Abstract
Large-diameter metal-on-metal articulations reportedly provide better stability and range of motion than smaller diameter bearings. We therefore asked whether a large-diameter (44- to 50-mm) metal-on-metal articulation (Durom®) would eliminate dislocation and provide similar functional scores and clinical and radiographic failure rates as those with 28-mm articulation. We prospectively followed 181 patients (207 hips) who had a large-diameter articulation implanted between May 2006 and November 2007. We compared these patients with a historical control of 54 patients who had a small-diameter (28-mm Metasul®) articulation. All patients had a Harris hip score and a self-assessment of outcome and radiographic followup. The minimum followup was 1 year (mean, 1.6 years; range, 1–2 years). During the followup period, we performed revisions on 29 patients (30 hips [15%]) with 21 of 29 (72%) having radiographic criteria of loosening. Thirteen retrieved cups and acetabular tissue were examined histologically. Twenty-eight of 151 unrevised patients had radiographic impending failure; 12 without revision had clinical failure. Eight patients (nine hips) had both clinical failure and impending radiographic failure. Cup inclination was 41.3° ± 5.4° and anteversion was 20.2° ± 7°. The revision rate and quality of clinical results were unacceptable as compared with our historical controls. We do not recommend use of the Durom® implant.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Large head metal-on-metal conventional THA has recently been clinically used with the anticipation that it would reduce dislocation, improve range of motion, and provide good durability by low wear. Metal-on-metal articulation for THA has been one subject of research for us [5, 9, 12, 19, 23]. Our only repeatable complication with this articulation was dislocation (using 28- and 32-mm heads), so the large-diameter head was attractive [5, 12, 19]. We had not experienced any hypersensitivity [23]. Therefore, we were asked to conduct a prospective clinical study of the Durom Metasul® large-diameter head metal-on-metal acetabular component (Zimmer, Warsaw, IN). The cup was approved by the US Food and Drug Administration in April 2006, and we began implanting the cup in May 2006. The cup was approved in Europe with a CE mark and was used outside the United States primarily for surface replacement with reported satisfactory results from Canada [26]. Much of the use in Europe was with surface replacement. With the increasing use of metal-on-metal surface replacement, there was less concern about ions and cancer expressed by surgeons, so patients had high acceptance of metal-on-metal.
We therefore asked whether conventional THA with the Durom® acetabular component for large-diameter head metal-on-metal articulation would (1) eliminate dislocation; (2) perform better in terms of range of motion and level of activity; and (3) have low rates of osteolysis equivalent to that in our historic controls of 28-mm Metasul® articulations using the Converge® porous-coated metal shell (Zimmer).
Patients and Methods
We prospectively followed 181 patients (207 hips) who had primary THA using the Durom® acetabular component between May 2006 and November 2007. The patients were selected because of the cost of the implant and its prospective indications for patients younger than 60 years of age and patients older than 65 years of age with small acetabula (ie, less than 52 mm). Contraindications included patients with known renal compromise and women of childbearing age. One patient (one hip) died within the first year of postoperative followup for reasons not related to hip surgery and was excluded from the study. This exclusion left 180 patients with 206 THAs. The criteria of small acetabula gave a preponderance of women (119 of 180 patients) (Table 1). We had prior Institutional Review Board approval.
Table 1.
Demographics
| Parameter | Men (61 patients; 70 hips) | Women (119 patients; 136 hips) |
|---|---|---|
| Age (years) | 55.8 ± 11.6 | 63.4 ± 10.4 |
| Weight (kg) | 80.2 ± 14.6 | 77.4 ± 11.4 |
| Body mass index (kg/m2) | 28.8 ± 4.3 | 28.2 ± 6.1 |
| Diagnosis (hips) | OA = 53 (60), DDH = 4 (4), AVN = 4 (6), RA = 0 | OA = 102 (116), DDH = 9 (11), AVN = 5 (6), RA = 3 (3) |
Values are mean ± standard deviation; OA = osteoarthritis; DDH = developmental dysplasia of the hip; AVN = avascular necrosis of hip; RA = rheumatoid arthritis.
