Abstract
Porous surfaces are intended to enhance osteointegration of cementless implants. Tantalum has been introduced in an effort to enhance osseointegration potential of uncemented components. We therefore compared the clinical outcome of acetabular components with two different porous surfaces. We retrospectively reviewed 283 patients (295 hips) who underwent cementless revision hip arthroplasty with either an HA-coated titanium cup (207 patients, 214 hips) or porous tantalum cup (79 patients, 81 hips). The minimum followup was 24 months in both groups (titanium: average 51.8 months, range, 24–98 months; tantalum: average, 35.4 months, range, 24–63 months). The titanium and tantalum groups had a mechanical failure rate (clinical plus radiographic) of 8% and 6%, respectively. In hips with minor bone deficiency (type 1, 2A, 2B using the classification of Paprosky et al.), 6% of titanium cups and 4% of tantalum cups failed. In hips with major bone deficiency (type 2C, 3), 24% of titanium cups and 12% of tantalum cups developed failure. In the major bone deficiency group, the tantalum cups had fewer numbers of lucent zones around the cup. Eighty-two percent of titanium cups that failed did so at 6 months postoperatively or later, whereas 80% of tantalum cups that failed did so in less than 6 months. Radiographically in the major group, tantalum cups yielded better fixation.
Level of Evidence: Level III, retrospective comparative study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The increasing number of total hip arthroplasty surgeries inevitably has led to an increased number of revision total hip surgeries. The demand for hip revision procedures is projected to double by the year 2026 [12]. Component selection continues to evolve, particularly in cases of major bone loss on the acetabular side, in which the surgeon encounters the dual tasks of restoring bone stock and obtaining stable fixation. The high survival rates of cementless implants in primary THA and the inconsistent reported survival rates for cemented revisions has resulted in surgeons shifting towards using cementless components for acetabular revision surgery and subsequently higher survival rates [18, 25]. Biologic fixation is possible with a variety of different cementless designs: plasma-sprayed titanium (with or without hydroxyapatite), sintered beads, and titanium fiber mesh [10, 21].
Acetabular components made of porous tantalum (trabecular metal), with a porosity of 75% to 85% have more recently become available and have had low failure rates. Van Kleunen et al. [27] reported no patients with aseptic failures among 97 revisions using a trabecular metal for revisions at a minimum of 2 years followup. Kim et al. [9] reviewed 46 acetabular revisions with Paprosky type 2 and 3 bone defects and reported one failure at a mean followup of 40 months in a patient who had a revision for a Paprosky Type 3B defect. Malkani et al. [14] studied a series of 22 patients with combined segmental and cavitary bone loss, Paprosky type 2 or 3. At a mean followup of 39 months, 21 of 22 patients had a well-fixed and functioning implant with ingrowth along the tantalum surface despite compromised host bone. There were no cases of aseptic loosening or dislocation at that time. The question is whether the low short-term failure rates in these three small series reflect an improvement over alternatives.
We therefore asked whether in the presence of any degree of bone deficiency (1) tantalum acetabular cups with bone ingrowth capability have less chance of mechanical failure than HA-coated titanium cups which have bone ongrowth potential, (2) tantalum cups show better osteointegration with less number of lucent zones in radiographic evaluation, and (3) whether the time to failure differed between the two groups.
Patients and Methods
We retrospectively retrieved the data of all patients who underwent cementless revision THA for aseptic failure of the acetabular cup between January 2000 and May 2006. Two hundred eighty-three patients (295 hips) had at least 2 years of followup or had mechanical failure of the acetabular component in shorter followup. The indications leading to index acetabular component revision in the titanium group (214 hips) were loosening in 163 (76%), instability due to malpositioned cup in 21 (10%), instability with loosening in 22 (10%), and osteolysis in eight (4%) hips. In the tantalum group (81 hips), the indications for acetabular cup revision were loosening in 69 (85%), instability attributable to malpositioned cup in six (7%), instability with loosening in four (5%), and osteolysis in two (3%) hips. We excluded 59 cases from the study. Of these, 11 cases were lost to followup before 2 years, nine patients had died within 2 years of surgery (without mechanical failure), seven hips had succumbed to septic failure, and the remaining 32 had a history of infected THA managed with two-stage revision surgery. Prior to the study we obtained Institutional Review Board approval.
