Abstract
Patient demand and surgeon interest in hip resurfacing has recently increased, but surgeons in the United States are relatively inexperienced with this procedure. We determined the learning curve associated with hip resurfacing and compared the rate of early complications of the first 650 hip resurfacings between five experienced hip surgeons and a national safety survey database study we previously published, which included 89 surgeons and 537 hip resurfacings. Patient demographics and adverse events were recorded. Specific features on pre- and postoperative radiographs were measured in a blinded fashion by a single observer. There were 13 major complications (2.0%), which is 3.7 times lower than our national safety survey complication rate of 7.4%. All fractures occurred in the first 25 cases performed. The complication rate was higher for the first 25 procedures (5.6%) compared with the second 25 procedures (1.6%). For experienced hip surgeons, the learning curve for avoiding early complications was short, 25 cases or less. The learning curve for achieving the desired component positioning radiographically was much longer, 75 to 100 cases or more. If achieving some ideal component position proves important for long-term function and implant survival, improved instrumentation and surgical techniques would be necessary to shorten the learning curve.
Level of Evidence: Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Current-generation hip resurfacing systems using metal-on-metal cobalt-chromium alloy components were introduced more than 10 years ago outside the United States. In Australia, hip resurfacing arthroplasties comprised 7.8% of hip arthroplasty procedures performed in 2007, and in Europe, the rates range between 6% and 9% [15]. Before May 2006, when the Food and Drug Administration (FDA) approved the first metal-on-metal hip resurfacing device, only a small group of surgeons in the United States [2, 4, 11, 40] were experienced with the hip resurfacing surgical technique either on a limited basis in conjunction with Investigational Device Exemption studies or on an off-label basis. Soon after FDA approval, hip resurfacing in the United States was performed on a more widespread basis by surgeons largely unfamiliar with the surgical technique.
The Australian hip registry indicates there is an increased risk of early revision after total hip resurfacing during the first 6 to 12 months postoperatively. Excluding these early revisions during the first year postoperatively, the rate of subsequent revision does not differ between hip resurfacing and conventional THA in properly selected patients [7]. These high early revision rates during the first 12 months are believed related to the more challenging surgical technique and the accuracy of component positioning [12, 35, 36].
A recent safety survey study reviewed the results of the first 537 hip resurfacing procedures performed in the United States by 89 surgeons and concluded these surgeons, with previously limited experience in hip resurfacing, had a complication rate of 7.4% at 1 year [21]. However, that study included a large number of surgeons with an unknown level of arthroplasty experience and a small number of cases per surgeon (during their early learning curve).
The clinical outcomes of hip resurfacing are extremely sensitive to patient selection [1, 3, 9, 13, 28, 32] and the technical details of the surgical technique [9, 30, 36, 43, 44]. It is well established that notching of the femoral neck, exposed cancellous bone, and varus placement of the femoral component increase the likelihood of femoral neck fracture [5, 9, 27, 36, 43]. Numerous studies demonstrate cup inclination or abduction angle greater than 50° to 55° is associated with high levels of serum metal ions and higher rates of early failure [19, 22, 23, 31]. Therefore, accurate component placement is critical to avoid complications after hip resurfacing. Although patient demand and surgeon interest in hip resurfacing has recently increased, most surgeons in the United States are relatively inexperienced with this procedure and may have higher rates of such complications early in their learning curve.
We therefore determined (1) the learning curve associated with this challenging surgical technique and (2) the rate of early complications by hip specialists compared with the national safety survey data.
Patients and Methods
We identified five experienced joint reconstructive surgeons at different sites across the United States, who had each performed more than 100 hip resurfacing procedures using the Birmingham hip resurfacing system (Smith & Nephew, Memphis, TN). The search criteria included high-volume joint reconstruction surgeons in the United States who had access to a well-maintained joint registry, radiographic database, no previous training or substantial experience performing hip resurfacing using other devices, and were willing to participate (Table 1). Each of these surgeons visited an expert in hip resurfacing and observed several hip resurfacing procedures just before starting to perform them on their own; and each surgeon had a technical specialist present for their first 10 cases. Three surgeons had performed more than 150 hip resurfacings and the other two had performed over 100 hip resurfacings between June 2006 and October 2007. None of these hip resurfacings were performed using computer-assisted navigation. Given the retrospective nature of the study patient selection criteria were not established and varied between the surgeons. We obtained Institutional Review Board approval at Washington University School of Medicine to conduct this multicenter research study.
Table 1.
Surgeon demographics
| Surgeon number | Years in practice | Number of joint arthroplasties per year | Percentage of practice doing joint arthroplasties | Percentage of practice doing hip arthroplasties | Number of hip arthroplasties per year | Fellowship-trained |
|---|---|---|---|---|---|---|
| 1 | 17 | 325 | 95 | 45 | 180 | No |
| 2 | 23 | 600 | 99 | 40 | 250 | Yes |
| 3 | 22 | 360 | 99 | 50 | 180 | Yes |
| 4 | 17 | 395 | 60 | 60 | 240 | No |
| 5 | 28 | 300 | 70 | 75 | 250 | No |
| Average | 21.4 | 396 | 84.6 | 54 | 220 |
At a minimum of 1 year followup, we reviewed the first 650 hip resurfacing procedures performed by the five surgeons, and all had complete clinical and radiographic records; no patients were seen in followup specifically for this study. The mean age of the patients was 52.6 years (range, 29–81 years), and only 20 patients (17 men, three women) (3.1%) were aged 65 years or older at the time of surgery. Overall, there were 469 men (72.2%) and 181 women (27.8%). The majority of patients had a diagnosis of osteoarthritis (90.3%), and only 3.2% had a diagnosis of avascular necrosis (Table 2).