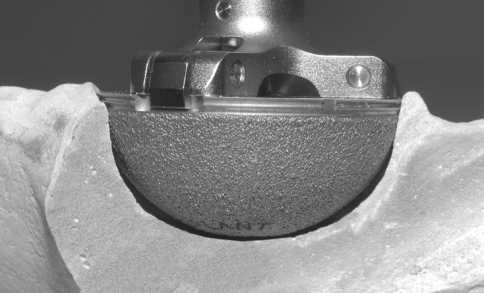
The acetabular component in all hips was the Durom® metal-on-metal cup, which is a wrought-forged high-carbon cobalt-chromium alloy with 3-mm thick walls (Fig. 1). The external geometric shape is not a hemisphere with a sector angle of 165° and a flattened dome that flares toward the periphery so it is 2 mm wider at the rim than the dome. Two-millimeter wide sharp circumferential fins for rotational stability is the most peripheral rim structure. These fins are impacted into the bone. The cup has no screw holes. The fixation surface is a plasma spray coating, which has a roughness of 20 to 50 μm. The plasma spraying of the cup enables the titanium alloy implant to retain most of its fatigue strength as compared with the sintered cups. Also, plasma spray provides good early stability as a result of its high frictional interface with the adjacent bone and its closed-pore structure prevents particulate debris migration [3, 13]. The metal femoral head was the Protasul Cobalt Chrome Metasul® alloy [5, 9, 12]. Head diameter was 44 to 50 mm in 164 hips, 52 to 56 mm in 33 hips, and greater than 56 mm in nine hips. The femoral component was the Anatomic Porous Replacement stem (Zimmer) in 160 hips; the Zweymüller Alloclassic stem (Zimmer) in 42 hips; and a cemented Apollo stem (Zimmer) in four hips.
Fig. 1.
Peripheral press-fit of the Durom® cup may prevent contact with the prepared acetabular bone, particularly when preparation was performed with a hemispheric reamer of the same size. The sharp peripheral fins of the flared cup with a sector angle of 165° do not permit predictable, repeatable bone preparation, especially with recommended hemispheric reamers and hemispheric preparation.
All arthroplasties were performed by three surgeons (LDD, WTL, MJH). The same postoperative analgesic and rehabilitation protocol was used in all patients [20]. All patients were operated on using a posterior approach [16]. The recommended technique was not described as different from any other cup (Zimmer product brochure). The instructions described reaming the acetabulum into a hemisphere. However, we learned quickly the hemispheric preparation of the acetabulum must be compromised because the cup is not a hemisphere. The hemispheric reamer was only partially engaged in the acetabular bone. In 83 hips, femoral preparation was performed first to estimate the femoral stem anteversion and the cup anteversion was adjusted for a combined anteversion of 25° to 45° [10]. In 123 hips, the acetabulum was targeted to 20° to 25° of anteversion and prepared first. The Navitrack Imageless Computer Navigation System (Orthosoft, Montreal, Canada) was used to intraoperatively assess the cup position for all patients [27]. The unique features of this technique are as follows: (1) the acetabulum was reamed with 2-mm graduated hemispheric reamers. Reaming was begun with a reamer size 3 mm smaller than the anticipated cup size. Reamers were not fully seated because the cup is not a hemisphere. The lunate bone was usually left partially intact for optimum bony contact in Zone II [7]; (2) the trial cup of the last reamer used (50-reamer, 50-cup trial) was placed and inspected through the slots for bone contact and coverage by bone. Posterosuperior protrusion of the rim (the circumferential fins were uncovered) by 3 mm was accepted when necessary to keep inclination at 45° or less. If this trial was tight in the acetabular cavity, with hard cortical bone, the peripheral acetabular cortical bone was reamed 1 mm more (51 mm) for a 50-mm cup to accommodate the flare of 2 mm. If the trial was grossly loose, we then reamed with a 51- and/or 52-mm reamer as necessary for a tight fit of the 52-mm trial; (3) acetabular inclination and anteversion were determined by touching the rim of the press-fit trial in six spots by computer navigation. A second option for aligning the cup was to position a Converge® cup trial with a holder fitted with computer navigation optical trackers. Inclination was targeted to 40° and anteversion between 15° and 30° depending on stem anteversion. Whatever the option used to determine the desired cup position, a methylene blue mark was made on the acetabular bone to act as a guide for the holder of the Durom® cup; (4) the cup was implanted using the rim holder. With the cup partially seated, inclination and anteversion were measured by touching the computer navigation pointer guide to the rim of the cup. The sound of the mallet blows and the resistance of the cup were the auditory and palpatory clues for cup seating. With the holder removed, the rim was again touched to measure inclination and anteversion. In the first 15 hips, if the numbers were not acceptable, the edge of the cup was hit using a bone tamp and mallet to improve its position. We determined the peripheral circumferential fins would cut through bone doing this, so this technique was abandoned. Thereafter, we accepted inclination to 50° and anteversion within 5° of the ideal position. Five cups were not within these parameters, were removed, and the entire trial and cup placement steps were repeated. If the press-fit was not rigid, as tested by tapping on the edge of the cup with a bone tap, we converted to a porous-coated hemispheric cup with which we could use screws (n = 5); (5) our goal was coverage of the cup anterosuperior to prevent impingement in flexion/adduction/internal rotation; the inferior medial edge of the cup should be inside the transverse acetabular ligament to ensure the center of rotation was not lateralized; (6) the femoral stem was implanted and the modular head size needed to restore leg length and offset selected (measured both manually and by computer navigation). Offset had to be sufficient to prevent impingement of the lesser trochanter to the ischium and the greater trochanter to the ilium in abduction/external rotation and flexion/adduction/internal rotation; and (7) an anterior-posterior pelvis radiograph was taken before the patient left the operating room to confirm the component positions, leg length, and offset.