We divided the patients into two groups based on the type of cementless cup inserted during the revision operation. The first group of patients had a hemispherical HA-coated titanium acetabular cup (Stryker Orthopaedics, Mahwah, NJ) with bone ongrowth capability. The second group had an elliptical tantalum acetabular cup, which is commercially available under the trademark Trabecular Metal (TM; Zimmer, Warsaw, IN) and has bone ingrowth capability. The hemispherical HA-coated titanium acetabular cup is produced from titanium-aluminum-vanadium alloy and coated with HA and titanium using the plasma spray process. The outer perimeter of the cup has a 1-mm expansion to enhance peripheral press-fit. The hemispherical tantalum acetabular cup has a 3-D structure of TM composed of a series of interconnected dodecahedron pores that are on average 550 Am in diameter [26]. The diameter of its equator is 2 mm larger than its polar diameter. Acetabular tantalum augments are available and are designed as an alternative to bone graft when major bone defects exist. These augments can provide mechanical support for the revision shell in situations where the cup by itself might not have reliable press-fit fixation [24].
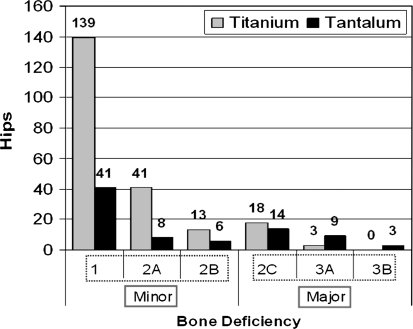
There were 155 men (55%) and 128 women (45%). The average age was 72 years (range, 34–91 years) and 66 years (range, 26–88 years) for the titanium and tantalum groups respectively. Minimum followup was 24 months (mean, 51.8 months; range, 24–98 months) in the titanium group, and 24 months (mean, 35.4 months; range, 24–63 months) in the tantalum group. There were 207 patients (214 hips) in the titanium and 79 patients (81 hips) in the tantalum groups. There were three patients who had a bilateral revision surgery with a titanium cup in one side and a tantalum cup in the other side. As a result, those patients were included in both groups. We observed no differences between the titanium and tantalum groups in regard to gender, BMI, age, and utilization of bone graft or screws. The majority of the hips (84%) had minor bone deficiencies (Type 1-2B using the classification of Paprosky et al. [19]). In the minor bone deficiency group there were 193 (78%) titanium and 55 (22%) tantalum cases. The major bone deficiency group (Type 2C-3B) consisted of 21 (45%) titanium and 26 (55%) tantalum cases (Fig. 1).
Fig. 1.
The distribution of the titanium and tantalum cups in the groups of Paprosky et al. classification is shown in this figure.
Prophylactic antibiotics were administered to all patients based on our institutional protocol. All operations were performed in supine position through a direct lateral approach. Then the hip was dislocated and the acetabular component was assessed. If the cup was loose or malpositioned it was carefully removed, with great care taken to protect the neurovascular structure and underlying host bone. A sliding trochanteric osteotomy was performed in 49 (17%) of the hips which was then fixed by cable(s). The femoral component was revised if the stem was loose or malpositioned. Bone graft was used for 16% and 20% of titanium and tantalum cups, respectively. Structural bone graft (distal femur in all cases) was used in one titanium cup revision (Type 3A), and six tantalum cup revisions (four with Type 3A bone deficiency, and two with Type 3B bone deficiency). All other cases had nonstructural morselized allograft bone. Tantalum acetabular augments were used in seven cases (four with Type 2C and three with Type 3A acetabular bone deficiency). Only one augment was used in conjunction with a distal femoral allograft. We found two cases in the tantalum group classified with a Type 3B bone deficiency and pelvic discontinuity. A distal femoral allograft and cup-cage technique [3, 8] was performed in these cases.