Table 2.
Patient demographics
| Variable | Surgeon | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Mean age of patients (years) | 49.7 (range, 35–65) | 50.7 (range, 29–64) | 51.8 (range, 34–81) | 55.7 (range, 34–74) | 53.7 (range, 30–81) |
| Older than 55 years | 24/100 (M: 21; F: 3) | 27/100 (M: 20; F: 7) | 45/150 (M: 36; F: 9) | 80/150 (M: 63; F: 17) | 61/150 (M: 32; F 29) |
| Older than 65 years | 0 | 0 | 4/150 (M: 3; F: 1) | 13/150 (M: 12; F: 1) | 3/150 (M: 2; F: 1) |
| Gender | |||||
| Male | 78/100 | 71/100 | 113/150 | 115/150 | 92/150 |
| Female | 22/100 | 29/100 | 37/150 | 35/150 | 58/150 |
| Side of operation | |||||
| Left | 55/100 | 44/100 | 65/150 | 71/150 | 75/150 |
| Right | 45/100 | 56/100 | 85/150 | 79/150 | 75/150 |
| Diagnosis | |||||
| Osteoarthritis | 92/100 (92%) | 91/100 (91%) | 133/150 (88.7%) | 132/150 (88%) | 139/150 (92.7%) |
| Avascular necrosis | 1/100 (1%) | 4/100 (4%) | 9/150 (6%) | 7/150 (4.7%) | 0 |
| Rheumatoid arthritis | 0 | 1/100 (1%) | 1/150 (0.7%) | 0 | 1/150 (0.7%) |
| Developmental dysplasia of the hip | 7/100 (7%) | 3/100 (3%) | 2/150 (1.3%) | 8/150 (5.3%) | 9/150 (6%) |
| Posttraumatic arthritis | 0 | 1/100 (1%) | 5/150 (3.3%) | 1/150 (0.7%) | 1/150 (0.7%) |
| Legg-Calvé-Perthes disease | 0 | 0 | 0 | 2/150 (1.3%) | 0 |
M = male; F = female.
Deidentified patient demographics, radiographic measurements, and adverse events were entered into a master database. The preoperative and 6-week postoperative radiographs on a consecutive series of 650 hips were retrospectively compiled, reviewed, and measured by one blinded joint reconstruction fellow (RMN) who has previous experience making radiographic measurements around the hip. All patients had standardized preoperative and 6-week postoperative radiographs. An independent biostatistician (JQZ) was used to analyze the data and run the statistical modeling.
Standardized anteroposterior (AP) pelvis radiographs with the patient supine and the feet internally rotated 15° to 20° were used at all sites. The xray beam was centered on the pubic symphysis at a standardized distance of 100 cm. Preoperative AP pelvis radiographs were measured to determine the neck-shaft angle (NSA). The NSA was determined by measuring the angle between the midline of the proximal femoral diaphysis and the anatomic axis of the femoral neck [46] (Fig. 1). The 6-week postoperative AP pelvis radiographs were measured to determine the femoral stem-shaft angle (SSA), the presence or absence of a femoral notch, and acetabular inclination. The femoral SSA was measured as the angle between the midline of the proximal femoral diaphysis and the axis of the femoral component stem (Fig. 2). We presumed the ideal positioning of the femoral component is in slight valgus compared with the preoperative NSA [20, 29, 36, 43]. The acetabular component inclination was measured as the angle between a horizontal line across the bottom of both ischial tuberosities and a line tangent to the superior and inferior edges of the acetabular component (Fig. 2) [14, 26]. In this study, acetabular inclination was divided into three groups: (1) less than 45°; (2) 46° to 55°; and (3) greater than 55°.
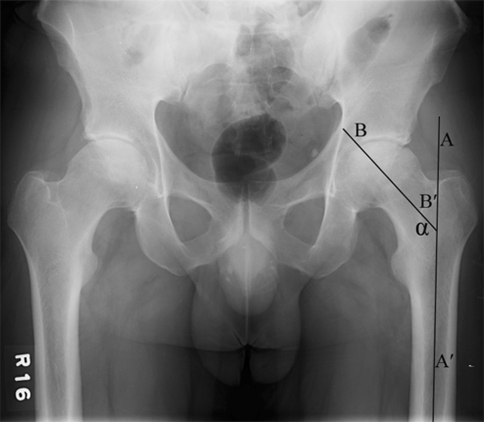
Fig. 1.
The femoral neck-shaft angle (NSA) was measured on the preoperative anteroposterior pelvic radiographs. The NSA was defined as the angle (α) between the midline of the proximal femoral diaphysis (AA′) and the anatomic axis of the neck (BB′).
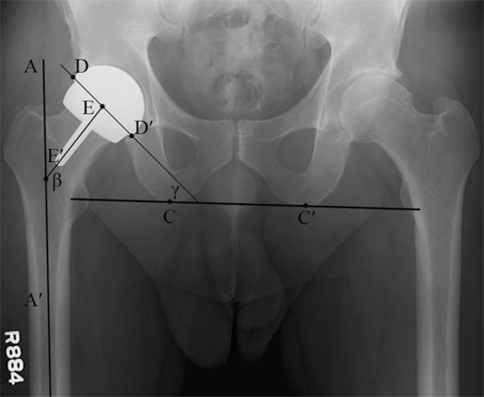
Fig. 2.