We obtained the Harris hip score [15] preoperatively and postoperatively at 3 months, 6 months, 1 year, and 2 years and, using a questionnaire independently completed by all patients, graded their outcome from poor to excellent. Activity was categorized as unlimited, active community ambulation (ability to walk six to eight blocks), limited community ambulation (ability to walk two blocks), household ambulation (ability to walk in the home and yard), or wheelchair-bound [11]. We defined clinical failure as Harris hip score below 70 or self-assessment grade of fair or poor similar to Blumenfeld and Bargar [2]. Symptoms common in patients with loose cups were persistent weightbearing pain on arising from a seated position; groin or buttock pain with weightbearing; pain in straightening up from a bent position; and pain with single-limb stance (such as putting on pants).
A supine anteroposterior pelvic radiograph centered over the symphysis pubis and an iliac oblique lateral radiograph were taken at each clinical followup [24]. Radiographs were made by the same technicians throughout the study. One observer (ZW) not involved in the surgery made all radiographic observations. Inclination of the cup was measured by the method of Callaghan et al. [4] and anteversion by the modified technique of Ackland et al. [1]. Fixation and osteolysis of the cup were measured by the zones of DeLee and Charnley [7]. Cup loosening was diagnosed when any of the following were present: (1) any evidence of a circumferential radiolucent line of 1 mm or wider; (2) appearance of a completely new radiolucent line in any zone; (3) progressive radiolucent line into a new zone; or (4) migration of the cup by more than 2 mm of vertical or horizontal shift or change in inclination of more than 5° [2, 25]. Impending failure was radiographic evidence of loosening without revision. Femoral radiolucent lines and osteolysis were reported in each of seven Gruen zones on the anterior-posterior and lateral radiographs [14, 17].
Thirteen patients had the cup and tissue specimens of the capsule and acetabular membrane of 13 revisions sent to the J. Vernon Luck Orthopedic Research Laboratory to be studied by Patricia Campbell, PhD. None of the cups grossly had bone fixation at revision. Three capsular samples were sent from the anterior, posterior, and medial acetabulum. The acetabular membrane was very thin and removed by curette.
Descriptive statistics were performed using SPSS Software (SPSS, Chicago, IL) to calculate mean and standard deviation of all study parameters. No statistical comparisons were made.
Results
Unexpectedly, we performed revisions in 29 of the 180 patients (16%) and 30 of the 206 hips (15%) for acetabular component loosening within 2 years of implantation. All 29 patients with revision had clinical failure (Harris hip score range 42 to 67). Ten revisions were performed in the first postoperative year because of clinical failures with four of these 10 hips having radiographic signs of loosening. Twenty revisions were performed in the second postoperative year: seven in hips that failed in first year of followup (six of seven having radiographic signs of loosening) and 13 in 13 new patients who developed clinical failure in the second year (11 of 13 having radiographic signs of loosening). Twelve of the remaining 151 patients had a clinical grade of poor or fair and/or a Harris hip score less than 70 (clinical failure). One revision was performed for deep infection successfully treated with two-stage reimplantation. All 13 retrieved cups examined in detail had no evidence of bone on the fixation surface. The capsular tissue had little metal particulate debris and no evidence of hypersensitivity with no perivascular lymphocytes. The capsular and acetabular tissue had inflammation. The total number of clinical failures was 41 of 180 patients (23%). Twenty-eight of the 151 patients (29 of 176 hips) who had not been revised had radiographic evidence of impending failure. Eight patients (nine hips) had both clinical failure and impending failure. Overall, 21 of 180 patients (11.7%) with 22 of 206 hips (10.7%) had radiographic failure at the time of revision; eight patients (eight hips [4%]) had revision for clinical failure alone. Twenty-eight of 151 patients (18.5%) with 29 of 179 hips (16.2%) had radiographic impending failure at final followup. Fifty-five of the 196 hips (28%) not revised at 1 year postoperatively had gaps or radiolucent lines and 41 of 78 (52.6%) hips not revised between 1 and 2 years had gaps or radiolucent lines. During postoperative Year 1, 16 of 180 patients (16 hips [7.8%]) had radiographically loose cups with four revised and 12 classified as impending failure. In the second postoperative year, three of these 12 patients had revision and 16 new hips in 15 patients developed impending failure. Ten of these had new radiolucent lines and six had progressive radiolucent lines (Table 2).
