Abstract
A novel low-stiffness extensively porous-coated total hip femoral component was designed to achieve stable skeletal fixation, structural durability, and reduced periprosthetic femoral stress shielding. In short- to intermediate-term clinical review, this implant achieved secure biologic fixation and preserved periprosthetic bone. We retrospectively reviewed all 102 prospectively followed patients (106 implants) with this implant to document the longer-term implant survivorship, clinical function, fixation quality, and periprosthetic bone preservation. Ninety-seven patients with 101 implants had current followup or were followed to patient death (range, 1–14 years; average, 10 years). Eighty-six living patients were followed for an average implant survivorship of 10 years. There were no known femoral implant removals. The average Harris hip score at 10-year followup was 98. Radiographs demonstrated secure implant fixation and maintenance of periprosthetic bone. These data suggest this implant design provided long-term function characterized by extensive fixation, structural durability, and radiographic appearance of maintained periprosthetic cortical thickness and density.
Level of Evidence: Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The femoral components utilized in THA have been subject to numerous and substantial design changes throughout the nearly 50 years of their clinical use. Throughout much of the history of THA, common design motivations have included improved implant fixation, prolonged structural durability, and reduced implant-related femoral stress shielding. With traditional metal femoral implants, increased strength and extensive fixation are commonly linked to higher implant stiffness and an increased tendency of bone loss due to periprosthetic stress shielding [6, 7, 9, 15, 23]. In the 1980s and early 1990s, a variety of composite total hip designs were proposed, developed, and evaluated [5, 12, 13, 17–19, 21, 22, 26] to reduce the risk of stress shielding. These composite implants were generally considered low-stiffness devices since their bending stiffness was approximately 50% that of a similar titanium alloy implant. Although it was stated “the future of joint replacement lies in the development of new composite materials” [27], most of these implants produced disappointing results, including 78%, 30%, 10%, and 9% revision within 10 years [1, 16, 20, 25] and 58% “clinical failure” at 8 years [14]. The limitations of these composite implant designs have included failure to achieve or maintain skeletal fixation and structural implant failure.
A novel approach to femoral total hip implant design utilizing an extensive porous titanium fiber metal fixation surface, a CoCrMo structural core, and an intervening polymer layer was developed to simultaneously achieve stable skeletal fixation, structural durability, and reduced periprosthetic femoral stress shielding (Fig. 1). This low-stiffness prosthesis has approximately 25% and 50% of the bending stiffness of a matching-size CoCrMo and titanium alloy implant, respectively (Fig. 2). A prospective clinical trial of the implant was initiated in 1994 at 11 clinical centers in the United States for the primary treatment of noninflammatory degenerative joint disease. One hundred sixty-four patients receiving 170 low-stiffness stems were enrolled in this study in accordance with a US Food and Drug Administration (FDA) approved Investigational Device Exemption (IDE) protocol. A report with 24-month minimum followup was submitted to the FDA documenting a 94% followup of living eligible patients with no revisions of the investigational implant. A contemporaneous clinical trial of this implant was also conducted at 10 clinical centers in Europe and Australia. A total of 366 patients receiving 386 investigational implants were enrolled in these two studies. At a followup of 3 months to 6 years (mean, 2.4 years), there were no implant-related failures, but the authors stated longer-term clinical study was required to assess the implant’s ability to withstand repetitive loading in vivo [10]. An assessment of periprosthetic bone quality, via dual-energy xray absorptiometry (DEXA), around this low-stiffness stem at 2 years’ followup in 65 patients with 68 implants (patients included in the Europe-based clinical study) observed bone retention compared to a proximally coated titanium implant [11]. Finally, a report on the clinical results (average 6.2 years’ followup) of 28 patients (patients included in the United States IDE protocol) demonstrated no implant-related failures [2]. Desirable periprosthetic bone retention at 2 years as assessed by DEXA was also reported. Additionally, two postmortem retrieved implants demonstrated excellent histologic evidence of bone ingrowth. Again, the authors concluded longer-term followup was important.