Component positioning was assessed on the postoperative anteroposterior pelvic radiograph. The femoral stem-shaft angle (SSA) (β) was measured as the angle between the midline of the proximal femoral diaphysis (AA′) and the axis of the femoral component stem (EE′). The acetabular component inclination (γ) was measured as the angle between a horizontal line across the bottom of both ischial tuberosities (CC′) and a line tangent to the superior and inferior edges of the acetabular component (DD′).
Lateral radiographs from the 6-week postoperative visit were also measured. Two of the surgeons only routinely performed frog-lateral radiographs and the other three surgeons only used shoot-through crosstable lateral radiographs. The crosstable lateral radiographs were taken with the patient placed supine and the contralateral hip and knee flexed to elevate the thigh in a vertical position. The patient’s pelvis was adjusted to prevent rotation. The xray beam was angled 45° from the long axis of the body in the cephalad direction and centered on the femoral head. Acetabular anteversion was measured on the crosstable lateral images for the three surgeons who use them (Fig. 3). We divided the acetabular anteversion measurements into two groups: (1) less than or equal to 30°; and (2) greater than 30°. The frog-leg lateral view was taken with the patient supine, the ipsilateral knee flexed, and the leg abducted so the sole of the foot contacted the contralateral leg at the level of the knee; then the leg was externally rotated while ensuring the pelvis did not rotate away from the plane of the table. The xray beam was directed anterior to posterior and centered on the femoral head. The anteversion on the crosstable lateral radiograph was determined as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the line of long axis plane of the body [6, 49] (Fig. 3).
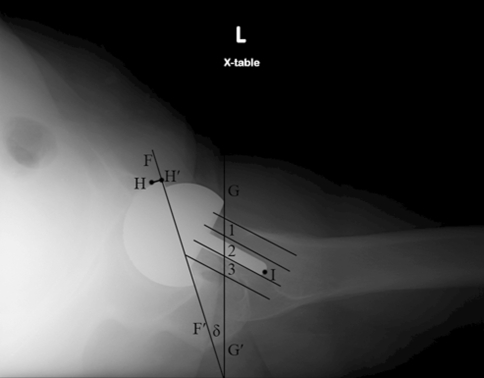
Fig. 3.
Component position was assessed on postoperative lateral radiographs. The acetabular component anteversion was defined as the angle (δ) between the projected long axis of the acetabular opening (FF′) and a line drawn perpendicular to the line of long axis plane of the body (GG′). The amount of the acetabular component lacking bony coverage on lateral radiographs was measured as the distance between the anterior edge of acetabular component and the anterior edge of acetabular bone (HH′). The femoral neck was divided into three equal zones: anterior (Zone 1), central (Zone 2), and posterior (Zone 3). The zones were determined by making tangential lines to the anterior and posterior cortex of the femoral neck at the apex of their curvature and then dividing the distance between these two lines into three equal zones. The position of the femoral stem tip (I) was determined to be in the anterior, center, or posterior zone.
To determine the femoral stem position on the lateral radiographs, the femoral neck was divided into three equal zones—anterior, central, and posterior—by making tangential lines to the anterior and posterior cortex of the femoral neck at the apex of their curvature and then dividing the distance between these two lines into three equal zones (Fig. 3). On the lateral radiographs of some patients, the tip of the femoral stem was touching the anterior or posterior cortex of the femoral neck. The presence or absence of this unique finding was documented on all patients because we were uncertain of the clinical importance of this observation (Fig. 4A–B) and could not find that it had been previously described in the literature.
Fig. 4A–B.
The tip of femoral stem touching the (A) anterior or (B) posterior femoral neck cortical bone is shown. We are uncertain as to the clinical importance of these findings.
To explore the learning curve characteristics, the patients were classified and analyzed in increments of 25 for the first 100 cases per surgeon (1–25, 26–50, 51–75, 76–100) with each incremental group containing a total of 125 cases (25 cases for each of the five surgeons). Thereafter, the three surgeons performing 150 cases had each of their remaining 50 cases pooled into one group of 150 cases (cases 101–150 for each of the three surgeons). Major complications for this study were defined as femoral neck fracture, dislocation, component failure, infection, or nerve injury.
To examine the outcomes across strata, e.g., the first 25 cases per surgeon, second 25 cases and so forth, Pearson’s Chi Square tests were carried out. In addition, complications and femoral component tip touching the femoral neck cortex occurred in a very small population of the patients, and Fisher’s exact tests were therefore adopted for better estimation. To picture learning curves, further examinations were carried out to determine improvements. The cutoff points were decided arbitrarily largely based on histogram demonstrations. For instance, if any undesirable outcome rates dropped sharply right after the first 25 cases, then the first 25 cases would be compared to all other cases. The same would be true if the first 50 cases were picked for comparison. The significance level was set to 0.05 two-sided. All statistical analyses were performed using SAS Version 9.1 (SAS Institute Inc, Cary, NC).
