Abstract
Perfluorinated carboxylates (PFCAs) are generally stable to metabolic and environmental degradation and have been found at low concentrations in environmental and biological samples. Renal clearance of PFCAs depends on chain length, species, and, in some cases, gender within species. While perfluoroheptanoate (C7) is almost completely eliminated renally in both male and female rats, renal clearance of perfluorooctanoate (C8) and perfluorononanoate (C9) is much higher in female rats. Perfluorodecanoate (C10) mainly accumulates in the liver for both genders. Therefore, we tested whether PFCAs with different chain lengths are substrates of rat renal transporters with gender-specific expression patterns. Inhibition of uptake of model substrates was measured for the basolateral organic anion transporter (Oat)1 and Oat3 and the apical Oat2, organic anion transporting polypeptide (Oatp)1a1, and Urat1 with 10μM PFCAs with chain lengths from 2 to 18 (C2–C18) carbons. Perfluorohexanoate (C6), C7, and C8 inhibited Oat1-mediated p-aminohippurate transport, with C7 being the strongest inhibitor. C8 and C9 were the strongest inhibitors for Oat3-mediated estrone-3-sulfate transport, while Oatp1a1-mediated estradiol-17β-glucuronide uptake was inhibited by C9, C10, and perflouroundecanoate (C11), with C10 giving the strongest inhibition. No strong inhibitors were found for Oat2 or Urat1. Kinetic analysis was performed for the strongest inhibitors. Oat1 transported C7 and C8 with Km values of 50.5 and 43.2μM, respectively. Oat3 transported C8 and C9 with Km values of 65.7 and 174.5μM, respectively. Oatp1a1-mediated transport yielded Km values of 126.4 (C8), 20.5 (C9), and 28.5μM (C10). These results suggest that Oat1 and Oat3 are involved in renal secretion of C7–C9, while Oatp1a1 can contribute to the reabsorption of C8 through C10, with highest affinities for C9 and C10.
Keywords: Oat1, Oat2, Oat3, Urat1, Oatp1a1, kidney transporter
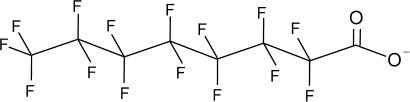
Perfluorinated carboxylates (PFCAs) are fatty acid analogues with fully fluorinated carbon backbones. A representative structure of perfluorooctanoate (C8) is shown in Figure 1. The chain lengths for most commercially available PFCAs range from 2 to 18 carbons (C2–C18). PFCAs are generally stable to metabolic and environmental degradation and have been found at low concentrations in samples of environmental and biological media. Although trifluoroacetate (C2) can occur naturally, C2 and most other PFCAs are present in the environment as a result of various commercial uses. PFCAs can also arise from degradation of other polyfluorinated chemistries (D'Eon et al., 2006; D'Eon and Mabury, 2007; Fasano et al., 2006; Wallington et al., 2006), and they are more resistant to natural degradation processes as compared to hydrocarbon carboxylates due to the presence of multiple carbon-fluorine bonds (Lemal, 2004).
FIG. 1.
Structure of C8.
The toxicological properties of perfluoroalkyl carboxylates have been studied extensively (Andersen et al., 2008; Kennedy et al., 2004; Kudo and Kawashima, 2003; Lau et al., 2007; Olsen et al., 2009; Vanden Heuvel et al., 1991), and differences in biological activity between congeners may be determined, in part, by pharmacokinetic differences in renal clearance. Renal clearance of PFCAs is influenced by chain length, isomeric structure (branched vs. linear), species, and, in some cases, gender within species (Butenhoff et al., 2004; Chang et al., 2008; Chengelis et al., 2009; De Silva et al., 2009; Kennedy et al., 2004; Kudo and Kawashima, 2003; Vanden Heuvel et al., 1991).