Fig. 1A–C.
Photographs show the Epoch® femoral implant in (A) proximal transverse cross section, (B) frontal view, and (C) longitudinal cross section. The implant is constructed with a CoCrMo alloy structural core, an exterior titanium fiber metal fixation surface, and an intervening thermoplastic layer. The thermoplastic material is radiolucent.
Fig. 2.
A graph shows a comparison of the proximal implant bending stiffnesses of CoCrMo, titanium alloy, and Epoch® implants for matching-size implants from 14 mm to 18 mm. The low-stiffness Epoch® implant has approximately 25% and 50% of the bending stiffness of a corresponding-size CoCrMo and titanium alloy implant, respectively.
Based on the early data, we hypothesized the longer-term clinical performance of this novel low-stiffness femoral stem would be characterized by (1) high implant survivorship; (2) good hip function; (3) radiographic evidence of persistent bone apposition; and (4) minimum periprosthetic osseous changes.
Materials and Methods
Four clinical centers that were originally a part of an 11-center FDA IDE investigation participated in a review of their patients who had the Epoch® stem (Zimmer, Inc, Warsaw, IN) implanted between December 1994 and January 1999. Seven of the original IRB centers did not participate in this current study due either to the clinical investigator’s retirement from clinical practice or to the prior termination of the original study’s IRB approval. After the completion of the IDE study, with a mandated minimum 2-year postoperative followup, investigators at these four centers continued to pursue patient followup at an interval of not less than every 2 years. At one of the four centers, the IRB terminated the study in the patients’ tenth postoperative year, and at the other three centers, the study remained open. To be included in the study, patients needed to provide informed consent, be agreeable to long-term followup, have unilateral untreated hip disease, and be candidates for primary THA with noninflammatory degenerative joint disease. One hundred two patients (33 females, 69 males) received 106 implants through a posterior approach (Table 1); these numbers reflect 62% of patients and 62% of implants from the original 11-center IDE investigation. Four patients in the study group had bilateral THAs (protocol violations) with a CoCr-beaded AML® implant (DePuy Orthopaedics, Inc, Warsaw, IN) on one side and the lower-stiffness Epoch® implant on the other side. The average patient age at the time of operation was 58 years (range, 23–78 years). The average patient body mass index and weight were, respectively, 27 (range, 18–42) and 183 lbs (range, 105–270 lbs). Eighty-four of the patients had osteoarthritis, 11 avascular necrosis of the femoral head, four posttraumatic arthritis, two each hip dysplasia and ankylosing spondylitis, and one each Legg-Calvé-Perthes, rheumatoid arthritis (a protocol violation), and a slipped capital femoral epiphysis.
Table 1.
Patient enrollment, implants at final followup, patient deaths, and patients lost to followup at each investigational center
| Clinical site | Patients enrolled | Implants | Implants at followup | Implants at death | Implants lost to followup | Known implant removals |
|---|---|---|---|---|---|---|
| Cleveland, OH | 28 | 28 | 23 | 3 | 2 (7.1%) | 0 |
| Virginia Beach, VA | 19 | 21 | 17 | 2 | 2 (10.5%) | 0 |
| Paramus, NJ | 29 | 29 | 26 | 2 | 1 (3.5%) | 0 |
| Alexandria, VA | 26 | 28 | 24 | 4 | 0 (0%) | 0 |
| Total | 102 | 106 | 89 | 11 | 5 (4.7%) | 0 |
In 2007, we summarized the patient data from the four centers and considered the data current followup if the patients had been seen within the previous 2 years and, at one center, with patients considered as having completed the study if they had been followed into their tenth postoperative year. In early 2008, we attempted to contact all patients who had not returned for a scheduled office followup within the previous 2 years and for those who indicated they could not or would not return for followup. Eleven patients with 11 hips (10% of the implants) had died (Table 2) with their index implant in place. Effort to contact five patients (five hips or 5% of the implants) not known to be deceased was unsuccessful (Table 2). Contact was successful with 86 living patients with 90 implants (95% of the implants and 95% of the implants not associated with a deceased patient).
