Abstract
Early versions of uncemented femoral total hip stems were often associated with thigh pain thought to be due to micromotion between the implant and bone in the distal uncoated regions. An extensively coated stem was introduced in 1992 to reduce that risk. We therefore asked whether second-generation extensively porous-coated cementless femoral stems in patients younger than 50 years of age would (1) be durable in terms of revisions; (2) provide high functional scores and reduce thigh pain; and (3) show radiographic signs of durability, including a reduction in stress shielding. We prospectively followed all 100 patients (115 hips) age 50 and younger treated with primary cementless total hip arthroplasties using a second-generation extensively porous-coated femoral stem between June 1994 and December 1999. The average age was 39.6 years (range, 17–50 years). The stems were mated to cementless acetabular components. Ninety patients were followed for a minimum of 5 years (mean, 8.6 years; range, 5–10 years). One stem was revised after a periprosthetic fracture. None were revised for loosening and all stems demonstrated bony ingrowth at last followup. No acetabular shell was revised for loosening and none was radiographically loose. Six acetabular liners were revised for wear (three each were 22-mm and 26-mm heads). This second-generation extensively porous-coated stem was durable at 5- to 10-year followup in this young active population.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The Prodigy femoral component (DePuy, Warsaw, IN) was developed in 1992 to potentially reduce the thigh pain (10%–20%) [8, 12] and stress shielding associated with the first-generation AML (DePuy) 5/8th porous-coated femoral component. Like the AML, the Prodigy was also a chrome cobalt implant, but the porous coating was extended along the entire length of the stem (in hopes of decreasing micromotion at the tip and thus thigh pain) [6] except for the polished bullet-shaped distal tip. Ten degrees of anteversion was incorporated in the neck of the device and the larger components had a medial diaphyseal cutout to reduce the flexural rigidity of the stem (in hopes of reducing stress shielding) [4] (Fig. 1).
Fig. 1.
The Prodigy implant with a medial cutout and extensive porous coating.
Midterm followup studies of the AML used in the younger patient population have reported bony ingrowth in up to 96% of cases and thigh pain incidence in some series has not exceeded 10%. In addition, the incidence of moderate to severe stress shielding has been reported in 55% (46% moderate, 9% severe) of cases in one young patient series [12, 14, 17]. Unfortunately, these case series are included with other devices and the minimum length of followup is not greater than 5 years.
We therefore asked whether the second-generation extensively porous-coated cementless femoral stem in patients younger than 50 years of age would (1) be durable in terms of revisions; (2) provide high functional scores and reduce thigh pain; and (3) show radiographic durability of fixation and reduction in stress shielding.
Materials and Methods
We retrospectively reviewed all 100 prospectively followed patients (115 hips) age 50 and younger treated with the Prodigy (DePuy) femoral component from June 1994 to December 1999. We used this implant in all patients under 50 years of age who had cementless THA during the study period (other than for patients with extremely small bones which required a miniature prothesis). The average age at the time of index THA was 39.6 years (range, 17–50 years). There were 63 men and 37 women. Five to 10 years after the index THA, 90 patients were alive (103 hips), five patients (seven hips) were dead (none of whom had additional revision), and five patients were lost to followup (five hips). The patients were followed for a minimum of 5 years (mean, 8.6 years; range, 5–10 years). Of the 90 living patients, 77 (89 hips) had a minimum 5-year followup radiograph; the other 14 hips in living patients all had at least a 1-year radiograph.
The preoperative clinical evaluation included documenting the level of activity, range of motion of the hip, level of pain, gait pattern, and level of performance of activities of daily living obtained through clinical examination as well as the use of a standard terminology questionnaire for the reporting of clinical and radiographic results [11]. The most common diagnoses were primary osteoarthritis in 39 hips and osteonecrosis in 35 hips (Table 1).
Table 1.
