Abstract
Background and aims
Studies have consistently demonstrated that variants in a number of candidate genes are significant determinants of lipid levels in adults. However, few studies have investigated the impact of these variants in children. Therefore, in the present investigation we examined the influence of ten common variants in the genes for lipoprotein lipase (LPL – S447X), cholesterol ester transfer protein (CETP – Taq1B) apolipoprotein (APO) E (ɛ2, ɛ3, ɛ4), APOA5 (−1131C > T and S19W), APOA4 (S347T) and APOC3 (−482C > T; 1100C > T and 3238G > C) on lipoprotein levels children from the Gene–Diet Attica Investigation on childhood obesity (GENDAI).
Methods and results
The ten variants selected were genotyped in 882 Greek children, mean age: 11.2 ± 0.7 years (418 females and 464 males). Genotypes were assessed using TaqMan technology. Significantly higher total cholesterol (TC) (p = 0.0001) and low-density lipoprotein cholesterol (LDL-C) (p < 0.0001) were observed in APOE ɛ4 carriers compared to ɛ3/ɛ3 homozygotes and ɛ2 carriers. The association of APOE genotype with TC and high-density lipoprotein cholesterol (HDL-C) ratio (p = 0.0008) was further modulated by body mass index. Carriers of the CETP TaqIB B2 allele had significantly higher HDL-C (p < 0.0001) and significantly lower TC: HDL-C ratio (p < 0.0001) compared to B1/B1 individuals. No significant associations were observed between APOA4, APOA5 and APOC3 variants and serum lipids.
Conclusion
This study demonstrates that these common variants are associated with lipid levels in this healthy paediatric cohort, suggesting that even in these young children there may be potential in predicting their lifelong exposure to an adverse lipid profile.
Keywords: Obesity, Apolipoproteins, Single nucleotide polymorphisms, Genetic variants, Lipids
Abbreviations: T2D, type 2 diabetes; GENDAI, Gene–Diet Attica Investigation on childhood obesity; APO, apolipoprotein; TG, triglyceride; LPL, lipoprotein lipase; CETP, cholesterol ester transfer protein; BMI, body mass index; SNPs, single nucleotide polymorphisms; HWE, Hardy–Weinberg equilibrium; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PCA, principal component analysis; PC1, first principal component; PC2, second principal component; MAF, minor allele frequency; GWAS, genome wide association studies; TGRL, triglyceride rich lipoproteins; NS, not statistically significant; CI, confidence intervals; IQR, inter quartile range; AA, amino acid
Introduction
In parallel with the increase in adult obesity, childhood obesity is a rapidly growing health problem worldwide [1]. Obesity in childhood is linked to many serious health complications usually seen in adulthood [2]. Co-morbidities include elevated blood pressure, increased prevalence of factors associated with type 2 diabetes (T2D) and lipid abnormalities [1]. The Gene–Diet Attica Investigation on childhood obesity (GENDAI) [3] was established to specifically explore the contribution of genetics and environmental factors in the development of childhood obesity. The GENDAI cohort consists of young children of both sexes attending school in the area of Attica, Greece. Preliminary assessment provided the impetus for a more detailed study of the metabolic syndrome phenotype in the GENDAI cohort with particular focus on the genetic contribution to inter-individual variation in plasma lipids in the young and the potential modulation of these genetic associations by environmental influences.
Genetic factors are considered to be important determinants of plasma lipoprotein levels in adults; however, the role of genetics in determining plasma lipoproteins in children and adolescents is less clear. The apolipoprotein (APO) cluster including APOA5, APOAIV, APOC3 and APOA1 on chromosome 11 is associated with variation in triglycerides (TG) in both children and adults and across a range of ethnic groups [4,5]. Variation in the APOE, lipoprotein lipase (LPL) and cholesterol ester transfer protein (CETP) genes has been consistently associated with variation in lipid levels in adults [6,7]. However, genetic variation in these gene loci explain only a modest proportion of inter-individual variability in fasting lipid levels [8]. We genotyped the GENDAI cohort for ten variants in the APOE, LPL, CETP genes and the APOA5/A4/C3 cluster, to examine if the reported effects could be replicated in children and assess if these associations could be further modulated by body mass index (BMI).
Methods
Study population
Participants were drawn from the children recruited in the GENDAI study. Briefly, a random sample of 2492 children attending school in the Attica region in Greece were invited to join the study. A total of 1138 children were recruited from November 2005 to June 2006. Due to the heterogeneity in allele frequencies between Greek and non-Greek Caucasians, only children of Greek nationality, mean age: 11.2 ± 0.7 years (n = 882; 418 males and 464 females), were included in the present study. Details of recruitment and data collection have been previously described [3]. All persons gave their informed consent prior to inclusion in the study. The study was approved by the Institutional Review Board of Harokopio University and the Greek Ministry of Education [3].