Table 2.
Date when last seen and period of implant survivorship for patients lost to followup and patients who died with their implant in vivo
| Lost to followup patients | Deceased patients | Reoperations | |||||
|---|---|---|---|---|---|---|---|
| Patient | Last seen | Followup (years) | Patient | Last seen | Followup (years) | Patient | Reason |
| 107 | 2002 | 7.4 | 105 | 2005 | 10.1 | 100 | Femoral fracture/cable removal |
| 130 | 2002 | 3.9 | 116 | 2007 | 1.1 | 108 | Wear/polyethylene exchange |
| 1008 | 1997 | 0.0 | 126 | 2007 | 6.7 | 118 | Wear/polyethylene exchange |
| 300 | 2000 | 5.5 | 1006 | 1998 | 1.1 | 827 | Wear/polyethylene exchange |
| 302 | 2001 | 6.4 | 305 | 2001 | 6.0 | ||
| 307 | 2000 | 6.5 | |||||
| 1019 | 2003 | 5.0 | |||||
| 815 | 1999 | 1.9 | |||||
| 820 | 2004 | 6.6 | |||||
| 828 | 2007 | 8.3 | |||||
| 829 | 2006 | 7.1 | |||||
| Average 5.2 | Average 5.5 | ||||||
All patients received Trilogy® acetabular components (Zimmer, Inc), either metal or ceramic femoral heads, and a polyethylene bearing surface. The polyethylene components were made from compression-molded GUR® 1050 resin. The earliest treated patients received gamma-sterilized-in-air polyethylene components while some of the later treated patients received gamma-sterilized-in-nitrogen polyethylene components. None of the patients in this series received highly crosslinked polyethylene components.
We routinely followed the patients at 3 months, 6 months and then yearly during the FDA monitored period of the study and then every 2 years thereafter. At the office-based followup, implant survivorship was determined, a Harris hip score was assessed, and anteroposterior (AP) and lateral radiographs were obtained. An interview was administered during telephone contact to ascertain implant survivorship and to assess hip function. In 15 cases of living patients, recent radiographs were not available and the only patient information obtained was by telephone contact.
Sequential postoperative radiographs were reviewed by a single observer (RDC). Radiographic evidence of any change in implant position of greater than 2 mm, implant endocortical contact, radiolucency of greater than 1 mm at the implant-bone interface, and femoral bone condition such as marked change in cortical thickness, calcar resorption, and focal osteolysis were noted. Medial and lateral endocortical implant contact within the straight reamer prepared distal stem femoral cavity [11] was judged complete if on AP radiographs both the medial and lateral surfaces of the distal stem were in contact with femoral cortical bone for at least 5 cm of stem length. Endocortical implant contact was judged partial if cortical bone contact occurred only medially or laterally or for less than 5 cm or absent if no contact occurred.
In addition to descriptive statistics, a Kaplan-Meier estimate was performed for femoral implant survivorship and for index procedure survivorship free of reoperation for any reason.
Results
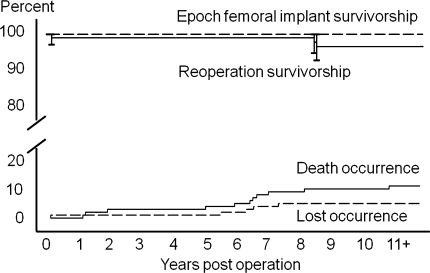
The survivorships of the Epoch® femoral components and index procedure survivorship without reoperation for any reason was 100% and 96% (CI 93% to 100%), respectively at 10 years (Fig. 3). There were no known femoral implant removals within the 102 patients with 106 implants. In all reoperated cases, the Epoch® implant was well fixed and was left in situ (Table 2). The 90 index implants not associated with patient death or loss functioned in vivo for between 8 and 14 years (average, 10 years).