Preoperative diagnoses
| Diagnosis | Number of patients | Number of hips |
|---|---|---|
| Osteoarthritis | 32 | 39 |
| Osteonecrosis | 31 | 35 |
| Developmental dysplasia of the hip | 12 | 14 |
| Legg-Calve-Perthes | 6 | 8 |
| Posttraumatic | 9 | 9 |
| Slipped capital femoral epiphysis | 2 | 2 |
| Cerebral palsy complications | 2 | 2 |
| Inflammatory arthritis | 1 | 1 |
| Septic arthritis | 1 | 1 |
| Juvenile rheumatoid arthritis | 1 | 1 |
| Ankylosing spondylitis | 1 | 1 |
| Spondyloepiphyseal dysplasia | 1 | 1 |
| Femoral neck nonunion | 1 | 1 |
| Total | 100 | 115 |
All surgery was performed by the senior author (JJC). We used a posterolateral approach in all cases. The femoral canal was underreamed by 0.5 mm to the diameter of the coating of the implant used. Intraoperative radiographs (typically a single view) were obtained with a broach in place to ensure proper fit and positioning. The canal was then machined for a larger size if there was any concern on the intraoperative radiograph that the component would be undersized or was malpositioned. The Prodigy femoral components were mated with 43 Harris-Galante I acetabular components (Zimmer, Warsaw, IN) or with 72 Duraloc Sector acetabular components (DePuy). We used 22-mm and 26-mm modular femoral heads.
Postoperatively, we recommended that the patients use crutches with partial weightbearing for 6 weeks and then progress to full weightbearing as tolerated.
Patients were routinely followed every 2 years, with closer followup warranted for those with symptoms. The latest followup evaluation was conducted at 5 to 10 years after the index THA. Patients returned to the clinic for followup or, if they were unable to return, sent current radiographs for evaluation. Thirty patients were evaluated in person and 60 were interviewed by telephone using standard terminology for reporting results and completed Harris hip scores [10] and UCLA activity level scores [1]. Thigh pain was specifically evaluated through the standard terminology questionnaire.
Two of us (JJC, CMM) reviewed all the radiographs with interpretation reported by consensus. Early postoperative and interval followup radiographs included anteroposterior projections of the pelvis that included the tip of the femoral prosthesis and lateral projections of the femur that included the hip. All observations were based on the anteroposterior radiographs from the early postoperative period and those at latest followup. Of the 90 living patients, 103 hips, 77 patients with 89 hips (86% of hips) had a minimum 5-year followup radiograph. Correction for magnification was completed by standardizing all measurements against the known size of the femoral head.
We evaluated the stems for radiographic evidence of (1) bone ingrowth; (2) stable fibrous fixation; or (3) unstable fibrous fixation according to the criteria of Engh et al. [7]. Femoral component subsidence was determined using the relationship of the top of the lesser trochanter to the medial aspect of the stem collar defined as a decrease of at least 5 mm (with magnification considered) between the initial postoperative radiograph and those from the last followup [2]. Osteolysis was defined as any nonlinear radiolucency at the bone-prosthesis interface that was at least 5 mm squared according to the seven femoral zones defined by Gruen et al. [9]. Radiolucencies were also recorded according to the seven femoral zones of Gruen [9]. Femoral component stress shielding was defined using a modification of the criteria defined by Engh and Bobyn [6]. Mild stress shielding was limited to the upper third of the implant. Moderate stress shielding extended to the middle one-third and severe stress shielding extended below the middle one-third. The acetabular components were evaluated for bone prosthesis radiolucencies and acetabular component migration according to the criteria of Massin et al. [13]. The definition of acetabular osteolysis was the same as that of femoral osteolysis.