Results
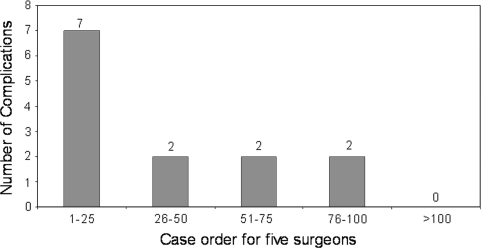
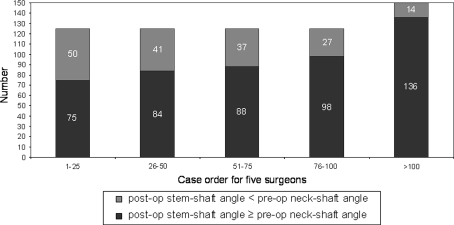
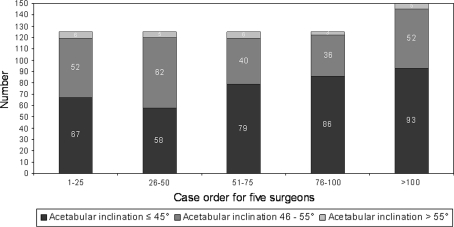
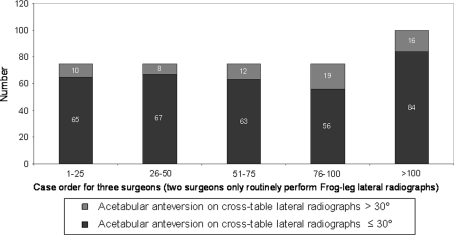
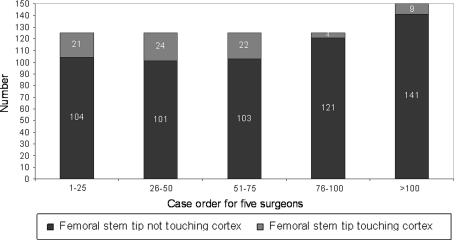
There were 13 major complications (2%) with seven hips (1.1%) requiring reoperation (Table 3). The major complication rate was greater (p < 0.002) for the surgeons’ first 25 cases compared to the second 25 cases (seven of 125 or 5.6% versus two of 125 or 1.6%, respectively) (Fig. 5). We observed 169 hips (26%) with the femoral component placed in relative varus; of these 169 femoral stems in varus, 106 hips (16.3%) had 1° to 5° of varus, and 63 hips (9.7%) had greater than 5° of varus (Fig. 6). The learning curve for avoiding relative femoral component varus placement continued to gradually improve but did not improve until comparing the surgeons’ first 100 cases to the next 50 cases (31% versus 14% respectively, p < 0.004). We found 267 hips (41%) with an acetabular component inclination greater than 45 degrees; of these 267 acetabular components there were 24 hips (4%) that had greater than 55 degrees of inclination (Fig. 7). The learning curve for optimal acetabular component inclination showed a difference (p < 0.0008) between the surgeons’ first 50 cases and their second 50 cases (50% versus 66% respectively). The acetabular anteversion measurements on crosstable lateral radiographs did not show an improvement over the surgeons’ learning curve, but there was a trend towards improvement after the first 75 cases for the surgeons (Fig. 8). We observed 16% of cases after the surgeons’ first 100 cases with acetabular version greater than 30°. Review of the lateral radiographs found that the tip of the femoral stem touched the femoral neck cortex in 80 patients (12.3%) with the majority of cases having the tip touch the anterior cortex (76 of 80 or 95%) (Fig. 9). The learning curve to avoid having the tip of the femoral component touch the femoral neck cortex on lateral radiographs was different (p < 0.0001) between the surgeons’ first 75 cases and their second 75 cases (21% versus 4% respectively).
Table 3.
Major complications
| Case number | Age (years) | Gender | Diagnosis | Type of complication | Outcome |
|---|---|---|---|---|---|
| 2 | 50 | M | DDH | Femoral neck fracture | Converted to THA |
| 2 | 58 | M | OA | Nerve injury | Unresolved |
| 5 | 49 | M | AVN | Femoral neck fracture | Converted to THA |
| 12 | 59 | F | DDH | Dislocation | Closed reduction |
| 17 | 65 | F | OA | Dislocation | Closed reduction |
| 17 | 56 | M | OA | Femoral neck fracture | Converted to THA |
| 25 | 60 | M | OA | Nerve injury | Recovery |
| 27 | 50 | M | DDH | Dislocation | Closed reduction |
| 35 | 62 | F | OA | Dislocation | Converted to THA |
| 57 | 41 | M | OA | Infection | Converted to THA |
| 63 | 56 | F | OA | Nerve injury | Recovery |
| 77 | 47 | M | OA | Dislocation | Converted to THA |
| 80 | 34 | F | DDH | Failure acetabular bone ingrowth | Converted to THA |
M = male; F = female; AVN = avascular necrosis; DDH = developmental dysplasia of the hip; OA = osteoarthritis.
Fig. 5.
Total major complications observed for all 5 surgeons were divided into groups of 25 patients per surgeon to establish the learning curve for major complications. The histograms show improvement (p < 0.002) between the surgeons’ first cohort of 25 patients and their second cohort of 25 patients.
Fig. 6.
Measurements from anteroposterior radiographs comparing the stem-shaft angle (SSA) to the preoperative neck-shaft angle (NSA) demonstrate a gradual improvement in the learning curve for femoral component positioning during the surgeons first 100 patients. Over 9% of femoral components were still placed in relative varus after the surgeons’ first 100 procedures.