Table 2.
Distribution of radiolucent lines in nonrevised Durom hips
| RLL pattern around the Durom cup | At 1-year followup (total hips = 196) | At 2-year followup (total hips = 78) |
|---|---|---|
| Absent RLL | 141 | 37 |
| Nonprogressive postoperative gaps | 43 | 12 |
| Progressive postoperative gaps | 2 | 6 |
| Anteroposterior and lateral Zone 3 RLL | 2 | 9 |
| Anteroposterior and lateral Zone 2 RLL | 4 | 8 |
| Anteroposterior and lateral Zone 1 RLL | 3 | 3 |
| Single-zone anteroposterior or lateral zone RLL | 1 | 3 |
RLL = radiolucent line(s).
One patient had posterior hip dislocation at 2 weeks, which was successfully treated with closed reduction.
One hundred thirty of the 180 patients (72%) improved their function from the preoperative grade. The overall mean postoperative Harris hip score of 151 unrevised patients at a mean 1.6-year followup was 87 ± 13 compared with a mean preoperative score of 45 ± 16.8; the mean Harris pain score was 40.6 ± 4.3 with 129 of 151 having no pain; the mean Harris function score for these 151 patients was 41.9 ± 6.6. Twelve patients (7.9%) used an assistive device with two bilateral (walker or crutches) and 10 a single device (one crutch or cane). One hundred twenty-nine patients (146 hips) were walking unlimited distances; 12 patients (18 hips) were active community ambulators; five patients (six hips) were limited community ambulators; three patients (four hips) were household ambulators; and two patients (two hips) were wheelchair-bound. During the first postoperative year, 12 patients not revised had a clinical grade of fair or poor or a Harris hip score less than 70. During the second postoperative year, 11 of these 12 developed radiographic signs of loosening and seven were revised. In patients not revised during the second postoperative year, 15 patients with 16 hips developed radiographic impending failure and seven patients with eight hips became clinical failures.
We observed no osteolysis on either anteroposterior or lateral radiographs in any zone of any hip. Radiographic cup inclination was a mean of 41.3° ± 5.4° (range, 28°–52°) and cup anteversion was a mean of 20.2° ± 7° (range, 13°–36°). Computer navigation inclination was a mean of 37.4° ± 4.3° and anteversion was a mean of 22.8° ± 5.1° (range, 12°–35°). There was one loose femoral component because of intraoperative fracture, which was revised.
There were three femoral fractures with one intraoperative and two seen on postoperative radiographs. The intraoperative fracture was fixed with cables and the fractures seen postoperatively were treated with protective weightbearing.
Discussion
Large-diameter metal-on-metal articulations were introduced in part to provide better stability and range of motion than smaller diameter bearings. We therefore asked whether conventional THA with Durom® acetabular components for metal-on-metal articulation and large-diameter femoral heads would eliminate dislocation and provide functional scores and low clinical and radiographic failure rates similar to our historic controls of 28-mm Metasul® in Converge® acetabular components. Although the primary aim of the study was to compare the results, unexpected high rates of aseptic loosening compelled us to report the study at a very early stage.
The limitation to these results could be our technique. However, the mean inclination was 41.3° with three cups above 50° on radiographs and none above 50° by computer navigation. Mean radiographic anteversion was 20.2° ± 7° and by computer navigation was 22.8° ± 5.1°. Pathologic examination of 13 retrieved specimens showed none with any bone attachment to the fixation surface, which means the surface either did not promote bone fixation well or was not in contact with bone as evidenced by Zone 2 gaps. We had 41 hips with Zone 2 postoperative gaps with 36 of these persistent at their last followup and 14 had progressed. The plasma spray fixation surface was untested and differed from that used outside the United States.