Fig. 3.
Kaplan-Meier femoral implant survivorship and survivorship without reoperation for any reason are shown in the top half of the graph; 95% confidence intervals are indicated by error bars. The temporal occurrence of patient deaths (11%) and loss to followup (4.7%) are shown in the bottom half of the graph.
The Harris hip scores were obtained at each time interval for patients returning for office visits; the average Harris hip score at 10-year followup was 98 (Table 3). The incidence of reported thigh pain was 82% in patients preoperatively, which dropped to zero at 10 years (Table 3).
Table 3.
Harris hip scores and incidence of reported thigh pain recorded at various postoperative intervals
| Interval | Harris hip score | Thigh pain incidence |
|
|---|---|---|---|
| Mean | Standard deviation | ||
| Preoperative | 50.8 | 11.1 | 82% |
| 2 years | 98.0 | 2.8 | 4% |
| 4 years | 98.1 | 4.1 | 3% |
| 6 years | 98.2 | 4.1 | 6% |
| 8 years | 98.5 | 3.1 | 0% |
| 10 years | 97.9 | 3.8 | 0% |
On radiographic examination, all Epoch® implants appeared bone ingrown, showing evidence of trabecular or cortical bone apposition and absence of either a complete stable or unstable soft tissue interface. Initial medial and lateral endocortical implant contact with cortical bone along the distal stem was judged complete in 97 (92%) of 106 cases (Fig. 4). In all cases where this endocortical implant contact was initially achieved, it was also observed to be preserved on the most recent radiograph. Partial endocortical contact was achieved with the distal stem in eight (8%) patients while the absence of endocortical contact occurred in one patient. A small stable region of proximal/lateral radiolucency was commonly observed (Fig. 5). In some cases, this radiolucency extended distally to include a line along the bone-implant interface at the proximal/lateral porous metal fixation surface. This radiolucency typically first appeared on radiographs as early as 6 months postoperatively. This stable radiolucent region, which was present and unchanged in some cases for more than 10 years, was observed with 31 (29%) implants. A progressive region of radiolucency associated with wear-related femoral head penetration into the gamma-sterilized-in-air acetabular polyethylene was observed on the last available radiograph in seven (9%) patients.
Fig. 4A–C.
Followup AP radiographs demonstrate (A) absent distal stem endocortical contact, (B) partial distal stem endocortical contact, and (C) complete distal stem endocortical contact.
Fig. 5A–C.
Arrows identify a proximal-lateral radiolucency that was present at (A) 6 months postimplantation and remained unchanged at (B) 5 and (C) 10 years postimplantation.
Periprosthetic bone retention appeared better with an Epoch® than the AML® (Fig. 6). Recent DEXA data were available in one female patient now aged 59 years with a well-functioning 12-year postoperative left Epoch® THA (Fig. 7). As measured by DEXA and when compared to her contralateral normal proximal right femur, the bone mineral density and bone area in her periprosthetic right femur appear nearly unchanged 12 years after receiving an Epoch® femoral prosthesis (Table 4).
Fig. 6.
An AP radiograph shows a patient who received a right Epoch® implant 12 years ago and a left AML® implant 17 years ago.
Fig. 7.
An AP radiograph shows a patient who received a left Epoch® implant 12 years ago. The DEXA-measured bone mineral density and area for this patient’s proximal femurs are reported in Table 4.
Table 4.
Comparable bone mineral density and bone area in a 12-year postoperative Epoch®-treated femur and the untreated contralateral hip in a 59-year-old female patient*
| Hip | DEXA-measured area | Bone mineral density (g/cm2) | Bone area (cm2) |
|---|---|---|---|
| Epoch® treated | Trochanter | 1.038 | 7.50 |
| Femoral shaft | 1.620 | 8.91 | |
| Untreated | Trochanter | 1.016 | 9.13 |
| Femoral shaft | 1.841 | 7.32 |
* An anteroposterior radiograph of this patient’s hips is shown in Fig. 6; DEXA = dual-energy xray absorptiometry.