Experimental procedures
Single nucleotide polymorphism selection and genotyping
A salting-out procedure [9] was used to isolate DNA samples from whole blood. Ten single nucleotide polymorphisms (SNPs) in six candidate genes; LPL S447X (rs328), CETP Taq1B (rs708272), APOE (rs7412, rs429358), APOA5 −1131C > T (rs662799) and S19W (rs3135506), APOA4 S347T (rs675) and APOC3 −482C > T (rs2854117), 1100C > T (rs4520) and 3238C > G (rs5128) were genotyped using TaqMan technology (Applied Biosciences, ABI, Warrington UK). Reactions were performed on 384-well microplates and analysed using ABI TaqMan 7900HT software. Primers and MGB probes are available upon request.
Statistical methods
Hardy–Weinberg equilibrium (HWE) was examined by chi-square goodness of fit test. A p value of <0.05 was taken as deviation from HWE. Plasma levels of insulin, TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) and all anthropometric measures were natural log-transformed. For association studies a p value of <0.01 was taken as statistically significant. Setting a threshold of significance was the chosen method above Bonferroni corrections, since the candidate genes studied had been selected for based on a priori hypothesis and biological plausibility. A p value of <0.05 was taken as statistically significant in Principal Component Analysis (PCA). The majority of statistical analyses were performed using Intercooled Stata 8.2 for Windows (StataCorp LP, Texas, USA). Haplotype association analysis was carried out using Thesias [10]. PCA was carried out using SAS (SAS Institute Inc., Cary, NC). Throughout this study we used the first principal component (PC1) and the second principal component (PC2) because both components explained more than 80% of the variation and their eigenvalues were greater than the average eigenvalue [11].
Results
Subjects were classified as obese, overweight and non-overweight according to the International Obesity Task Force [12]. The non-overweight group includes normal weight (n = 533) and underweight (n = 2) children and for the purposes of this study has been called the normal weight group (n = 535). Table 1 summarises the baseline characteristics of a selection of lipid and anthropometric measures, comparing the overweight and obese children (n = 343) in the study to their normal weight counterparts (n = 535). Measures of blood pressure, insulin, TG, height and insulin resistance were significantly higher (p < 0.0001) and HDL-C significantly lower (p < 0.0001) in the overweight and obese group compared to their normal weight counterparts. The mean BMI of the mothers and fathers of the children was 24.6 ± 4.8 and 27.2 ± 3.6, respectively. Children whose parents were both overweight (BMI ≥ 25) had a BMI 2.2 units larger than children whose parents were both of a normal weight (BMI < 25) (p < 0.00001) (Appendices Table 1a). Children of parents who were hypercholesterolemic (Cholesterol >240 mg/dl) had significantly higher cholesterol levels compared to children of parents who's cholesterol levels were normal (p < 0.00001) and the same observation was observed between parents and their children with LDL levels (p = 0.003) (Appendices Table 1c and 1b, respectively).
Table 1.
Comparisons between normal weight and overweight/obesea subjects.
| Variable | Normal-weight | n | Overweight and obese | n | p value |
|---|---|---|---|---|---|
| Gender (male/female) (%male) | 236/297 (44.1%) | 535 | 180/163 (52.5%) | 343 | 0.015c |
| Age (years) | 11.2 ± 0.6d | 534 | 11.1 ± 0.6d | 340 | NSb |
| Tanner genitals or breast score | 2 (IQR 2–3)e | 532 | 3 (IQR 2–3)e | 338 | 0.06c |
| Tanner pubic hair score | 2 (IQR 2–3)e | 532 | 2 (IQR 2–3)e | 338 | NSc |
| Variable | Normal weightf | n | Overweight and obesef | n | p valueg |
|---|---|---|---|---|---|
| Weight (kg) | 39.1 (38.6, 39.6) | 535 | 53.1 (52.2, 53.9) | 343 | <0.0001 |
| Height (cm) | 147.8 (147.2, 148.4) | 535 | 150.8 (149.3, 150.0) | 343 | <0.0001 |
| Waist circumference (cm) | 63.5 (63.0, 64.0) | 535 | 77.1 (76.1, 77.9) | 342 | <0.0001 |
| Triceps skin fold (mm) | 15.0 (14.6, 15.4) | 535 | 25.7 (25.1, 26.4) | 340 | <0.0001 |
| Subscapular skin fold (mm) | 8.2 (8.0, 8.4) | 535 | 16.0 (15.5, 16.6) | 340 | <0.0001 |
| Body fatness % (Slaughter eq.) | 21.8 (21.3, 22.3) | 455 | 39.1 (37.9, 40.4) | 270 | <0.0001 |
| Systolic blood pressure (mmHg) | 117.2 (116.0, 118.5) | 531 | 123.9 (122.2,125.5) | 340 | <0.0001 |
| Diastolic blood pressure (mmHg) | 72.8 (71.8, 73.9) | 529 | 77.6 (76.2, 79.0) | 340 | <0.0001 |
| Insulin (uIU/mL) | 5.8 (5.6, 6.1) | 513 | 8.8 (8.3, 9.3) | 325 | <0.0001 |
| Glucose (mg/dL) | 85.2 (84.4, 86.0) | 525 | 84.6 (83.6, 85.6) | 332 | NS |
| Insulin resistance (HOMAIR-IR) | 1.22 (1.16, 1.28) | 512 | 1.84 (1.73, 1.96) | 332 | <0.0001 |
| TG (mmol/L) | 0.63 (0.61, 0.65) | 526 | 0.75 (0.75, 0.81) | 332 | <0.0001 |
| TC (mmol/L) | 4.79 (4.72, 4.86) | 526 | 4.81 (4.72, 4.89) | 332 | NS |
| HDL-C (mmol/L) | 1.38 (1.36, 1.41) | 526 | 1.27 (1.24, 1.29) | 332 | <0.0001 |
| LDL-C (mmol/L) | 3.07 (3.02, 3.13) | 526 | 3.13 (3.06, 3.21) | 332 | NS |
| Birthweight (g) | 3241.6 (2167.67, 4184.40) | 467 | 3297.3 (2319.14, 4403.03) | 305 | NS |
NS, not statistically significant; TG, total triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; IQR, inter quartile range and CI, confidence intervals.