Results
Of the original 115 hips, 10 had reoperations at 5- to 10-year followup (Table 2). Six were for polyethylene wear, two for periprosthetic fracture, and two for dislocation. Five of the six hips that had reoperation for wear had a Harris-Galante I acetabular component and one had a Duraloc Sector acetabular component. The average time to reoperation for these six patients was 9.5 years (range, 8–10.4 years). All six had bone-ingrown acetabular shells. Five underwent cementation of a new liner into the shell and femoral head exchange. One required removal of the ingrown shell with reinsertion of a cementless shell and constrained liner. This patient had Down syndrome with limited mental ability and lax soft tissues. Two hips were reoperated on for periprosthetic femoral fracture, one with retention of the femoral component and plating of the femur and one with removal of the component and placement of a longer stem. The patient who required revision of a component fell down an entire flight of steps 4 weeks postoperatively and had a short spiral fracture at the tip of the stem, which was treated by removal of the prosthesis that had not yet fully bone ingrown and placement of a longer femoral component. The second fracture occurred after an automobile accident 10 years postoperatively and was treated with plating and retention of the ingrown femoral component. Two patients underwent reoperation for recurrent dislocation and both were treated with a femoral head and liner exchange with no further dislocations. No hip was revised for infection or aseptic loosening.
Table 2.
Reoperations
| Time of reoperation from index primary THA (years) | Indication for reoperation | Components revised |
|---|---|---|
| 0.1 | Dislocation | Head and liner exchange |
| 0.1 | Periprosthetic fracture | Stem revision |
| 0.5 | Dislocation | Head and liner exchange (cemented constrained liner) |
| 8.0 | Liner wear | Head and liner exchange |
| 8.9 | Liner wear | Head and liner exchange |
| 9.6 | Liner dissociation/liner wear | Head and liner exchange |
| 9.9 | Liner dissociation/liner wear | Constrained liner and shell |
| 10.1 | Periprosthetic fracture | Original components retained |
| 10.1 | Liner wear | Head and liner exchange |
| 10.4 | Liner dissociation/liner wear | Head and liner exchange |
The average preoperative and final followup Harris hip scores were 46 points (range, 17–77 points) and 84 points (range, 42–100 points), respectively. Final followup UCLA activity scores averaged 6.2 (range, 2–10). Hence, the average patient performed moderate activity. Over 40% of patients performed some sport with 21.5% performing some impact-loading activities. Thigh pain was present in 10 patients (all mild) at final followup.
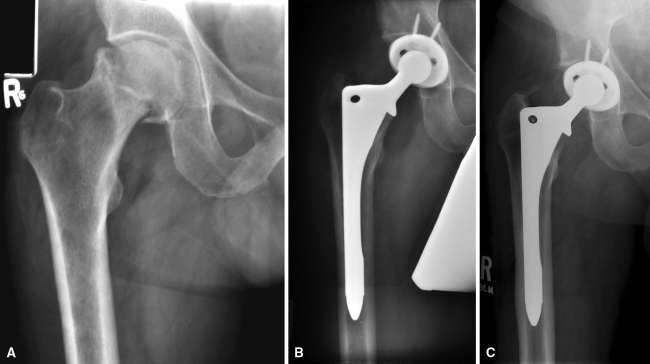
Radiographic evaluation of the 89 hips with minimum 5-year radiographs demonstrated bony ingrowth in all hips; the remaining 14 hips in living patients all had at least a 1-year radiograph and all of these demonstrated bony ingrowth of the prostheses. For the remainder of the radiographic analysis, we only include the 88 hips with minimum 5-year radiographs excluding the one hip revised early for periprosthetic fracture. Femoral stress shielding was mild in 53 hips (60% of hips) (Fig. 2A–C), moderate in 26 hips (29.5% of hips), and severe in one hip (1.1% of hips). Proximal radiolucencies, Zones I and/or VII, occurred in 18 hips, 20% of hips. Distal tip radiolucencies (Zone IV) occurred in six hips, 7%. In the intermediate zones (II, III, V, and VI), radiolucencies were present in 17 hips (19%). All were in the area of the medial stem cutout. Proximal femoral osteolysis occurred in 14 hips, 16% of hips, and there were no cases of distal femoral osteolysis. Radiographic evaluation of the acetabular constructs demonstrated 31 hips (35% of hips) with bone-prosthesis radiolucencies. None of these were more than 1 mm in width and none were circumferential to include the screws (two or three screws were used in all cases). No acetabular components had migrated.