The mechanism of failure of fixation in these cups may be biologic or mechanical. Biologically, the plasma spray fixation surface may require nearly perfect contact conditions to conduct bone osseointegration onto it. We did have 129 of 206 (62.6%) hips that had no radiographic gaps or radiolucent lines and Harris hip scores between 80 and 100 at the end of 1 year. This result is much lower than what we reported with 54 hips at a mean 3 years of followup with 28-mm Metasul® articulations and had 97% patients with improved function (mean Harris hip score 93) and no radiographic acetabular component loosening [8], and the clinical improvement and revision rates at 5 or more years in three other studies of 35 of 39 hips (90% patients with Harris hip score greater than 90 and no radiographic loosening) [22], 95 of 98 hips (97% patients with improved clinical results in terms of pain, range of motion, function, and no acetabular component loosening) [6], and 56 of 60 patients (93% with Harris hip score greater than 90 and no acetabular component loosening) [18]. The clinical and radiographic findings were not the same as the previously reported rapid failure of the InterOp® cup (Sulzer, Austin, TX) [2, 21]. InterOp® cups had rapid progressive radiolucent lines and cup migration as early as 6 weeks postoperatively. The Durom® cups had absent radiographic evidence of loosening in six of 10 cups revised in the first postoperative year. The pathology of the InterOp® cup failures was consistent with biologic failure [2].
The mechanical design has features similar to a threaded cup with a flare design, which can concentrate fixation at the rim. The rim fixation is only the uncoated sharp fins. The flare design may prevent the fixation surface from contact throughout prepared acetabular bone. Using reamers that do not match the shape of the cup compounds this risk (Fig. 1). Preferential peripheral contact in a less than hemispheric cup may have created a cantilever-type effect with micromotion of the fixation surface with maximum motion at the dome, particularly with a Zone 2 gap. The mechanical fit and stability of the cup was not studied in cadaver bones by the company who manufactured it.
Our experience with this cup has educated us that future use of new products must be supported with biologic proof of fixation with the fixation surface used; cadaver studies that show sectioning of the bone-implant construct for evidence of total contact of implant to bone with the recommended preparation; and mechanical studies that show stability of the implant within bone. These testing requirements would have likely exposed the design and technically demanding features of this implant. In addition, the designing surgeons should provide validated clinical data with a minimum 1-year followup as soon as possible. Companies must be quick to admit product failure. These authors have experienced two cup design failures (Interop® and Durom®) and in both situations, the company blamed the technique of the surgeon for months before admitting to a product problem. A national registry would protect surgeons from companies deflecting blame onto the technique. We do not recommend use of the Durom® implant.
Acknowledgments
We thank Ulrike Ehrenpfordt, BS, and Jason Vu, BS, for their assistance in data collection.
Footnotes
One or more of the authors (LDD) have received funding from the Dorr Arthritis Research and Education Foundation and Zimmer, Inc.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ackland MK, Bourne WB, Uhthoff HK. Anteversion of the acetabular cup. Measurement of angle after total hip replacement. J Bone Joint Surg Br. 1986;68:409–413. doi: 10.1302/0301-620X.68B3.3733807. [DOI] [PubMed] [Google Scholar]
- 2.Blumenfeld TJ, Bargar WL. Early aseptic loosening of a modern acetabular component secondary to a change in manufacturing. J Arthroplasty. 2006;21:689–695. doi: 10.1016/j.arth.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Bourne RB, Rorabeck CH, Burkart BC, Kirk PG. Ingrowth surfaces. Plasma spray coating to titanium alloy hip replacements. Clin Orthop Relat Res. 1994;298:37–46. [PubMed] [Google Scholar]
- 4.Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD, Jr, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982. A two to five-year follow-up. J Bone Joint Surg Am. 1985;67:1074–1085. [PubMed] [Google Scholar]