Fig. 7.
Measurements of acetabular component inclination demonstrate an improvement (p = 0.0008) in the surgeons’ learning curve between the first 50 patients and the second cohort of 50 patients.
Fig. 8.
Measurements of acetabular component anteversion on cross-table lateral radiographs did not demonstrate a substantial learning curve for the surgeons.
Fig. 9.
The position of the tip of the femoral component on lateral radiographs improved after the first 75 procedures. We do not currently know if this radiographic finding will ultimately be clinically important.
The overall major complication rate was relatively low (2.0%) for all five surgeons and individual complication rate was similar among all five surgeons. There were three femoral neck fractures (0.46%), which all occurred in the first 25 cases performed by the surgeons and, in all three cases, there was relative varus placement of the femoral component with postoperative SSA less than preoperative NSA (Table 4). Two of these patients had radiographic evidence of femoral neck notching on their postoperative radiographs. There were five hip dislocations (0.77%) and four of these occurred in the first 35 cases performed by the surgeons (Table 5). In these patients with dislocations, three were female and three patients had a diagnosis of developmental dysplasia of the hip. Acetabular inclination was greater than 45° in four of the hips with dislocations. All hip dislocations were initially treated with closed reduction, but two were believed unstable and were converted to a THA. There were three nerve injuries (0.46%) and two of these occurred in the first 25 cases performed by the surgeons (Table 6). Only two of these were a direct result of the surgical procedure, and one was the result of compression of the peroneal nerve at the level of the fibular head. There were two additional major complications: one deep infection (0.15%) and one apparent acetabular component failure of fixation (0.15%).
Table 4.
Femoral neck fractures
| Case number | Age (years) | Gender | Diagnosis | Outcome | Preoperative NSA | Postoperative SSA | Femoral notch |
|---|---|---|---|---|---|---|---|
| 2 | 50 | M | DDH | Converted to THA | 130 | 129 | No |
| 5 | 49 | M | AVN | Converted to THA | 145 | 140 | Yes |
| 17 | 56 | M | OA | Converted to THA | 138 | 137 | Yes |
NSA = neck-shaft angle; SSA = stem-shaft angle; M = male; DDH = developmental dysplasia of the hip; AVN = avascular necrosis; OA = osteoarthritis.
Table 5.
Dislocations
| Case number | Age (years) | Gender | Diagnosis | Treatment | Acetabular inclination | Acetabular version |
|---|---|---|---|---|---|---|
| 12 | 59 | F | DDH | Closed reduction | 51 | 32 |
| 17 | 65 | F | OA | Closed reduction | 42 | 17 |
| 27 | 50 | M | DDH | Closed reduction | 69 | N/A* |
| 35 | 62 | F | OA | Converted to THA | 49 | 19 |
| 77 | 47 | M | DDH | Converted to THA | 48 | N/A* |
* Patient only had frog lateral images so acetabular version could not be measured; F = female; M = male; DDH = developmental dysplasia of the hip; OA = osteoarthritis.
Table 6.
Nerve injuries
| Case number | Age (years) | Gender | Diagnosis | Nerve deficit | Outcome |
|---|---|---|---|---|---|
| 2 | 58 | M | OA | Complete peroneal | *Unresolved |
| 25 | 60 | M | OA | Peroneal | Full recovery |
| 63 | 56 | F | OA | Peroneal at fibular head | Full recovery |
* Patient has refused followup and is being treated at another institution for nerve injury; M = male; F = female; OA = osteoarthritis.
Discussion
Evidence has accumulated indicating optimal component position is an important aim of hip resurfacing and failure to achieve this goal is associated with negative consequences. The component positioning parameters most strongly associated with a low early complication rate and high survivorship long-term include acetabular component inclination and femoral stem shaft angle [19, 22, 23, 31, 37, 38, 43, 47]. Therefore, we used these measures to determine the learning curves. Other radiographic measures suggested as possibly important in successful resurfacing include acetabular version and the femoral stem position on lateral imaging (impingement, retroversion, and touching the femoral cortex) [10, 15, 24, 33, 38, 39, 42, 47]. The data supporting the importance of these measures is less compelling, so in this study, they were termed “secondary goals.” In this study, we measured the preoperative and postoperative radiographs to determine the learning curve associated with hip resurfacing by a group of hip specialists, and we compared the rate of early, major complications between this group and our previously published national safety survey data [21].
The limitations of our study include the fact that the measurements were performed by a single reviewer, there was no defined standardization of patient selection by the surgeons, and it has a relatively short clinical followup period. Although all images were measured in a blinded and randomized order to avoid potential bias, there were no intra- or interobserver measurements performed and it is well known that plain radiographs have a certain degree of variability as a result of subtle differences in patient positioning and imaging technique. However, we included multiple experienced hip surgeons at different geographic locations throughout the United States each performing 100 or more hip resurfacing procedures, had well-maintained joint registries, and 100% availability of pre- and postoperative radiographs. Longer followup is necessary to determine implant survivorship and the effect of femoral and acetabular component positioning on functional scores and wear rates.