Overweight and obesity were defined according to the International Obesity Task Force recommendations, using international reference values.
Assessed by ANOVA.
Assessed by χ2.
Mean (±standard deviation).
Median (IQR).
Mean (95% CI).
Assessed by linear regression, adjusted for Tanner status and gender.
Genotype frequencies
There were no allele frequency differences between boys and girls for any of the ten variants (data not shown). The genotype and minor allele frequency (MAF) for the ten variants are shown in Table 2. The genotype distribution of APOC3 1100C > T deviated from those expected under HWE (p = 0.02). There was a borderline statistically significant difference in allele frequency distribution of the LPL S447X variant between normal weight children and their overweight and obese counterparts, MAF 0.14 (95% confidence intervals (CI) 0.11, 0.16) vs. 0.11 (95% CI 0.08, 0.14) (p = 0.02).
Table 2.
Allele and genotype frequencies of candidate gene variants in Greek children.
| Gene | rs Number | AA and nucleotide change | Genotype | n | % | MAF (95% CI) |
|---|---|---|---|---|---|---|
| CETP | 708272 | Taq1B G > A | GG-B1/B1 | 252 | 35.44 | 0.4 |
| GA-B1/B2 | 351 | 49.37 | (0.37, 0.42) | |||
| AA-B2/B2 | 108 | 15.19 | ||||
| APOE | 429358 | C112R | C112/C158-ɛ2 | 34 | 7.78 | ɛ2 −0.09 |
| 7412 | R158C | C112/R158-ɛ3 | 335 | 76.66 | (0.07, 0.10) | |
| R112/R158-ɛ4 | 68 | 15.56 | ɛ4 −0.14 | |||
| (0.10, 0.18) | ||||||
| APOC3 | 2854117 | −482C > T | CC | 304 | 48.41 | 0.31 |
| CT | 262 | 41.72 | (0.28, 0.33) | |||
| TT | 62 | 9.87 | ||||
| APOC3 | 4520a | 1100C > T | CC | 321 | 50.63 | 0.28 |
| CT | 277 | 43.69 | (0.25, 0.30) | |||
| TT | 36 | 5.68 | ||||
| APOC3 | 5128 | Sst1 | GG-S1S1 | 477 | 76.44 | 0.13 |
| 3238G > C | GC-S1S2 | 137 | 21.96 | (0.11, 0.14) | ||
| CC-S2S2 | 10 | 1.6 | ||||
| APOA4 | 675 | S347T | TT | 352 | 57.8 | 0.24 |
| AT | 221 | 36.29 | (0.22, 0.26) | |||
| AA | 36 | 5.91 | ||||
| APOA5 | 662799 | −1131T > C | TT | 556 | 84.5 | 0.08 |
| TC | 97 | 14.74 | (0.07, 0.10) | |||
| CC | 5 | 0.76 | ||||
| APOA5 | 3135506 | S19W | GG | 582 | 90.23 | 0.05 |
| GC | 62 | 9.61 | (0.04, 0.06) | |||
| CC | 1 | 0.16 | ||||
| LPL | 328 | S447X | CC | 540 | 76.49 | 0.13 |
| CG | 154 | 21.81 | (0.11, 0.14) | |||
| GG | 12 | 1.7 | ||||
AA, amino acid; MAF, minor allele frequencie; CI, confidence intervals; APO, apolipoprotein; LPL, lipoprotein lipase and CETP, cholesterol ester transfer protein.
Borderline deviation from Hardy–Weinberg Equilibrium (p = 0.02).