- 5.Dastane MR, Long WT, Wan Z, Chao L, Dorr LD. Metal-on-metal hip arthroplasty does equally well in osteonecrosis and osteoarthritis. Clin Orthop Relat Res. 2008;466:1148–1153. doi: 10.1007/s11999-008-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaunay CP. Metal-on-metal bearings in cementless primary total hip arthroplasty. J Arthroplasty. 2004;19:35–40. doi: 10.1016/j.arth.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Dorr LD, Hilton KR, Wan Z, Markovich GD, Bloebaum R. Modern metal on metal articulation for total hip replacements. Clin Orthop Relat Res. 1996;333:108–117. doi: 10.1097/00003086-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Dorr LD, Long WT, Sirianni L, Campana M, Wan Z. The argument for the use of Metasul as an articulation surface in total hip replacement. Clin Orthop Relat Res. 2004;429:80–85. doi: 10.1097/01.blo.0000150343.66755.79. [DOI] [PubMed] [Google Scholar]
- 10.Dorr LD, Malik A, Dastane M, Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009;467:119–127. doi: 10.1007/s11999-008-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorr LD, Wan Z, Gruen T. Functional results in total hip replacement in patients 65 years and older. Clin Orthop Relat Res. 1997;336:143–151. doi: 10.1097/00003086-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Dorr LD, Wan Z, Longjohn DB, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg Am. 2000;82:789–798. doi: 10.2106/00004623-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Emerson RH, Jr, Sanders SB, Head WC, Higgins L. Effect of circumferential plasma-spray porous coating on the rate of femoral osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1291–1298. doi: 10.2106/00004623-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 15.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 16.Inaba Y, Dorr LD, Wan Z, Sirianni L, Boutary M. Operative and patient care techniques for posterior mini-incision total hip arthroplasty. Clin Orthop Relat Res. 2005;441:104–114. doi: 10.1097/01.blo.0000193811.23706.3a. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RC, Fitzgerald RH, Jr, Harris WH, Poss R, Muller ME, Sledge CB. Clinical and radiographic evaluation of total hip replacement. A standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–168. [PubMed] [Google Scholar]
- 18.Kim SY, Kyung HS, Ihn JC, Cho MR, Koo KH, Kim CY. Cementless Metasul metal-on-metal total hip arthroplasty in patients less than fifty years old. J Bone Joint Surg Am. 2004;86:2475–2481. doi: 10.1302/0301-620X.86B7.15255. [DOI] [PubMed] [Google Scholar]
- 19.Long WT, Dorr LD, Gendelman V. An American experience with metal-on-metal total hip arthroplasties: a 7-year follow-up study. J Arthroplasty. 2004;19:29–34. doi: 10.1016/j.arth.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Maheshwari AV, Boutary M, Yun AG, Sirianni LE, Dorr LD. Multimodal analgesia without routine parenteral narcotics for total hip arthroplasty. Clin Orthop Relat Res. 2006;453:231–238. doi: 10.1097/01.blo.0000246545.72445.c4. [DOI] [PubMed] [Google Scholar]
- 21.Manning DW, Ponce BA, Chiang PP, Harris WH, Burke DW. Isolated acetabular revision through the posterior approach: short-term results after revision of a recalled acetabular component. J Arthroplasty. 2005;20:723–729. doi: 10.1016/j.arth.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Migaud H, Jobin A, Chantelot C, Giraud F, Laffargue P, Duquennoy A. Cementless metal-on-metal hip arthroplasty in patients less than 50 years of age: comparison with a matched control group using ceramic-on-polyethylene after a minimum 5-year follow-up. J Arthroplasty. 2004;19:23–28. doi: 10.1016/j.arth.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Shahrdar C, Campbell P, Mirra J, Dorr LD. Painful metal-on-metal total hip arthroplasty. J Arthroplasty. 2006;21:289–293. doi: 10.1016/j.arth.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Southwell DG, Bechtold JE, Lew WD, Schmidt AH. Improving the detection of acetabular osteolysis using oblique radiographs. J Bone Joint Surg Br. 1999;81:289–295. doi: 10.1302/0301-620X.81B2.9334. [DOI] [PubMed] [Google Scholar]
- 25.Udomkiat P, Wan Z, Dorr LD. Comparison of preoperative radiographs and intraoperative findings of fixation of hemispheric porous-coated sockets. J Bone Joint Surg Am. 2001;83:1865–1870. doi: 10.2106/00004623-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Vendittoli PA, Mottard S, Roy AG, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br. 2007;89:441–448. doi: 10.1302/0301-620X.89B4.18054. [DOI] [PubMed] [Google Scholar]
- 27.Wan Z, Boutary M, Dorr LD. Precision and limitation of measuring two-dimensional wear on clinical radiographs. Clin Orthop Relat Res. 2006;449:267–274. doi: 10.1097/01.blo.0000218758.35181.93. [DOI] [PubMed] [Google Scholar]