The regimen for thromboprophylaxis consisted of administration of warfarin on the day of surgery and continued for 6 weeks aiming for an international normalized ratio (INR) of 1.8 to 2.0. Patients were given intravenous first-generation cephalosporin (or vancomycin for those with allergy) before the skin incision and for 24 hours postoperatively. Patients were generally mobilized within 48 hours after surgery and followed THA precautions. They were kept 10% weight bearing with crutches for 6 weeks. Thereafter the crutches were discarded, and full weight bearing was allowed. Following surgery 198 (67%) of the patients were sent to a rehabilitation center.
Clinical followup and anteroposterior and lateral radiographs were obtained at 6 weeks, 6 months, 2 years, and then every 2 years after surgery. Two of us (SMJ and BB) reviewed all radiographs and made all radiographic observations. Three of us (WJH, SMJ, and BB) classified the preoperative acetabular bone deficiencies using the classification of Paprosky et al. [19]. Subsequently based on the type of bone deficiency, we divided the hips into two groups: minor including Paprosky 1, 2A and 2B, and major including Paprosky 2C, 3A and 3B. We (SMJ and BB) used the three acetabular zones of DeLee-Charnley model and the Anderson Orthopaedic Research Institute radiographic criteria to assess radiographic bone ingrowth [5, 17]. We conducted a power analysis. According to the available failure rates in major bone deficiency group, our study is underpowered.
We considered revision surgery for cup loosening as a clinical failure, and combined clinical and radiographic failure as mechanical failure. The functional scores of revision THAs are affected by both the femoral and acetabular components and such scores have little meaning for isolated components, therefore we centered our attention on re-revisions and radiographic observations. Cups were considered radiographic failures if any of the following criteria were identified: migration of cup more than 3 mm in either horizontal or vertical directions, breakage of screws, variation of cup angle greater than 5°, or radiolucent lines of 2 mm or more in all DeLee-Charnley zones. In both the minor and major bone deficiency groups we counted the number of the lucent zones around the cups that had a width of 2 mm or greater. We considered failures occurring less than 6 months or 6 months or more after surgery as early and late failures, respectively. Time to failure was defined as the period from index revision surgery to the date of mechanical failure.
Descriptive analysis was performed to report mean and standard deviations for the continuous variables such as age and body mass index (BMI). Frequency distribution (%) was reported for the categorical variables including gender (male/female), bone graft (yes/no), screws (yes/no), and failure (early/late). We compared the average measurements for the continuous variables such as age and BMI among dependent variables including clinical failure (yes/no), radiological failure (yes/no), and mechanical failure (yes/no). We performed nonparametric tests to compare these continuous variables across the outcomes. Categorical variables including group (titanium/tantalum), gender (male/female), bone graft (yes/no), screws (yes/no), and failure (yes/no) were compared across these dependent variables including clinical failure (yes/no), radiological failure (yes/no), and mechanical failure (yes/no). The independent categorical variables including group (titanium/tantalum), gender (male/female), bone graft (yes/no), screws (yes/no), and failure (yes/no) were compared using Fisher exact test since the number in either one of the cells was less than five. The independent average values of the continuous variables such as age and BMI were compared for lucent zone categories (including 0 versus 1 versus 2 versus 3) as a dependent variable using GLM analysis. All results have been reported as F statistics and p values. However, the categorical variables such as group (titanium versus tantalum), sex (males versus females), bone graft (yes versus no), screws (yes versus no), and failure (early versus late) were compared across the different categories of lucent zones using Fisher exact test. We used Kaplan-Meier survivorship analysis to estimate the probability of retention of the titanium and tantalum acetabular components from the time of the index revision to aseptic revision of the cup or definite radiographic loosening of the acetabular component. We compared the rate of survival between titanium and tantalum cups using the log-rank test. We used version 9.1 of SAS® software (SAS Institute Inc, Cary, NC) for the statistical analysis.