As an example of the differences that can occur in elimination kinetics, in humans, the geometric mean half-life of C8 serum elimination in a group of retired production workers was 3.5 years (95% confidence interval, 3.0–4.1), with no obvious gender differences (Olsen et al., 2007). This compares to cynomolgus monkeys, for which the serum C8 elimination half-life is estimated to be several weeks (Butenhoff et al., 2004). However, in male rats, the serum elimination half-life of C8 is several days, as compared to hours for female rats (Kennedy et al., 2004; Kudo and Kawashima, 2003). In contrast, the estimated elimination half-life of perfluorobutyrate (C4) is approximately 3–4 days for humans and 1.5 days for cynomolgus monkeys (Chang et al., 2008), again with no obvious gender differences in either species. In male and female rats exposed to C4, serum elimination half-lives are approximately 6–9 and 2–4 h, respectively.
Because PFCAs are not known to undergo metabolic conversion (Lau et al., 2007), differences in elimination kinetics are hypothesized to be due to differences in renal elimination as a result of differences in renal transport processes (Andersen et al., 2006; Katakura et al., 2007; Kudo et al., 2001; Nakagawa et al., 2008, 2009; Tan et al., 2008). Since the rat is one of the most commonly used species in toxicological studies, it is important to identify the involved transport systems and elucidate their role in the renal elimination of PFCAs to further understand potential toxicological effects and extrapolate the findings to humans.
The renal organic anion transport system is a very important system in facilitating the daily elimination of toxic compounds and reabsorption of useful anionic metabolites. There are several major transporter families that are involved in the transport of organic anions in the kidney: the organic anion transporter (Oat) family, the organic anion transporting polypeptide (Oatp) superfamily, the multidrug resistance protein (Mdr), and the multidrug resistance–associated protein (Mrp) superfamilies (Sekine et al., 2006). In the rat proximal tubule, these transporters are expressed in a polarized manner with specific transporters being differentially localized in the basolateral membrane and apical membrane. Transporters localized at the basolateral membrane include Oat1, Oat3 (Kojima et al., 2002; Tojo et al., 1999), and Oatp4c1 (Mikkaichi et al., 2004). Transporters localized at the brush border membrane include Oat2 (Kojima et al., 2002), Urat1 (Rizwan and Burckhardt, 2007), Oat5 (Anzai et al., 2005), Oatp1a1 (Bergwerk et al., 1996), Mdr1a/b (Sekine et al., 2006), Mrp2 (Schaub et al., 1997), and Mrp4 (van Aubel et al., 2002). Gender-dependent expression patterns occur with several of these transporters, including Oat1, Oat2, Oat3 (Buist et al., 2002; Cerrutti et al., 2002; Ljubojevic et al., 2007, 2004), Oatp1a1 (Kato et al., 2002; Lu et al., 1996), and Mdr1b (Lu and Klaassen, 2008). Among these transporters, Oat1, Oat3, and Oatp1a1 have been shown to transport C8 (Katakura et al., 2007; Nakagawa et al., 2008; Yang et al., 2009).
Given the in vivo evidence that there are gender-dependent renal elimination patterns for PFCAs with chain lengths from 7 to 10 (Kudo et al., 2001), we hypothesized that PFCAs with different chain lengths are substrates of different Oats and that the location and gender-specific expression of those transporters would contribute to the renal elimination pattern. In this study, we investigated the roles of the five rat renal Oats Oat1, Oat2, Oat3, Urat1, and Oatp1a1 in transporting PFCAs with different chain lengths (C2–C18). Initial experiments studied the potential of C2–C18 PFCAs to inhibit known substrates of the transporters. Based on those results, PFCA uptake and transport kinetic studies were performed, focusing on four PFCAs (perfluoroheptanoate [C7] to perfluorodecanoate [C10]).
MATERIALS AND METHODS
Materials.