Numerous studies have correlated acetabular component inclination greater than 50° to 55° with increased metal ion levels, metallosis, pseudotumors, and early failure [19, 22, 23, 31, 34]. In this study, acetabular inclination less than 45° was achieved in 58.9% of cases and it appears the learning curve continued to slowly improve through the first 150 cases. In a similar radiographic learning curve study looking at the first 40 hip resurfacings performed by a single surgeon without computer assistance, Witjes et al. [48] reported finding steeper acetabular component abduction angles measured during the first cohort of 10 patients (mean, 52°) compared with later cohorts. Their study had a relatively small number of patients and the differences among the four groups did not reach statistical significance [48]. We found a similar trend but with slower improvement in acetabular component abduction angles, and our data were alarming in the fact that 13.3% of hips, following the first 500 hip resurfacings performed, had acetabular components placed in greater than 50° of inclination. Because acetabular inclination greater than 50° is one of the biggest risk factors for metal ion production, there is a need to improve acetabular component positioning at a minimum to potentially avoid future problems related to increase metal ion production.
Achieving relative femoral component valgus has been well described in the literature to be an important factor in avoiding fracture [16, 42, 44]. Several published articles have examined the learning curve associated with optimal placement of the femoral component in hip resurfacing [17, 38, 41, 48]. Three of these studies used some form of computer navigation to help with femoral head preparation [17, 38, 41]. In these studies, the authors concluded the addition of computer assistance shortened the learning curve, provided a more reliable and accurate method of positioning the femoral component in valgus, and reduced the potential risk of notching of the femoral neck. We observed a large number of femoral components (169 hips [26%]) placed into relative varus, including 63 hips (9.7%) with greater than 5° of varus, and a persistent level of varus in 9.3% of cases after the first 500 hip resurfacings. Thus, there is still room for improvement in femoral component positioning and our findings may support the use of some form of computer assistance to shorten the learning curve and improve the accuracy of femoral component placement.
We had an overall major complication rate of 2% (13 of 650) and a revision rate of 1.1% (seven of 650) at a minimum of 12 months followup. This is lower than the previously reported US national safety survey study of 537 hip resurfacing cases performed by 89 surgeons with a 7.4% complication rate at 12 months [21]. Most major complications (seven of 13 [54%]) in this study occurred during the first 25 cases performed by these surgeons. There was a statistically significant drop in the complication rate between the first grouping of 25 patients and the second grouping of 25 patients suggesting improved results with surgeon experience. In the Australian registry, the revision rate for hip resurfacing (excluding infection) was 1.7% at 1 year and 1.2% for conventional THA [7]. In our study, the overall rate of revision (excluding infection) was 0.9%, and these findings compare favorably to the Australian Registry and to other published reports [2, 8, 18, 20, 24, 25, 45].
Patient demand and surgeon interest in hip resurfacing has recently increased, but surgeons in the United States are relatively inexperienced with this procedure. The stakes are much higher with hip resurfacing than with THA because failure to achieve optimal component positioning can lead to early failure and with the current nonmodular hip resurfacing components, there are few options available to surgeons short of complete revision once a patient develops problems related to suboptimal component position.
There is a high degree of variability in the reported incidence of femoral neck fracture, femoral loosening, metal ion production, metal hypersensitivity, aseptic lymphocytic vasculitis-associated lesions, pseudotumors, and hip impingement (groin pain) after hip resurfacing. It is conceivable, but not proven at this point, that much of this variability may be explained by the relative inconsistency of component positioning observed in this study of high-volume, experienced hip surgeons. If this proves to be the case, a higher degree of precision and accuracy may be necessary to achieve the consistently high level of function and implant survival anticipated for this procedure.
Our data show there is a long learning curve associated with hip resurfacing and the trends we observed from radiographic measurements indicate some parameters continue to improve beyond the first 100 cases performed by experienced hip surgeons. As the number of surgeons performing hip resurfacing continues to expand, we hope this technically demanding procedure will be approached with caution to avoid early, major complications and malpositioning of the components.
Acknowledgments
We thank Jean (Qin) Zhang, Division of Biostatistics at Washington University School of Medicine, for her help with the statistical analyses.
Footnotes
One of the authors (RMN) has received institutional research support from Smith & Nephew (Memphis, TN). One of the authors (PJB) is a consultant for Stryker Orthopaedics (Mahwah, NJ) and Smith & Nephew. One of the authors (CAE) is a consultant for LifeNet (Virginia Beach, VA), Smith & Nephew, and DePuy Orthopaedics (Warsaw, IN) and receives institutional support from Inova Health System (Fairfax, VA). One of the authors (SJR) is a consultant for Smith & Nephew. One of the authors (JSR) is a consultant for Smith & Nephew but has received no remuneration. One of the authors (RLB) is a designer (royalty income) for Smith & Nephew and receives institutional research support from Smith & Nephew.
Washington University institutional review board approved the protocol for this investigation and all investigations were conducted in conformity with ethical principles of research.
This work was performed at Washington University, St Louis, MO, USA.