Fig. 2A–C.
A 48-year-old male with osteonecrosis of the right hip is shown. (A) The preoperative radiograph shows collapse of the head. (B) Postoperative radiograph showing the THA. (C) Radiograph at 10 years demonstrating bony ingrowth with mild proximal stress shielding. The patient walks unlimitedly without support and performs manual labor.
Discussion
The Prodigy femoral component was developed as a second-generation extensively coated device, which was designed after the 5/8th porous-coated AML femoral component. It was hoped the Prodigy femoral component would decrease thigh pain and increase bony ingrowth by extending the porous coating and incorporating a polished bullet tip and would decrease stress shielding by providing medial diaphyseal stem relief to decrease the flexural rigidity of the stem. We therefore asked whether the second-generation extensively porous-coated cementless femoral stem in patients younger than 50 years of age would (1) be durable in terms of revisions; (2) provide high functional scores with a reduction in thigh pain; and (3) show radiographic signs of durability, including a reduction in stress shielding.
There are several limitations of our study. First, we have no comparison group with first-generation extensively coated stems in younger patients and, as previously stated, the studies with the reported results using the AML in younger patients are not a truly analogous comparison because these stems were not always mated to a cementless acetabular component, were included with other extensively coated designs, and different clinical scores were used. Second, we did not measure for inter- and intraobserver variability of radiographic measurement, including stress shielding, but instead agreed on findings through consensus. There are difficulties in determining stress shielding resulting from such variables as quality of radiographs and observer interpretation. However, we attempted to limit the effects of such variables through the evaluation of bone remodeling patterns using an established grading system [6].
None of our patients were revised for femoral or acetabular aseptic loosening; one femoral component was revised because of an early traumatic periprosthetic fracture. Other than for two periprosthetic fractures and two liner and head exchanges for dislocation, all other reoperations in this series were related to wear of the acetabular liner. Six hips were revised for this indication. This compares favorably with the revision results in other series in younger patients (Table 3).
Table 3.
Comparison of cementless stems in THA in the young (50 years of age and younger)
| Report | Femoral component | Age (years)* | Length of followup | Revision rate of femoral component for aseptic loosening | Bone ingrown | Thigh pain | Stress shielding |
|---|---|---|---|---|---|---|---|
| Capello et al. [3] (2003) | Omnifit-HA | 39 (16–49) | Minimum 10 years | 0.9% | |||
| Duffy et al. [5] (2001) | PCA HG-I Porous Osteonics Omnifit | 32 (17–39) | 10.1 years | 12.2% | |||
| Kronick et al. [12] (1997) | AML (various types) Prodigy | 37.6 (14–50) | 8.3 years | 0.6% | 96% | 6% | 9% severe |
| McAuley et al. [14] (2004) | AML Prodigy Solution | 40 (16–50) | 6.9 years | ||||
| McLaughlin et al. [15] (2000) | Taperloc | 37 (20–50) | 10.2 years | 0% | 98% | 2% | 73% |
| Mont et al. [16] (1993) | PCA | Younger than 45 years old | 4.6 years | 2.3% | 89% | 23% | |
| Nercessian et al. [17] (2001) | AML | 48 (25–64) | 10.5 years | 8% | |||
| Moyer et al. [current study] | Prodigy | 39 (17–50) | 8.6 years | 0% | 100% | 8.7% | 60% mild, 30% moderate, 1% severe |
* Ranges in parentheses.
Functional scores demonstrated the average patient performed moderate activity. Over 40% of patients performed some sport with 21.5% performing some impact-loading activities. Thigh pain was present in 10 patients (all mild and not activity-limiting) at final followup. However, there is no difference in rates of thigh pain when compared with the rates reported in this population using an AML component.