Discussion
A novel low-stiffness extensively porous-coated total hip femoral component (Epoch®) was designed to achieve stable skeletal fixation, structural durability, and reduced periprosthetic femoral stress shielding. It has been demonstrated in short- to intermediate-term clinical review this implant can achieve secure biologic fixation and periprosthetic femoral bone preservation. We conducted a longer-term clinical review of a subset of patients who originally enrolled in the FDA-approved IDE investigation to determine whether this low-stiffness femoral stem would be characterized by (1) high implant survivorship; (2) good hip function; (3) durable implant fixation; and (4) minimum periprosthetic osseous changes.
Our study is limited first by the fact that all patients were not available for an office visit, physical examination, and radiographic examination at the most recent followup. Only limited patient information and implant survivorship were obtainable from those patients most recently contacted by telephone. However, we lost only 5% of patients to followup and the remaining patients had an average documented implant performance of 10 years. Further, the patients in this study were prospectively followed at multiple centers. Second, DEXA analysis permitting quantitative bone density measurement was not generally available in this later followup period. In a few patients with bilateral THAs, direct radiographic comparison of femoral bone quality associated with both the low-stiffness implant and a higher-stiffness metal implant was permitted.
High clinical failure rates have been reported in the use of composite implants with bending stiffness substantially lower than traditional CoCrMo or titanium implants [1, 14, 16, 20, 25]. The 10-year 100% survival of the Epoch® implant stands in contrast with those results and confirms earlier expectations that this implant concept would provide a durable hip reconstruction. With one exception (a femoral fracture), the hip reoperations within this patient group were performed for polyethylene wear and related osteolysis. Since most of the patients in this study received their implants in the mid 1990s, most of the polyethylene components utilized were sterilized by exposure to gamma radiation in air. All of the patients in this study who underwent a reoperation for progressive osteolysis or acetabular polyethylene component exchange initially received gamma-sterilized-in-air polyethylene components. This material utilization combined with patient age helps account for the wear-related surgical intervention [3, 8, 24]. The improved wear performance of newer generations of polyethylene acetabular components can be expected to improve the future performance of this implant as it may with other THA systems.
Thigh pain after THA is reported and its origin may in some cases be related to the relatively high bending stiffness of the implant within the femur [4]. The reported extremely low incidence of thigh pain in this patient group is likely attributable to the low bending stiffness of this implant. High Harris hip scores confirm this implant can result in excellent overall hip function.
The observed extensive long-term fixation of this implant [2, 10, 11, 26] stands in contrast to the fixation failures of other composite implants [1, 14, 16, 20, 25]. The Epoch® implant’s anatomic proximal shape, straight cylindrical distal stem, and associated bone preparation instruments contributed to the implant fitting and filling the proximal femur within this male-dominated primary THA patient population. Good initial femoral cavity fit and fill likely contributed to the establishment of good initial fixation, which was preserved over time by the implant’s low stiffness and resulting periprosthetic bone retention. The observed stable radiolucency lateral of the proximal-most portion of some implants appeared to be the product of femoral preparation resulting from the rasp removing excessive bone in the implant shoulder region followed by the development of a sclerotic surface on the rasped bone cavity.
It is reported periprosthetic bone retention associated with metal femoral total hip components is influenced by component bending stiffness and extent of porous fixation [6, 7, 15]. It has been previously reported, while the Epoch® implant achieved extensive bone ingrowth fixation, it also retained, as measured by DEXA in 16 patients, more periprosthetic bone than a more rigid implant [2, 11]. Additionally, the postmortem histologic examination of this implant demonstrated extensive bone ingrowth and resulting excellent implant fixation [2]. Our study confirms these features of implant function are preserved at longer followup. The longer-term radiographic examination in this study of bilateral THA patients with a fully porous-coated CoCr implant on one side and this more flexible fully porous-coated implant on the other side confirms the earlier observations of periprosthetic bone retention.