References
- 1.Amstutz HC, Ball ST, Le Duff MJ, Dorey FJ. Resurfacing THA for patients younger than 50 years: results of 2- to 9-year followup. Clin Orthop Relat Res. 2007;460:159–164. doi: 10.1097/BLO.0b013e318041f0e7. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz HC, Beaule PE, Dorey FJ, Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 3.Amstutz HC, Campbell PA, Duff MJ. Fracture of the neck of the femur after surface arthroplasty of the hip. J Bone Joint Surg Am. 2004;86:1874–1877. doi: 10.2106/00004623-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Amstutz HC, Grigoris P, Dorey FJ. Evolution and future of surface replacement of the hip. J Orthop Sci. 1998;3:169–186. doi: 10.1007/s007760050038. [DOI] [PubMed] [Google Scholar]
- 5.Anglin C, Masri BA, Tonetti J, Hodgson AJ, Greidanus NV. Hip resurfacing femoral neck fracture influenced by valgus placement. Clin Orthop Relat Res. 2007;465:71–79. doi: 10.1097/BLO.0b013e318137a13f. [DOI] [PubMed] [Google Scholar]
- 6.Arai N, Nakamura S, Matsushita T. Difference between 2 measurement methods of version angles of the acetabular component. J Arthroplasty. 2007;22:715–720. doi: 10.1016/j.arth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Australian Orthopaedic Association. AOA Joint Replacement Registry. Updated January 2008. Available at: http://www.aoa.org.au/jointregistry.asp. Accessed September 29 2008.
- 8.Back DL, Dalziel R, Young D, Shimmin A. Early results of primary Birmingham hip resurfacings. J Bone Joint Surg Br. 2005;87:324–329. doi: 10.1302/0301-620X.87B3.15556. [DOI] [PubMed] [Google Scholar]
- 9.Beaule PE, Dorey FJ, LeDuff M, Gruen T, Amstutz HC. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin Orthop Relat Res. 2004;418:87–93. doi: 10.1097/00003086-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Beaule PE, Harvey N, Zaragoza E, Duff MJ, Dorey FJ. The femoral head/neck offset and hip resurfacing. J Bone Joint Surg Br. 2007;89:9–15. doi: 10.2106/JBJS.F.00681. [DOI] [PubMed] [Google Scholar]
- 11.Beaule PE, LeDuff M, Campbell P, Dorey FJ, Park SH, Amstutz HC. Metal-on-metal surface arthroplasty with a cemented femoral component: a 7–10 year follow-up study. J Arthroplasty. 2004;19(Suppl 3):17–22. doi: 10.1016/j.arth.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Beaule PE, Poitras P. Femoral component sizing and positioning in hip resurfacing arthroplasty. Instr Course Lect. 2007;56:163–169. [PubMed] [Google Scholar]
- 13.Boyd HS, Ulrich SD, Seyler TM, Marulanda GA, Mont MA. Resurfacing for Perthes disease: an alternative to standard hip arthroplasty. Clin Orthop Relat Res. 2007;465:80–85. doi: 10.1097/BLO.0b013e318156bf76. [DOI] [PubMed] [Google Scholar]
- 14.Brodner W, Grubl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19(Suppl 3):66–70. doi: 10.1016/j.arth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Buergi ML, Walter WL. Hip resurfacing arthroplasty: the Australian experience. J Arthroplasty. 2007;22(Suppl 3):61–65. doi: 10.1016/j.arth.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Campbell P, Beaule PE, Ebramzadeh E, LeDuff M, Smet K, Lu Z, Amstutz HC. The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res. 2006;453:35–46. doi: 10.1097/01.blo.0000238777.34939.82. [DOI] [PubMed] [Google Scholar]
- 17.Cobb JP, Kannan V, Brust K, Thevendran G. Navigation reduces the learning curve in resurfacing total hip arthroplasty. Clin Orthop Relat Res. 2007;463:90–97. doi: 10.1097/BLO.0b013e318126c0a5. [DOI] [PubMed] [Google Scholar]
- 18.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. doi: 10.1302/0301-620X.86B2.14600. [DOI] [PubMed] [Google Scholar]
- 19.Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 20.Smet KA. Belgium experience with metal-on-metal surface arthroplasty. Orthop Clin North Am. 2005;36:203–213. doi: 10.1016/j.ocl.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Valle CJ, Nunley RM, Raterman SJ, Barrack RL. Initial American experience with hip resurfacing following FDA approval. Clin Orthop Relat Res. 2009;467:72–78. doi: 10.1007/s11999-008-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart AJ, Buddhdev P, Winship P, Faria N, Powell JJ, Skinner JA. Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after metal-on-metal hip resurfacing. Hip Int. 2008;18:212–219. doi: 10.1177/112070000801800304. [DOI] [PubMed] [Google Scholar]
- 23.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 24.Lavigne M, Rama KR, Roy A, Vendittoli PA. Painful impingement of the hip joint after total hip resurfacing: a report of two cases. J Arthroplasty. 2008;23:1074–1079. doi: 10.1016/j.arth.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Lilikakis AK, Vowler SL, Villar RN. Hydroxyapatite-coated femoral implant in metal-on-metal resurfacing hip arthroplasty: minimum of two years follow-up. Orthop Clin North Am. 2005;36:215–222. doi: 10.1016/j.ocl.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009 May 2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 27.Marker DR, Seyler TM, Jinnah RH, Delanois RE, Ulrich SD, Mont MA. Femoral neck fractures after metal-on-metal total hip resurfacing: a prospective cohort study. J Arthroplasty. 2007;22(Suppl 3):66–71. doi: 10.1016/j.arth.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 28.McMinn D, Daniel J. History and modern concepts in surface replacement. Proc Inst Mech Eng [H] 2006;220:239–251. doi: 10.1243/095441105X68944. [DOI] [PubMed] [Google Scholar]