Association with quantitative traits
Significant data for lipid and anthropometric variables, according to genotype, are presented in Table 3. APOE genotype, defined by two variant sites at residues 112 and 158 creating the ɛ2, ɛ3 and ɛ4 alleles, was significantly associated with TC (p = 0.0001) with ɛ2 carriers having TC plasma levels 11.3% lower than ɛ3/ɛ3 subjects (p < 0.001) and ɛ4 carriers with 1.3% higher TC plasma levels than ɛ3/ɛ3 subjects (p = 0.522). APOE genotype was also significantly associated with LDL-C (p < 0.0001). ɛ2 carriers had LDL-C plasma levels that were 17.6% lower than ɛ3/ɛ3 subjects (p < 0.001) and ɛ4 carriers had a mean LDL-C plasma level that was 2.8% higher than ɛ3/ɛ3 subjects (p = 0.258). ɛ2 carriers were also observed with a significantly lower mean TC: HDL-C ratio of 3.26 (95% CI 3.1, 3.5) compared to 3.62 (95% CI 3.6, 3.7) and 3.81 (95% CI 3.7, 4.0) for ɛ3/ɛ3 and ɛ4 carriers, respectively (p = 0.0008). The CETP Taq1B variant was associated with significantly higher HDL-C (p < 0.0001) and lower TC: HDL-C ratio (p < 0.0001) in those who were carriers or homozygotes for the B2 allele.
Table 3.
Association of APOE, CETP Taq1B and LPL S447X with baseline plasma and anthropometric measures.
| APOE | Genotype (n) | LDL-C (mmol/L)a |
TC (mmol/L)b |
TC: HDL-C Ratioc |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| ɛ2 Carriers (33) | 2.58 (2.41, 2.76) | 4.27 (4.04, 4.49) | 3.29 (3.11, 3.48) | |
| ɛ3/ɛ3 (332) | 3.13 (3.07, 3.20) | 4.82 (4.74, 4.90) | 3.62 (3.56, 3.67) | |
| ɛ4 Carriers (67) | 3.22 (3.07, 3.37) | 4.88 (4.70, 5.07) | 3.78 (3.62, 3.93) | |
| p value | <0.0001 | 0.0001 | 0.0008 |
| CETP TAQ1B | Genotype (n) | HDL-C (mmol/L)d |
TC: HDL-C ratioc |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | ||
| B1B1 (248) | 1.27 (1.24, 1.30) | 3.75 (3.67, 3.83) | |
| B1B2 (348) | 1.37 (1.34, 1.40) | 3.53 (3.47, 3.60) | |
| B2B2 (108) | 1.40 (1.35, 1.46) | 3.45 (3.35, 3.56) | |
| p value | <0.0001 | <0.0001 |
| LPL S447X | Genotype (n) | Weight (kg)e |
|---|---|---|
| Mean (95% CI) | ||
| SS (534) | 44.25 (43.65, 44.87) | |
| SX (151) | 43.30 (42.21, 44.40) | |
| XX (12) | 39.42 (35.98, 43.20) | |
| p value | 0.02 |
APO, apolipoprotein; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; CI, confidence intervals; TG, total triglyceride; LPL, lipoprotein lipase; CETP, cholesterol ester transfer protein and BMI, Body mass index.
Only data with p values ≤0.02 has been presented. Tanner status was combined into one variable from the two Tanner measures to produce a mean Tanner score.
Adjusted for height.
Adjusted for height and gender.
Adjusted for gender, mean Tanner score and BMI.
Adjusted for height, gender and BMI.
Adjusted for height, mean Tanner score and gender.
The LPL S447X variant showed borderline significant association with weight (p = 0.02) with 447X homozygotes weighing almost 5 kg less than S447 homozygotes. Carriers of the APOA5 19W had 7.8% higher plasma TG levels compared to homozygotes for the common allele, but the association did not reach statistical significance (p = 0.038) (Appendices Table 2). Carriers of the APOA5 −1131 rare T allele had 6.1% higher TG levels compared to CC homozygotes, but again this did not reach statistical significance (p = 0.048) (Appendices Table 2). No significant associations were observed between the APOA4 T347S and APOC3 variants and serum lipids. Common haplotypes within the APOA5/A4/C3 cluster showed no significant effect on TG, TC HDL-C or LDL-C levels (Appendices Table 2).
Principal component analysis
The plasma and anthropometric measures used for PCA were combined based on their correlation structures (Appendices Table 3). The first PCA included HDL-C, TC and LDL-C with PC1 explaining 74% of the variation and PC2 an additional 25% of the variation. CETP Taq1B and APOE were identified as having a significant effect in both PC1 (p < 0.01) and PC2 (p < 0.001) (Table 4a). The second PCA included weight, waist circumference, hip circumference, triceps and subscapular skin folds. PC1 alone explained 84% of the total variation, identifying LPL S447X as significant (p = 0.04) (Table 4b). The final PCA combined measures of TG, insulin and insulin resistance with PC1 explaining 72% of the variation and PC2 explaining an additional 27%. PC2 identified the two variants in APOA5, −1131C > T and S19W as having a significant effect (p = 0.015 and p = 0.039, respectively) (Table 4c).
Table 4.
