangeal joints, engulfed the extensor tendons all around, and were free from any attachment with the neurovascular structures (Fig. 6). The skin over the lesions appeared normal but stretched out, adherent to the xanthoma without any subcutaneous tissue between, which necessitated dissection for complete removal of the lesions. The excised tissue was rubbery and friable. The postoperative period was uneventful, with delayed healing of the left index finger. Physiotherapy and finger mobilization exercises were continued to improve grip. The patient was investigated thoroughly for CHD (electrocardiography, echocardiography, treadmill test) and hyperlipidemia. His cholesterol was 310.0 mg/dL (normal, 156.0–200.0 mg/dL), serum triglyceride was 815.0 mg/dL (normal, ≤ 150.0 mg/dL), HDL-C was 50.0 mg/dL (normal, 35.0–60.0 mg/dL), LDL-C was 108.0 mg/dL (normal, ≤ 130.0 mg/dL), and VLDL-C was 152.0 mg/dL (normal, ≤ 40.0 mg/dL). The cholesterol:HDL-C ratio in the patient was 6.20, which signifies an average to moderate risk of having CHD develop. He was diagnosed as having combined hyperlipidemia with multiple tendon xanthomata, and was prescribed a two-drug therapy of cholesterol-lowering agents comprised of a statin and a fibrate derivative.
Fig. 6.
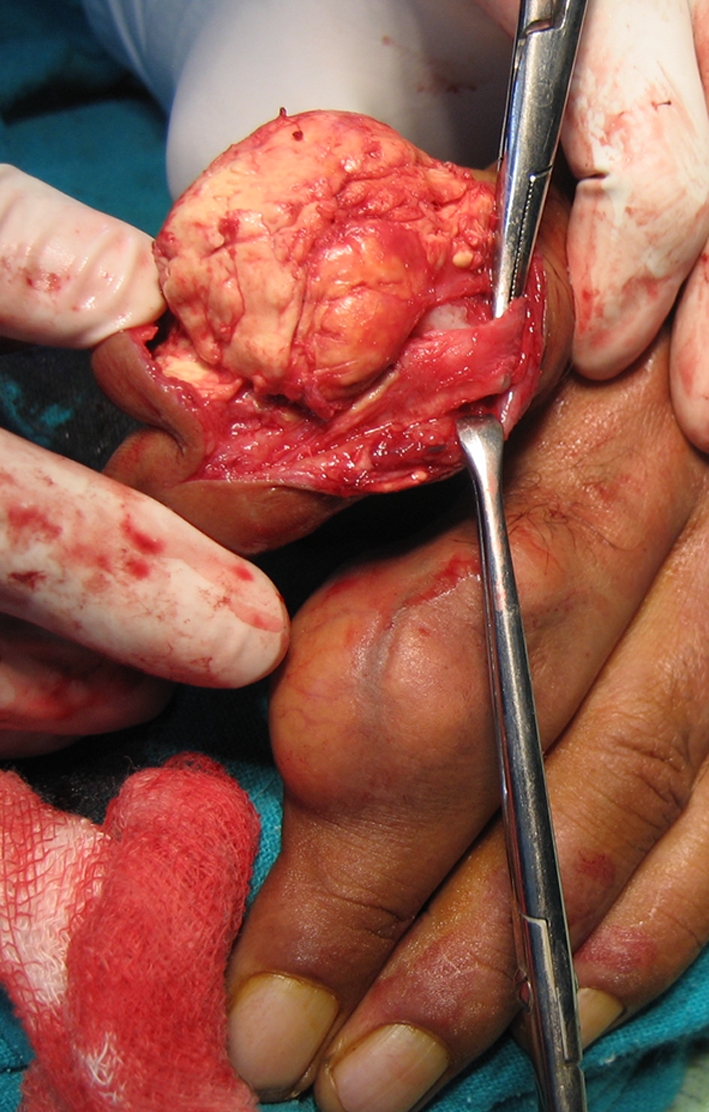
An intraoperative image shows the xanthoma in the left index finger without involvement of the neurovascular structures.