Results
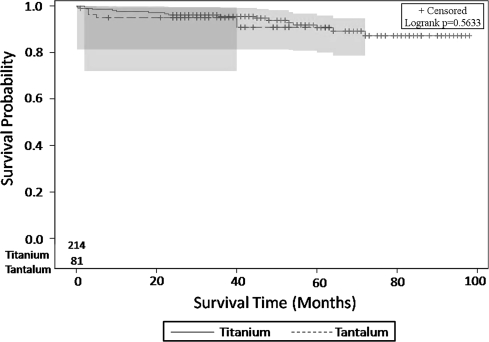
The survivorships of these two components were similar (Fig. 2). Eleven patients in the titanium group and one patient in tantalum group underwent re-revision surgery because of stem loosening but the cups were well-fixed and not revised. As a result these patients were not considered as failures of the cup for the analysis. Overall, the titanium group had a mechanical failure rate of 8% (17 of 214), including a clinical failure rate of 4% (nine of 214) and a radiographic failure rate of 4% (eight of 214) (Table 1). The tantalum group had a mechanical failure rate of 6% (five of 81), including a clinical failure rate of 4% (three of 81) and a radiographic failure rate of 3% (two of 81) (Table 1). In the minor deficiency group, titanium and tantalum cups had similar (p = 0.85) failure rates: the titanium cups had a mechanical failure rate of 6% (12 of 193), including seven (4%) clinical and five (2%) radiographic failures; the tantalum cups had a mechanical failure rate of 4% (two of 55) including one (2%) clinical and one (2%) radiographic failure. Similarly, in the major bone deficiency group both cups had similar failure rates (p = 0.27) despite the twofold higher incidence of failure in the titanium group (perhaps owing to an underpowered study): the titanium cups had a mechanical failure rate of 24% (five of 21) including two (10%) clinical and three (14%) radiographic failures, whereas the tantalum cups demonstrated a mechanical failure rate of 12% (three of 26) including two (8%) clinical and one (4%) radiographic failure.
Fig. 2.
This figure shows the Kaplan-Meier survivorship for titanium and tantalum acetabular cups with revision or definite radiographic loosening of the acetabular component as the end point. The survivorships of these two components were similar.
Table 1.
Characteristics of the failed acetabular cups
| Case | Age (years) | Gender | Type of acetabular cup | Type of bone deficiency | Time to failure (months) | Type of failure |
|---|---|---|---|---|---|---|
| 1 | 65 | M | Titanium | 2A | 0.25 | Clinical |
| 2 | 50 | M | Titanium | 1 | 9 | Clinical |
| 3 | 53 | M | Tantalum | 3B | 5 | Clinical |
| 4 | 66 | F | Titanium | 1 | 109 | Radiologic |
| 5 | 64 | M | Titanium | 2A | 48 | Radiologic |
| 6 | 58 | F | Titanium | 2C | 72 | Radiologic |
| 7 | 82 | F | Titanium | 1 | 53 | Clinical |
| 8 | 60 | F | Titanium | 1 | 60 | Clinical |
| 9 | 56 | M | Titanium | 2A | 4 | Clinical |
| 10 | 72 | M | Titanium | 1 | 64 | Radiologic |
| 11 | 55 | F | Titanium | 1 | 36 | Clinical |
| 12 | 83 | F | Titanium | 1 | 1 | Clinical |
| 13 | 60 | F | Titanium | 2C | 10 | Clinical |
| 14 | 66 | F | Tantalum | 1 | 3 | Clinical |
| 15 | 47 | F | Titanium | 1 | 23 | Radiologic |
| 16 | 55 | F | Titanium | 2C | 54 | Radiologic |
| 17 | 61 | M | Tantalum | 3A | 2 | Clinical |
| 18 | 82 | F | Tantalum | 2C | 40 | Radiologic |
| 19 | 88 | F | Titanium | 2B | 45 | Radiologic |
| 20 | 64 | M | Titanium | 3A | 22 | Radiologic |
| 21 | 57 | M | Titanium | 2C | 18 | Clinical |
| 22 | 82 | M | Tantalum | 1 | 3 | Radiologic |
In the major bone deficiency group, titanium cups had higher (p = 0.02) numbers of lucent zones compared to tantalum cups (Table 2). In the hips with minor bone deficiencies, titanium cups had a similar (p = 0.67) number of lucent zones 2 mm or larger in width than tantalum cups. All seven tantalum augments showed radiographic signs of osteointegration.