Principal component analysis.
| Variant | PC1 (74%) |
PC2 (27%) |
||
|---|---|---|---|---|
| F value | p value | F value | p value | |
| CETP Taq1B | 3.38 | 0.006 | 28.01 | <0.001 |
| APOE | 8.21 | 0.004 | 13.05 | <0.001 |
| a) HDL-C – LDL-C – TC | ||||
| Variant | PC1 (84%) |
|
|---|---|---|
| F value | p value | |
| LPL S447X | 4.24 | 0.04 |
| b) Weight – Waist – Hip – Triceps and Subscapular skin folds | ||
| Variant | PC1 (72%) |
PC2 (25%) |
||
|---|---|---|---|---|
| F value | p value | F value | p value | |
| APOA5 S19W | 1.52 | 0.218 | 4.29 | 0.039 |
| APOA5 −1131C > T | 0.07 | 0.798 | 5.93 | 0.015 |
| c) TG – insulin – insulin resistance | ||||
Principal component analysis was performed on three separate clusters. a) HDL-C, LDL-C and TC; b) weight, waist circumference, hip circumference, triceps skin fold and subscapular skin folds; c) TG, insulin and insulin resistanceHOMA. The variance for the first and second principal components (PC1 and PC2) is provided; F and p values are provided for each of the significant variants. LPL, lipoprotein lipase; HDL-C, high-density lipoprotein cholesterol; LDL-C; low-density lipoprotein cholesterol; TC, total cholesterol; CETP, cholesterol ester transfer protein; APO, apolipoprotein and TG, total triglyceride.
APOE variation interacts with BMI impacting on lipid measures
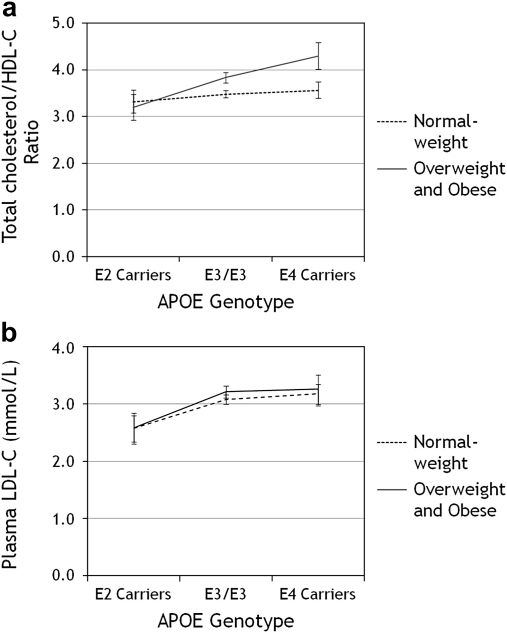
An interaction of APOE and BMI (dichotomised into Normal weight and Overweight/Obese) was impacting on the TC: HDL-C ratio in this young cohort (p = 0.008) was identified (Fig. 1a) and HDL-C levels (p = 0.01) (data not shown). ɛ4 carriers in the overweight and obese category had a poorer TC: HDL-C ratio of 4.3 (95% CI 4.0, 4.6) compared to ɛ2 carriers with a ratio of 3.2 (95% CI 2.9, 3.5). Children in the normal weight category had a TC: HDL-C ratio which was unaffected by APOE genotype. ɛ4 carriers had a mean ratio of 3.6 (95% CI 3.4, 3.8), ɛ3/ɛ3 3.5 (95% CI 3.4, 3.6) and ɛ2 carriers 3.3 (95% CI 3.1, 3.6). There was no interaction between BMI and APOE genotype on plasma LDL-C (Fig. 1b); TG or TC levels (data not shown). Gene–diet interactions examining all variants with a number of dietary measures were investigated, but there were no significant results (data not shown).
Figure 1.
a) Interaction between APOE genotype and BMI category on total cholesterol/HDL-C ratio (p = 0.008). Normal weight children: ɛ3/ɛ3 = 195, ɛ2 carriers = 20, ɛ4 carriers = 43; Overweight and obese children: ɛ3/ɛ3 = 139, ɛ2 carriers = 14, ɛ4 carriers = 25. b) Interaction between APOE genotype and BMI category on plasma LDL-C (Not significant). Normal weight children: ɛ3/ɛ3 = 195, ɛ2 carriers = 19, ɛ4 carriers = 43; Overweight and obese children: ɛ3/ɛ3 = 136, ɛ2 carriers = 14, ɛ4 carriers = 2. APO, apolipoprotein; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol, and LDL-C, low-density lipoprotein cholesterol.
Discussion
The results from this study demonstrate in this dataset of healthy Greek children, the significant association of candidate gene variants with baseline anthropometric and lipid measures. More than a third of the children in this cohort were overweight with obesity reaching a level of 9.5%. This is comparable to a study in central Greece assessing 11-year-olds weight status where a total of 30.3% were reported to be overweight and 6.7% obese [13]. The fact that parental BMI was positively associated with their child's BMI highlights the importance of family history and environment in the development of obesity. The overweight and obese children in the current study had significantly higher arterial blood pressure, lower HDL-C levels, higher TG and increased insulin compared to their the normal weight counterparts. Higher Tanner scores and heights of the overweight and obese group also suggest earlier onset of puberty, which is often frequently observed in overweight and obese children [14]. Any interaction between sexual maturity and effects of allelic variation on lipid levels that may have occurred could be accounted for in analyses from the Tanner measures.