Our study demonstrates desirably low stiffness, extensive bone ingrowth fixation, and structural durability can be simultaneously achieved in a femoral hip component. The capability of porous titanium to support bone ingrowth provided the means of skeletal fixation for this implant while the structural durability of forged CoCrMo alloy provided its strength. The use of a thermoplastic filler material between the two metal elements provided the opportunity to reduce the implant’s stiffness. In the first decade of its use, this metal and polymer composite appears to have adequate material interface and overall structural strength. The second decade of its function, use in different clinical situations, use in different implant geometries, and use in a broader patient population will further test the utility of this implant concept. All the authors who are currently in clinical practice (VMG, AHG, KBF, MAH, LCJ) use either the Epoch® or the newer VerSys® Epoch® Fullcoat Hip Prosthesis in selected cases where extensively porous-coated implants may be indicated.
Acknowledgments
We thank Kimberly J. Rowe and Daniel Reyner, PhD, for their assistance in this study.
Footnotes
One or more of the authors (MAH, AHG, VMG, LRJ, RDC) have received financial support from Zimmer, Inc, Warsaw, IN. One of more of the authors (MAH, AHG, VMG, LRJ, RDC) certifies that he or she has or may receive payments or benefits from a commercial entity related to this work.
This study has received IRB approval and was initiated as part of an FDA-approved Investigation Device Exemption. Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was conducted at Case Western University, Anderson Orthopaedic Clinic, Hartzband Center for Hip and Knee Replacement, and the Jordan-Young Institute.
References
- 1.Adam F, Hammer DS, Pfautsch S, Westermann K. Early failure of a press-fit carbon fiber hip prosthesis with a smooth surface. J Arthroplasty. 2002;17:217–223. doi: 10.1054/arth.2002.30285. [DOI] [PubMed] [Google Scholar]
- 2.Akhavan S, Matthiesen MM, Schulte L, Penoyar T, Kraay MJ, Rimnac CM, Goldberg VM. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88:1308–1314. doi: 10.2106/JBJS.E.00316. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann LS, Bargmann BC, Collier JP, Currier BH, Mayor MB. Current sterilization and packaging methods for polyethylene. Clin Orthop Relat Res. 1999;369:49–58. doi: 10.1097/00003086-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brown TE, Larson B, Shen F, Moskal JT. Thigh pain after cementless total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2002;10:385–392. doi: 10.5435/00124635-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cheal EJ, Spector M, Hayes WC. Role of loads and prosthesis material properties on the mechanics of the proximal femur after total hip arthroplasty. J Orthop Res. 1992;10:405–422. doi: 10.1002/jor.1100100314. [DOI] [PubMed] [Google Scholar]
- 6.Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop Relat Res. 1988;231:7–28. [PubMed] [Google Scholar]
- 7.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement: the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 8.Faris PM, Ritter MA, Pierce AL, Davis KE, Faris GW. Polyethylene sterilization and production affects wear in total hip arthroplasties. Clin Orthop Relat Res. 2006;453:305–308. doi: 10.1097/01.blo.0000229348.10458.79. [DOI] [PubMed] [Google Scholar]
- 9.Glassman AH, Bobyn JD, Tanzer M. New femoral designs: do they influence stress shielding? Clin Orthop Relat Res. 2006;453:64–74. doi: 10.1097/01.blo.0000246541.41951.20. [DOI] [PubMed] [Google Scholar]