Acknowledgments
We thank Satya S. G. Mohapatra, MD, for help in evaluating the radiographs and MRI, Ishani Mohapatra, MD, for help in analyzing the histopathologic data, and Vipul Vijay, Resident in Orthopedics, for collection of research materials.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Dr. Ram Manohar Lohia Hospital & PGIMER.
References
- 1.Artieda M, Cenarro A, Junquera C, Lasierra P, Lorenzo MJ, Pocovi M, Civeira F. Tendon xanthomas in familial hypercholesterolemia are associated with a differential inflammatory response of macrophages to oxidized LDL. FEBS Lett. 2005;579:4503–4512. doi: 10.1016/j.febslet.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 2.Baker DL, Stroup AS, Gilstrap CA. Tophaceous gout in patient with rheumatoid arthritis. J Am Osteopath Assoc. 2007;107:554–556. [PubMed] [Google Scholar]
- 3.Bude RO, Adler RS, Bassett DR. Diagnosis of Achilles tendon xanthoma in patients with heterozygous familial hypercholesterolemia: MR vs sonography. AJR Am J Roentgenol. 1994;162:913–917. doi: 10.2214/ajr.162.4.8141017. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain MA. Intra-articular rheumatoid nodules of the knee. J Bone Joint Surg Am. 1971;53:507–509. [PubMed] [Google Scholar]
- 5.Gentili A. The advanced imaging of gouty tophi. Curr Rheumatol Rep. 2006;8:231–235. doi: 10.1007/s11926-996-0030-6. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton WC, Ramsey PL, Hanson SM, Schiff DC. Osseous xanthoma and multiple hand tumors as a complication of hyperlipidemia. J Bone Joint Surg Am. 1975;57:551–553. [PubMed] [Google Scholar]
- 7.Harada-shiba M, Takagi A, Miyamoto Y, Tsushima M, Ikeda Y, Yokoyama S, Yamamoto A. Clinical features and genetic analysis of autosomal recessive hypercholesterolemia. J Clin Endocrinol Metab. 2003;88:2541–2547. doi: 10.1210/jc.2002-021487. [DOI] [PubMed] [Google Scholar]
- 8.Lee CK, Weiss AB. Xanthoma of the Achilles tendon. J Bone Joint Surg Am. 1980;62:666–669. [PubMed] [Google Scholar]
- 9.Lipsky PE. Rheumatoid arthritis. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16. New York, NY: McGraw-Hill; 2005. p. 2286. [Google Scholar]
- 10.Mahley RW, Weisgraber KH, Farese RV., Jr . Disorders of lipid metabolism. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams’ Textbook of Endocrinology. 10. New York, NY: WB Saunders; 2003. p. 1668. [Google Scholar]
- 11.Peh WC, Shek TW, Yip DK. Magnetic resonance imaging of subcutaneous diffuse neurofibroma. Br J Radiol. 1997;70:1180–1183. doi: 10.1259/bjr.70.839.9536912. [DOI] [PubMed] [Google Scholar]
- 12.Rader DJ, Hobbs HH. Disorders of lipoprotein metabolism. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill; 2005. p. 2286. [Google Scholar]
- 13.Rosenberg AE. Bones, joints and soft tissue tumors. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran: Pathologic Basis of Disease. 7. Philadelphia, PA: WB Saunders; 2004. pp. 1311–1315. [Google Scholar]
- 14.Tuli SM. Tuberculosis of Skeletal System (Bones, Joints, Spine and Bursal Sheaths) 3. New Delhi, India: JayPee Brothers Medical Publishers (P) Ltd; 2004. p. 184. [Google Scholar]
- 15.Yaghmai I. Intra- and extraosseous xanthomata associated with hyperlipidemia. Radiology. 1978;128:49–54. doi: 10.1148/128.1.49. [DOI] [PubMed] [Google Scholar]