Genetic factors are considered important determinants of plasma lipid levels in adults, demonstrated in several of the recent genome wide association studies (GWAS) in which a number of candidate genes have been confirmed [15,16]. The meta-analysis of 3 GWAS by Willer et al. (2008) identified strong associations with variants in APOA5/A4/C3/A1 cluster, APOE, CETP and LPL influencing plasma lipid concentrations. Although a number of associations comparable to those seen in adults were confirmed in this study, the role of genetic factors in the heterogeneity of plasma lipid levels in children is less clear. Replication of these variants in cohorts of children is needed.
In GENDAI, APOE genotypes were associated with differences in TC and LDL-C plasma levels and the TC: HDL-C ratio. The LDL-C and TC lowering effect of the ɛ2 allele reported in the recent meta-analysis [17] was also observed in this cohort of young Greek children. Carriers of the ɛ4 allele had LDL-C and TC levels that were 19.9% and 12.2% higher than carriers of the ɛ2 allele and 2.8% and 1.3% higher than ɛ3/ɛ3 subjects. The results of a 21-year longitudinal study on changes in serum lipids in 1233 Finns followed from childhood to adulthood consistently observed the ɛ2 allele to be associated with lower LDL-C levels and the ɛ4 allele with higher TC and LDL-C levels (p < 0.001 for all associations) in childhood. The LDL-C-lowering effect of the ɛ2 allele was an association that was tracked through to adulthood, having a greater effect with increasing age (p = 0.039). The association of the APOE genotype with plasma TC and LDL-C has been reported in children as young as 3 years old [18]. The fact that differences in lipid levels cannot be detected in children at birth by the APOE genotype leads to the conclusion that lipid levels are influenced by genetic and environmental factors in a child's very first years of life [18].
CETP is involved primarily in the transfer of lipids in reverse cholesterol transport, exchanging cholesterol from HDL-C for TG from TG-rich lipoproteins (TGRL) [19], thus influencing the TG content of TGRL particles and playing a major role in the determination of HDL-C levels [20]. Meta-analysis of CETP Taq1B has consistently shown association with HDL-C levels [21]. The association of the B2 allele with higher HDL-C levels was observed in this study. Homozygotes for the B2 allele had approximately 10% higher mean HDL-C levels compared to the B1/B1 individuals, comparable to that seen in adults [22]. A highly significant association of the Taq1B variant with TC: HDL-C ratios in the cohort were also observed, highlighting the importance of this particular genotype and its effects on HDL-C levels from a young age. In a cohort of 257 Dutch prepubescent boys and girls (aged 6.7–8.1 years) the same association with the Taq1B variant was reported, but dependant on APOE genotype [23].
LPL is a key lipolytic enzyme that plays a crucial role in the catabolism of triglycerides in TG-rich particles and the S447X variant in exon 9 results in premature truncation [24]. The LPL 447X genotype has been consistently associated in adult populations with a beneficial lipid profile conferring a protective effect against myocardial infarction [25]. Children homozygous for the rare 447X allele had approximately 2% lower TG levels than children who were homozygous for the common allele, but this did not reach statistical significance. The borderline association of the 447X allele with lower weight is interesting considering the significant difference in MAF (p = 0.02) between the normal weight and overweight children (MAF 0.14 and 0.11, respectively).
Numerous studies have investigated the association of genetic variation in the APOA5/A4/C3/A1 cluster on lipid levels in adults [5,26]. The TG raising effect of the APOA5 S19W variant seen in adults was also observed in this cohort, but this did not reach statistical significance. Previous studies have shown significant associations of the APOA5 −1131T > C promoter variant with TG levels [5], and although the association of this variant in the present study was not statistically significant, TG levels were 6.1% higher in children who were carriers of the rare allele. There was no significant association with any of the baseline lipid measures with the APO4 and three APOC3 variants examined. These findings corroborate with the data on the association of variants in the APOC3 gene with lipid levels in children in the Columbia Biomarkers Study [27]. Although, trends were observed with the APOC3 variants they did not reach statistical significance. In particular, carriers of the S2 allele of the APOC3 Sst1 variant was associated with higher TG levels, which is consistent with the recently published AVENA Study [28]. The lack of association in the case of both the APOA5 and APOC3 variants was due to insufficient power to detect the modest effect size these variants were having on TG levels. PCA was adopted to increase the power of detecting such associations, but did not provide any information over and above the results from the regression analysis, but all associations were confirmed. As well as the association of these variants with lipid levels, it is of importance that the effect and influence of these variants on plasma apolipoprotein levels is also investigated. In the present study we unfortunately did not have these measures.
Increased levels of obesity have been demonstrated to amplify genetic effects. Even in these young children, BMI through an interaction with APOE was modulating and determining the lipid parameters of the TC: HDL-C ratio, with the less beneficial ratio being found among ɛ4 carriers than among ɛ3/ɛ3 or ɛ2 carriers. The APOE genotype had little influence on the TC: HDL-C ratio in children of a normal BMI. A similar association was seen in a cohort of 266 healthy men with APOE ɛ2, ɛ3, ɛ4 genotype and TC, LDL-C and insulin levels. Individuals who were ɛ4 carriers had significantly higher (p = 0.04) TC, LDL-C and insulin levels compared ɛ3/ɛ3 or ɛ2 carriers, an association which was enhanced in the ɛ4 carriers as BMI increased [29]. These data suggest that effects of APOE alleles on lipids levels are partly dependent on and modulated by environmental variables such as BMI.