- 29.Mont MA, Seyler TM, Ulrich SD, Beaule PE, Boyd HS, Grecula MJ, Goldberg VM, Kennedy WR, Marker DR, Schmalzried TP, Sparling EA, Vail TP, Amstutz HC. Effect of changing indications and techniques on total hip resurfacing. Clin Orthop Relat Res. 2007;465:63–70. doi: 10.1097/BLO.0b013e318159dd60. [DOI] [PubMed] [Google Scholar]
- 30.Morlock MM, Bishop N, Ruther W, Delling G, Hahn M. Biomechanical, morphological, and histological analysis of early failures in hip resurfacing arthroplasty. Proc Inst Mech Eng [H] 2006;220:333–344. doi: 10.1243/095441105X69015. [DOI] [PubMed] [Google Scholar]
- 31.Morlock MM, Bishop N, Zustin J, Hahn M, Ruther W, Amling M. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg Am. 2008;90(Suppl 3):89–95. doi: 10.2106/JBJS.H.00621. [DOI] [PubMed] [Google Scholar]
- 32.Nunley RM, Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467:56–65. doi: 10.1007/s11999-008-0558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong KL, Kurtz SM, Manley MT, Rushton N, Mohammed NA, Field RE. Biomechanics of the Birmingham hip resurfacing arthroplasty. J Bone Joint Surg Br. 2006;88:1110–1115. doi: 10.1302/0301-620X.88B8.17567. [DOI] [PubMed] [Google Scholar]
- 34.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 35.Radcliffe IA, Taylor M. Investigation into the effect of cementing techniques on load transfer in the resurfaced femoral head: a multi-femur finite element analysis. Clin Biomech (Bristol, Avon) 2007;22:422–430. doi: 10.1016/j.clinbiomech.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Radcliffe IA, Taylor M. Investigation into the effect of varus-valgus orientation on load transfer in the resurfaced femoral head: a multi-femur finite element analysis. Clin Biomech (Bristol, Avon). 2007;22:780–786. doi: 10.1016/j.clinbiomech.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Richards CJ, Giannitsios D, Huk OL, Zukor DJ, Steffen T, Antoniou J. Risk of periprosthetic femoral neck fracture after hip resurfacing arthroplasty: valgus compared with anatomic alignment. A biomechanical and clinical analysis. J Bone Joint Surg Am. 2008;90(Suppl 3):96–101. doi: 10.2106/JBJS.H.00444. [DOI] [PubMed] [Google Scholar]
- 38.Romanowski JR, Swank ML. Imageless navigation in hip resurfacing: avoiding component malposition during the surgeon learning curve. J Bone Joint Surg Am. 2008;90(Suppl 3):65–70. doi: 10.2106/JBJS.H.00462. [DOI] [PubMed] [Google Scholar]
- 39.Schmalzried TP. Why total hip resurfacing. J Arthroplasty. 2007;22(Suppl 3):57–60. doi: 10.1016/j.arth.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 40.Schmalzried TP, Fowble VA, Ure KJ, Amstutz HC. Metal on metal surface replacement of the hip. Technique, fixation, and early results. Clin Orthop Relat Res. 1996;329(Suppl):S106–S114. doi: 10.1097/00003086-199608001-00011. [DOI] [PubMed] [Google Scholar]
- 41.Seyler TM, Lai LP, Sprinkle DI, Ward WG, Jinnah RH. Does computer-assisted surgery improve accuracy and decrease the learning curve in hip resurfacing? A radiographic analysis. J Bone Joint Surg Am. 2008;90(Suppl 3):71–80. doi: 10.2106/JBJS.H.00697. [DOI] [PubMed] [Google Scholar]
- 42.Shimmin A, Beaule PE, Campbell P. Metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2008;90:637–654. doi: 10.2106/JBJS.G.01012. [DOI] [PubMed] [Google Scholar]
- 43.Shimmin AJ, Back D. Femoral neck fractures following Birmingham hip resurfacing: a national review of 50 cases. J Bone Joint Surg Br. 2005;87:463–464. doi: 10.1302/0301-620X.87B4.15498. [DOI] [PubMed] [Google Scholar]
- 44.Shimmin AJ, Bare J, Back DL. Complications associated with hip resurfacing arthroplasty. Orthop Clin North Am. 2005;36:187–193. doi: 10.1016/j.ocl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. doi: 10.1302/0301-620X.87B2.15030. [DOI] [PubMed] [Google Scholar]
- 46.Vail TP, Glisson RR, Dominguez DE, Kitaoka K, Ottaviano D. Position of hip resurfacing component affects strain and resistance to fracture in the femoral neck. J Bone Joint Surg Am. 2008;90:1951–1960. doi: 10.2106/JBJS.F.00788. [DOI] [PubMed] [Google Scholar]
- 47.Williams D, Royle M, Norton M. Metal-on-metal hip resurfacing: the effect of cup position and component size on range of motion to impingement. J Arthroplasty. 2009;24:144–151. doi: 10.1016/j.arth.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Witjes S, Smolders JM, Beaule PE, Pasker P, Van Susante JL. Learning from the learning curve in total hip resurfacing: a radiographic analysis. Arch Orthop Trauma Surg. 2009 Apr 21 [Epub ahead of print]. [DOI] [PubMed]
- 49.Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop Relat Res. 1995;316:106–111. [PubMed] [Google Scholar]