Table 2.
Distribution of the lucent zones around cups in minor and major bone deficiency groups
| Group | Number of lucent zones | Chi square | p Value | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Minor group | ||||||
| Titanium cup | 169 (87.6%) | 10 (5.2%) | 9 (4.6%) | 5 (2.6%) | 0.18 | 0.67 |
| Tantalum cup | 48 (87.3%) | 5 (9.1%) | 1 (1.8%) | 1 (1.8%) | ||
| Major group | ||||||
| Titanium cup | 9 (42.9%) | 4 (19%) | 5 (23.8%) | 3 (14.3%) | 5.01 | 0.02 |
| Tantalum cup | 22 (84.6%) | 0 | 2 (7.7%) | 2 (7.7%) | ||
Titanium cups had a tendency (p = 0.05) to fail after 6 months from the index revision surgery, whereas tantalum cups had a tendency to fail before 6 months after the index revision surgery. In the titanium group, we observed 17 aseptic failures of which 14 failed more than 6 months, and 3 failed less than 6 months after index revision surgery. In the tantalum group, five cups developed aseptic failure of which one failed more than 6 months, and four failed less than 6 months after index revision surgery (Table 3).
Table 3.
Time to failure of titanium and tantalum acetabular cups
| Group | Time to failure | p Value | |
|---|---|---|---|
| Early (< 6 mo) | Late (≥ 6 mo) | ||
| Both groups | |||
| Titanium cup | 3 (17.7%) | 14 (82.4%) | 0.01 |
| Tantalum cup | 4 (80%) | 1 (20%) | |
| Major group | |||
| Titanium cup | 0 | 5 (100%) | 0.05 |
| Tantalum cup | 2 (66.7%) | 1 (33.3%) | |
| Minor group | |||
| Titanium cup | 3 (25%) | 9 (75%) | 0.05 |
| Tantalum cup | 2 (100%) | 0 | |
Discussion
Acetabular bone deficiency can complicate revision THA surgery, compromising biologic fixation and often limiting implant options [19]. Mild acetabular deficiencies can be reconstructed with an uncemented hemispherical cup with or without morselized bone graft. However, severe acetabular defects might require special techniques or implants such as structural graft, oblong cup, a cage, or a cup-cage construct, depending on the type of bone deficiency [3, 23]. Conventional orthopaedic implants have typically been fashioned from cobalt-chromium or titanium alloys. Numerous surface coatings and porous designs have been developed to enhance biological fixation of these implants to bone for use in orthopaedic procedures. These materials have inherent limitations such as low volumetric porosity, relatively high modulus of elasticity, and low frictional characteristics [13]. In addition, conventional metals are typically not bioactive. In order to encourage and enhance the bonding of these porous metallic components to bone, various bioactive coatings, such as hydroxyapatite (HA), have been developed [6]. These bioactive materials degrade over time and have the potential to debond from the underlying metallic surface [20]. With the aim of addressing the limitations of these metals and coatings, porous tantalum components and augments has been developed and are currently available for use in both primary and revision THA [22]. This material offers a low modulus of elasticity, high surface frictional characteristics, high porosity (80%), and excellent osseointegration properties (ie, bioactivity, biocompatibility, and in-growth properties) [1, 4]. However, long-term followup is not yet available and the higher cost of this material has limited its widespread use [1, 2, 11, 15, 16]. We therefore asked whether in the presence of any degree of bone deficiency (1) tantalum acetabular cups with bone ingrowth capability have less chance of mechanical failure than HA-coated titanium cups which have bone ongrowth potential, (2) tantalum cups show better osteointegration with less number of lucent zones in radiographic evaluation, and (3) whether the time to failure differed between the two groups.