Previous genetic studies have demonstrated that variants investigated in this study are significant determinants of serum lipid levels in adults. However, only a few studies have investigated the association of these variants in children. The effects in the GENDAI study are of similar magnitude to those observed in adults, suggesting that even in these young children there is potential in predicting their long-term exposure to an adverse lipid profile. Kathiresan et al. have developed a genotype score for use in CHD risk assessment [30]. Using 9 SNPs in genes that determining plasma LDL and HDL cholesterol levels, they reported that addition of a genotype score to a CHD risk algorithm improved risk reclassification, even after adjustment for baseline lipid levels. This result importantly suggested that lipid-associated SNPs may provide incremental information about an individuals' risk beyond a single lipid measure and furthermore, although individual SNPs exert only a modest affect on lipid variation, in combination they may have a substantial influence. The data from this present study suggest the influence of variants is exerted at a very young age, and thus reflecting a lifelong exposure.
Acknowledgements
The authors would like to thank the following investigators Ioanna Hatzopoulou, Maria Tzirkalli, Anastasia-Eleni Farmaki, Ioannis Alexandrou, Nektarios Lainakis, Evagelia Evagelidaki, Garifallia Kapravelou, Ioanna Kontele, Katerina Skenderi, for their assistance in physical examination, biochemical analysis and nutritional assessment. The study was supported by a research grant from Coca-Cola Hellas. MCS is supported by a Unilever/BBSRC Case studentship. SEH, FD and PJT are supported by the British Heart Foundation (PG2005/014).
Footnotes
Sources of support: the Gene–Diet Attica Investigation on childhood obesity (GENDAI) was supported by a research grant from Coca-Cola Hellas. MCS is supported by a Unilever/BBSRC Case studentship. SEH, FD and PJT are supported by the British Heart Foundation (PG2005/014).
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.numecd.2009.02.005.
Appendix. Supplementary information
References
- 1.Deckelbaum R.J., Williams C.L. Childhood obesity: the health issue. Obesity Research. 2001 Nov;9(Suppl. 4):239S–243S. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- 2.Dietz W.H. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998 Mar;101(3 Pt 2):518–525. [PubMed] [Google Scholar]
- 3.Papoutsakis C., Vidra N.V., Hatzopoulou I., Tzirkalli M., Farmaki A.E., Evagelidaki E. The Gene–Diet Attica investigation on childhood obesity (GENDAI): overview of the study design. Clinical Chemistry and Laboratory Medicine. 2007;45(3):309–315. doi: 10.1515/CCLM.2007.080. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk K.W., Rensen P.C., Voshol P.J., Havekes L.M. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Current Opinion in Lipidology. 2004 Jun;15(3):239–246. doi: 10.1097/00041433-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hallman D.M., Srinivasan S.R., Chen W., Boerwinkle E., Berenson G.S. Longitudinal analysis of haplotypes and polymorphisms of the APOA5 and APOC3 genes associated with variation in serum triglyceride levels: the Bogalusa Heart Study. Metabolism. 2006 Dec;55(12):1574–1581. doi: 10.1016/j.metabol.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Eichner J.E., Dunn S.T., Perveen G., Thompson D.M., Stewart K.E., Stroehla B.C. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. American Journal of Epidemiology. 2002 Mar 15;155(6):487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 7.Skoglund-Andersson C., Ehrenborg E., Fisher R.M., Olivecrona G., Hamsten A., Karpe F. Influence of common variants in the CETP, LPL, HL and APO E genes on LDL heterogeneity in healthy, middle-aged men. Atherosclerosis. 2003 Apr;167(2):311–317. doi: 10.1016/s0021-9150(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 8.Vincent S., Planells R., Defoort C., Bernard M.C., Gerber M., Prudhomme J. Genetic polymorphisms and lipoprotein responses to diets. Proceedings of the Nutrition Society. 2002;61(4):427–434. doi: 10.1079/pns2002177. [DOI] [PubMed] [Google Scholar]
- 9.Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988 Feb 11;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tregouet D.A., Garelle V. A new JAVA interface implementation of THESIAS: testing haplotype effects in association studies. Bioinformatics (Oxford, England) 2007 Apr 15;23(8):1038–1039. doi: 10.1093/bioinformatics/btm058. [DOI] [PubMed] [Google Scholar]
- 11.Rencher A. John Wiley and Sons; USA: 1998. Multivariate statistical inference and applications. [Google Scholar]