Radiographic stability of the implant was obtained in all patients with all patients demonstrating bone ingrowth on followup radiographs. The prevalence of moderate to severe stress shielding was 30.6% (27 hips with only one hip demonstrating severe stress shielding). In our study, the prevalence of bony ingrowth is higher and the prevalence of stress shielding (moderate or severe) is lower than the best results reported in this age population using the AML prosthesis (Table 3).
This second-generation extensively coated femoral component as well as the two acetabular components that we studied in this active young population (average UCLA scores of 6.2 with 70% of patients performing moderate activity) provided excellent clinical and radiographic durability, including a low incidence of thigh pain, with no acetabular or femoral components revised for aseptic loosening. Our data encourages the senior author to continue to use extensively porous-coated implants in his primary THAs in the younger population.
Acknowledgments
We thank Rhonda Chalus for her help with this study.
Footnotes
One or more of the authors (JJC) has received funding (royalties and consulting) from DePuy, Warsaw, IN. None of the authors were involved in the development of this implant.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241. [PubMed] [Google Scholar]
- 2.Callaghan JJ, Dysart SH, Savory CG. The uncemented porous-coated anatomic total hip prosthesis. Two-year results of a prospective consecutive series. J Bone Joint Surg Am. 1988;70:337–346. [PubMed] [Google Scholar]
- 3.Capello WN, D’Antonio JA, Feinberg JR, Manley MT. Ten-year results with hydroxyapatite-coated total hip femoral components in patients less than fifty years old. A concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85:885–889. doi: 10.2106/00004623-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Xenos JS, McAuley JP, Young A, Engh CA., Sr Second-generation porous-coated cementless total hip arthroplasties have high survival. Clin Orthop Relat Res. 2006;451:121–127. doi: 10.1097/01.blo.0000224047.71377.5e. [DOI] [PubMed] [Google Scholar]
- 5.Duffy GP, Berry DJ, Rowland C, Cabanela ME. Primary uncemented total hip arthroplasty in patients < 40 years old: 10- to 14-year results using first-generation proximally porous-coated implants. J Arthroplasty. 2001;16(Suppl 1):140–144. doi: 10.1054/arth.2001.28716. [DOI] [PubMed] [Google Scholar]
- 6.Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop Relat Res. 1988;231:7–28. [PubMed] [Google Scholar]
- 7.Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–128. [PubMed] [Google Scholar]
- 8.Engh CA, Sr, Culpepper WJ., II Femoral fixation in primary total hip arthroplasty. Orthopedics. 1997;20:771–773. doi: 10.3928/0147-7447-19970901-09. [DOI] [PubMed] [Google Scholar]
- 9.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 10.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 11.Johnston RC, Fitzgerald RH, Jr, Harris WH, Poss R, Muller ME, Sledge CB. Clinical and radiographic evaluation of total hip replacement. A standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–168. [PubMed] [Google Scholar]
- 12.Kronick JL, Barba ML, Paprosky WG. Extensively coated femoral components in young patients. Clin Orthop Relat Res. 1997;344:263–274. doi: 10.1097/00003086-199711000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 14.McAuley JP, Szuszczewicz ES, Young A, Engh CA., Sr Total hip arthroplasty in patients 50 years and younger. Clin Orthop Relat Res. 2004;418:119–125. doi: 10.1097/00003086-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin JR, Lee KR. Total hip arthroplasty in young patients. 8- to 13-year results using an uncemented stem. Clin Orthop Relat Res. 2000;373:153–163. doi: 10.1097/00003086-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Mont MA, Maar DC, Krackow KA, Jacobs MA, Jones LC, Hungerford DS. Total hip replacement without cement for non-inflammatory osteoarthritis in patients who are less than forty-five years old. J Bone Joint Surg Am. 1993;75:740–751. doi: 10.2106/00004623-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Nercessian OA, Wu WH, Sarkissian H. Clinical and radiographic results of cementless AML total hip arthroplasty in young patients. J Arthroplasty. 2001;16:312–316. doi: 10.1054/arth.2001.21503. [DOI] [PubMed] [Google Scholar]