Several limitations warrant mention. First, this is not a study aimed at comparing the outcome at long term. We continue to follow the cohort of revision THA patients carefully, and aim to report the longer followup in the future. Second, the mean followup period of the tantalum cups is less than that of the titanium group, resulting in findings that could favor the tantalum component. Third, despite the lower incidence of failures in the tantalum group, with the number of cases we found no statistical differences (possible Type II error). With the inclusion of further cases this difference may reach statistical significance. At this point, however, the findings confirm tantalum cups are useful for reconstruction of acetabulum with complex bone loss. Finally, the findings of this study cannot be generalized to all traditional acetabular components, particularly those with ingrowth capacity such as sintered CoCr beads, titanium mesh, and titanium beads.
Our observations demonstrate the tantalum cups have a lower frequency of radiolucencies around the cup. All the tantalum augments showed evidence of osseointegration. The difference in performance between the HA-coated titanium cup and the tantalum cup was less apparent in situations of minor bone deficiency where enough contact between cup and host bone can be achieved and line-to-line fit with adjunctive fixation provides good initial stability. However, in situations of severe bone deficiency where the contact between cup and the host bone is compromised and press-fit stability is less easily obtained, the tantalum cups appeared to have a superior performance. Our data with the tantalum cup mirrors that of other reports. Unger et al. [26] evaluated revision THA in 60 patients for whom TM monoblock acetabular cups were implanted. The radiographic assessment of the frequency of gaps and radiolucencies in their study provided evidence that excellent bone ingrowth occurs with the TM monoblock acetabular component in revision THA. Their cases consisted of 42 Grade 1-2B and 18 Grade 2C-3B Paprosky acetabular bone deficiencies. Gruen et al. [7] in radiographic evaluation of 414 primary THAs with 2- to 5-year followup showed the majority of the initial gaps seen postoperatively around porous tantalum monoblock acetabular component have been filled.
Whereas the tantalum cup has clear potential for bone ingrowth, the titanium cup utilized in this report had only bone ongrowth potential. The titanium surface comprises “hill and valleys” and is supplemented by a plasma spray HA surface, but is not a true bone ingrowth surface. It is quite clear from this report that a bone ingrowth surface has the potential for a higher success rate in terms of clinical and radiographic results in the revision situation, particularly in cases with severe bone deficiency. We believe a bone ongrowth surface is more than adequate for primary total hip arthroplasty but based on our data should not be utilized in revision situations where moderate to severe bone loss exists.
The pattern of failure of the two cups in this series was quite different. Eighty percent of the tantalum cups that failed did so within the first 6 months, while 82.3% of the titanium cups that failed did so after 6 months from the revision surgery. The tendency of the titanium cups to late failure is probably due to the inability of these cups to achieve true bone ingrowth. Whether this is due to the bone ongrowth surface or to dissolution of HA coating over time and the potential of delamination between the coating and implant surface is unknown. It is clear however, that if bone ingrowth is not achieved in the early postoperative period, failure may be inevitable.