- 10.Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;393:128–136. doi: 10.1097/00003086-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kärrholm J, Anderber C, Snorrason F, Thanner J, Langeland N, Malchau H, Herberts P. Evaluation of a femoral stem with reduced stiffness: a randomized study with use of radiostereometry and bone densitometry. J Bone Joint Surg Am. 2002;84:1651–1658. [PubMed] [Google Scholar]
- 12.Katoozian H, Davy DT, Arshi A, Saadati U. Material optimization of femoral component of total hip prosthesis using fiber reinforced polymeric composites. Med Eng Phys. 2001;23:503–509. doi: 10.1016/S1350-4533(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Granger C, Del Schutte H, Biggers SB, Jr, Kennedy JM, Jr, Latour RA., Jr Progressive failure analysis of laminated composite femoral prostheses for total hip arthroplasty. Biomaterials. 2002;23:4249–4262. doi: 10.1016/S0142-9612(02)00188-6. [DOI] [PubMed] [Google Scholar]
- 14.Maathuis PG, Visser JD. High failure rate of soft-interface stem coating for fixation of femoral endoprostheses. J Arthroplasty. 1996;11:548–552. doi: 10.1016/S0883-5403(96)80108-2. [DOI] [PubMed] [Google Scholar]
- 15.McAuley JP, Sychterz CJ, Engh CA. Influence of porous coating level on proximal femoral remodeling. Clin Orthop Relat Res. 2000;371:146–153. doi: 10.1097/00003086-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Morscher EW, Dick W. Cementless fixation of “isoelastic” hip endoprostheses manufactured from plastic materials. Clin Orthop Relat Res. 1983;176:77–87. [PubMed] [Google Scholar]
- 17.Mukherjee DP, Saha S. The application of new composite materials for total joint arthroplasty. J Long Term Eff Med Implants. 1993;3:131–141. [PubMed] [Google Scholar]
- 18.Otani T, Whiteside LA, White SE. Strain distribution in the proximal femur with flexible composite and metallic femoral components under axial and torsional loads. J Biomed Mater Res. 1993;27:575–585. doi: 10.1002/jbm.820270504. [DOI] [PubMed] [Google Scholar]
- 19.Ritter MA, Keating EM, Faris PM. A porous polyethylene-coated femoral component of a total hip arthroplasty. J Arthroplasty. 1990;5:83–88. doi: 10.1016/S0883-5403(06)80014-8. [DOI] [PubMed] [Google Scholar]
- 20.Runne WC, Sambeek KJ, Stierum JL, Tongerloo RB. Femoral endoprosthesis fixation with a soft, flexible low modulus stem coating (four- to six-year clinical results) Orthopedics. 1989;12:529–535. doi: 10.3928/0147-7447-19890401-07. [DOI] [PubMed] [Google Scholar]
- 21.Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;235:224–236. [PubMed] [Google Scholar]
- 22.Skinner HB. Isoelasticity and total hip arthroplasty. Orthopedics. 1991;14:323–328. [PubMed] [Google Scholar]
- 23.Sychterz CJ, Engh CA. The influence of clinical factors on periprosthetic bone remolding. Clin Orthop Relat Res. 1996;322:285–292. doi: 10.1097/00003086-199601000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Sychterz CJ, Orishimo KF, Engh CA. Sterilization and polyethylene wear: clinical studies to support laboratory data. J Bone Joint Surg Am. 2004;86:1017–1022. [PubMed] [Google Scholar]
- 25.Trebse R, Milosev I, Kovac S, Mikek M, Pisot V. Poor results from the isoelastic total hip replacement: 14–17-year follow-up of 149 cementless prostheses. Acta Orthop. 2005;76:169–176. doi: 10.1080/00016470510030535. [DOI] [PubMed] [Google Scholar]
- 26.Turner AW, Gillies RM, Sekel R, Morris P, Bruce W, Walsh WR. Computational bone remodelling simulations and comparisons with DEXA results. J Orthop Res. 2004;23:705–712. doi: 10.1016/j.orthres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Volz RG, Benjamin JB. The current status of total joint replacement. Invest Radiol. 1990;25:86–92. doi: 10.1097/00004424-199001000-00022. [DOI] [PubMed] [Google Scholar]