- 12.Cole T.J., Bellizzi M.C., Flegal K.M., Dietz W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clinical Research ed.) 2000 May 6;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magkos F., Piperkou I., Manios Y., Papoutsakis C., Yiannakouris N., Cimponerio A. Diet, blood lipid profile and physical activity patterns in primary school children from a semi-rural area of Greece. Journal of Human Nutrition and Dietetics. 2006 Apr;19(2):101–112. doi: 10.1111/j.1365-277X.2006.00675.x. [quiz 13–6] [DOI] [PubMed] [Google Scholar]
- 14.Lee J.M., Appugliese D., Kaciroti N., Corwyn R.F., Bradley R.H., Lumeng J.C. Weight status in young girls and the onset of puberty. Pediatrics. 2007 Mar;119(3):e624–e630. doi: 10.1542/peds.2006-2188. [DOI] [PubMed] [Google Scholar]
- 15.Kathiresan S., Manning A.K., Demissie S., D'Agostino R.B., Surti A., Guiducci C. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Medical Genetics. 2007;8(Suppl. 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willer C.J., Sanna S., Jackson A.U., Scuteri A., Bonnycastle L.L., Clarke R. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nature Genetics. 2008 Feb;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennet A.M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007 Sep 19;298(11):1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 18.Lehtimaki T., Porkka K., Viikari J., Ehnholm C., Akerblom H.K., Nikkari T. Apolipoprotein E phenotypes and serum lipids in newborns and 3-year-old children: the Cardiovascular Risk in Young Finns Study. Pediatrics. 1994 Oct;94(4 Pt 1):489–493. [PubMed] [Google Scholar]
- 19.Barter P.J., Brewer H.B., Jr., Chapman M.J., Hennekens C.H., Rader D.J., Tall A.R. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2003 Feb 1;23(2):160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 20.Barter P.J., Chang L.B., Newnham H.H., Rye K.A., Rajaram O.V. The interaction of cholesteryl ester transfer protein and unesterified fatty acids promotes a reduction in the particle size of high-density lipoproteins. Biochimica et Biophysica Acta. 1990 Jun 28;1045(1):81–89. doi: 10.1016/0005-2760(90)90206-d. [DOI] [PubMed] [Google Scholar]
- 21.Boekholdt S.M., Thompson J.F. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. Journal of Lipid Research. 2003 Jun;44(6):1080–1093. doi: 10.1194/jlr.R200018-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Ordovas J.M. Genetic polymorphisms and activity of cholesterol ester transfer protein (CETP): should we be measuring them? Clinical Chemistry and Laboratory Medicine. 2000 Oct;38(10):945–949. doi: 10.1515/CCLM.2000.139. [DOI] [PubMed] [Google Scholar]
- 23.Rump P., Mensink R.P., Hornstra G. Interaction between a common variant of the cholesteryl ester transfer protein gene and the apolipoprotein E polymorphism: effects on plasma lipids and lipoproteins in a cohort of 7-year-old children. Nutrition, Metabolism and Cardiovascular Diseases. 2002 Dec;12(6):317–324. [PubMed] [Google Scholar]
- 24.Hata A., Robertson M., Emi M., Lalouel J.M. Direct detection and automated sequencing of individual alleles after electrophoretic strand separation: identification of a common nonsense mutation in exon 9 of the human lipoprotein lipase gene. Nucleic Acids Research. 1990 Sep 25;18(18):5407–5411. doi: 10.1093/nar/18.18.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagne S.E., Larson M.G., Pimstone S.N., Schaefer E.J., Kastelein J.J., Wilson P.W. A common truncation variant of lipoprotein lipase (Ser447X) confers protection against coronary heart disease: the Framingham Offspring Study. Clinical Genetics. 1999 Jun;55(6):450–454. doi: 10.1034/j.1399-0004.1999.550609.x. [DOI] [PubMed] [Google Scholar]
- 26.Surguchov A.P., Page G.P., Smith L., Patsch W., Boerwinkle E. Polymorphic markers in apolipoprotein C-III gene flanking regions and hypertriglyceridemia. Arteriosclerosis Thrombosis and Vascular Biology. 1996 Aug;16(8):941–947. doi: 10.1161/01.atv.16.8.941. [DOI] [PubMed] [Google Scholar]
- 27.Talmud P.J., Berglund L., Hawe E.M., Waterworth D.M., Isasi C.R., Deckelbaum R.E. Age-related effects of genetic variation on lipid levels: the Columbia University BioMarkers Study. Pediatrics. 2001 Sep;108(3) doi: 10.1542/peds.108.3.e50. E50. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz J.R., Labayen I., Ortega F.B., Moreno L.A., Gonzalez-Lamuno D., Marti A. Birth weight and blood lipid levels in Spanish adolescents: influence of selected APOE, APOC3 and PPARgamma2 gene polymorphisms. The AVENA Study. BMC Medical Genetics. 2008;9:98. doi: 10.1186/1471-2350-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques-Vidal P., Bongard V., Ruidavets J.B., Fauvel J., Hanaire-Broutin H., Perret B. Obesity and alcohol modulate the effect of apolipoprotein E polymorphism on lipids and insulin. Obesity Research. 2003 Oct;11(10):1200–1206. doi: 10.1038/oby.2003.165. [DOI] [PubMed] [Google Scholar]
- 30.Kathiresan S., Melander O., Anevski D., Guiducci C., Burtt N.P., Roos C. Polymorphisms associated with cholesterol and risk of cardiovascular events. The New England Journal of Medicine. 2008 Mar 20;358(12):1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.