Despite the limitations, our data highlight findings that may have clinical importance. Based on the findings of this study and our clinical impression, it appears porous tantalum can be a valuable device in reconstruction of acetabulum in patients with bone loss. Although conventional titanium acetabular components perform well in most circumstances, tantalum acetabular cups may be considered during revision THA when moderate to severe acetabular deficiency exists. Longer term followup will be needed to confirm these early findings.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. Drs. Sharkey and Parvizi are consultant surgeons of Stryker Orthopaedics.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907–914. doi: 10.1302/0301-620X.81B5.9283. [DOI] [PubMed] [Google Scholar]
- 2.Bobyn JD, Toh KK, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999;14:347–354. doi: 10.1016/S0883-5403(99)90062-1. [DOI] [PubMed] [Google Scholar]
- 3.Boscainos PJ, Kellett CF, Maury AC, Backstein D, Gross AE. Management of periacetabular bone loss in revision hip arthroplasty. Clin Orthop Relat Res. 2007;465:159–165. doi: 10.1097/BLO.0b013e3181560c6c. [DOI] [PubMed] [Google Scholar]
- 4.Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop. 2002;31:216–217. [PubMed] [Google Scholar]
- 5.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 6.Dorairajan A, Reddy RM, Krikler S. Outcome of acetabular revision using an uncemented hydroxyapatite-coated component: two- to five-year results and review. J Arthroplasty. 2005;20:209–218. doi: 10.1016/j.arth.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O’Keefe TJ, Stulberg SD, Sutherland CJ. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty. 2005;20:369–378. doi: 10.1016/j.arth.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Hanssen AD, Lewallen DG. Modular acetabular augments: composite void fillers. Orthopedics. 2005;28:971–972. doi: 10.3928/0147-7447-20050901-29. [DOI] [PubMed] [Google Scholar]
- 9.Kim WY, Greidanus NV, Duncan CP, Masri BA, Garbuz DS. Porous tantalum uncemented acetabular shells in revision total hip replacement: two to four year clinical and radiographic results. Hip Int. 2008;18:17–22. doi: 10.1177/112070000801800104. [DOI] [PubMed] [Google Scholar]
- 10.Klika AK, Murray TG, Darwiche H, Barsoum WK. Options for acetabular fixation surfaces. J Long Term Eff Med Implants. 2007;17:187–192. doi: 10.1615/jlongtermeffmedimplants.v17.i3.20. [DOI] [PubMed] [Google Scholar]
- 11.Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–2175. doi: 10.1016/S0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 13.Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671–4681. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Malkani AL, Price MR, Crawford CH 3rd, Baker DL. Acetabular component revision using a porous tantalum biomaterial a case series. J Arthroplasty. 2008 Sep 26 [Epub ahead of print]. [DOI] [PubMed]
- 15.Matsuno H, Yokoyama A, Watari F, Uo M, Kawasaki T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001;22:1253–1262. doi: 10.1016/S0142-9612(00)00275-1. [DOI] [PubMed] [Google Scholar]
- 16.Miyaza T, Kim HM, Kokubo T, Ohtsuki C, Kato H, Nakamura T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials. 2002;23:827–832. doi: 10.1016/S0142-9612(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 17.Moore MS, McAuley JP, Young AM, Engh CA., Sr Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;444:176–183. doi: 10.1097/01.blo.0000201149.14078.50. [DOI] [PubMed] [Google Scholar]
- 18.Ng TP, Chiu KY. Acetabular revision without cement. J Arthroplasty. 2003;18:435–441. doi: 10.1016/S0883-5403(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 19.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9:33–44. doi: 10.1016/0883-5403(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 20.Reikerås O, Gunderson RB. Failure of HA coating on a gritblasted acetabular cup: 155 patients followed for 7–10 years. Acta Orthop Scand. 2002;73:104–108. doi: 10.1080/000164702317281503. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez JA. Acetabular fixation options: notes from the other side. J Arthroplasty. 2006;21:93–96. doi: 10.1016/j.arth.2006.02.152. [DOI] [PubMed] [Google Scholar]
- 22.Siegmeth A, Duncan CP, Masri BA, Kim WY, Garbuz DS. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res. 2009;467:199–205. doi: 10.1007/s11999-008-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporer SM, Paprosky WG, O’Rourke MR. Managing bone loss in acetabular revision. Instr Course Lect. 2006;55:287–297. [PubMed] [Google Scholar]
- 24.Stiehl JB. Trabecular metal in hip reconstructive surgery. Orthopedics. 2005;28:662–670. doi: 10.3928/0147-7447-20050701-13. [DOI] [PubMed] [Google Scholar]
- 25.Templeton JE, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen-year follow-up study. J Bone Joint Surg Am. 2001;83:1706–1711. doi: 10.2106/00004623-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Unger AS, Lewis RJ, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthroplasty. 2005;20:1002–1009. doi: 10.1016/j.arth.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Van Kleunen JP, Lee GC, Lementowski PW, Nelson CL, Garino JP. Acetabular revisions using trabecular metal cups and augments. J Arthroplasty. 2009 Mar 31 [Epub ahead of print]. [DOI] [PubMed]