Radiolabeled [3H]-estradiol-17β-glucuronide (E-17β-G), [3H]-p-aminohippurate (PAH), and [3H]-estrone-3-sulfate (E-3-S) were purchased from Perkin Elmer (Boston, MA). [14C]-uric acid was purchased from Moravek Biochemicals (Brea, CA). Unlabeled chemicals were obtained from Sigma-Aldrich (St Louis, MO). cDNAs of Oat1, Oat3, and Urat1 were purchased from Open Biosystem (Huntsville, AL). The Chinese hamster ovary (CHO)-Oatp1a1 cell line was kindly provided by Bruno Stieger from the University Hospital in Zurich, Switzerland (Eckhardt et al., 1999).
Tissue culture and transporter expression.
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA) were grown at 37°C in a humidified 5% CO2 atmosphere in Dulbecco's Modified Eagle Medium (DMEM) High Glucose (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Twenty-four hours before transfection, HEK293 cells were harvested by trypsinization and replated at 250,000 cells per well in 24-well plates (coated with 0.1 mg/ml poly-D-lysine). The transfection mixture consisted of 0.8 μg of plasmid DNA and 2 μl Lipofectamine 2000 (Invitrogen). Transfected cells were incubated at 37°C for 48 h before use.
CHO cells (CHO-wild-type and CHO-Oatp1a1) were grown at 37°C in a humidified 5% CO2 atmosphere in DMEM containing 1 g/l D-glucose, 2mM L-glutamine, 25mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, and 110 mg/l sodium pyruvate supplemented with 10% FBS (Hyclone), 50 μg/ml L-proline, 100 U/ml penicillin, and 100 μg/ml streptomycin. For uptake experiments, CHO-wild-type- and Oatp1a1-expressing cells were plated at 40,000 cells per well on 24-well plates, and 48 h later, medium was replaced with medium containing 5mM sodium butyrate to induce nonspecific gene expression. After another 24 h in culture, the cells were used for uptake experiments.
Oat2 was cloned from kidney RNA of Sprague-Dawley rats by reverse transcriptase PCR using gene-specific primers and subcloned into the pcDNA5/FRT vector. The cDNA sequence was verified by sequencing both strands individually. FlpIn-HEK293 cells (Invitrogen) were maintained in Ham's F12/DMEM (1/1) medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml Zeocin. FlpIn-HEK293 cells were plated on 6-well plates without antibiotics and then transfected as described in the manufacturer's instructions (Invitrogen). Twenty-four hours after transfection, cells were replated onto 10-cm plates in medium supplemented with 400 μg/ml hygromycin B for selection for 2 weeks with one subsequent 1:3 passage after 1 week. The pooled cells were tested for uptake of model substrates and used in PFCA transport studies. FlpIn-HEK293 cells stably transfected with pcDNA5/FRT empty vector were used as negative control.
Transport assay.
After washing the cells three times with prewarmed (37°C) HEK uptake buffer (142mM NaCl, 5mM KCl, 1mM KH2PO4, 1.2mM MgSO4, 1.5mM CaCl2, 5mM glucose, and 12.5mM HEPES, pH 7.4) or CHO uptake buffer (116.4mM NaCl, 5.3mM KCl, 1mM NaH2PO4, 0.8mM MgSO4, 5.5mM D-glucose, and 20mM HEPES, pH adjusted to 7.4 with Trizma base), 200 μl uptake buffer containing model substrates with or without PFCAs were added to initiate transport. After incubating for the indicated time periods, transport was terminated by two washes with ice-cold uptake buffer with 3% bovine serum albumin (BSA) followed by two washes with ice-cold uptake buffer without BSA. Then, cells were lysed with 300 μl 1% Triton X-100 in H2O at room temperature for 20 min. For radiolabeled substrates, 200 μl of cell lysate were transferred to 24-well scintillation plates (Perkin Elmer, Shelton, CT), and radioactivity was measured after adding Optiphase Supermix scintillation cocktail (Perkin Elmer) in a MicroBeta liquid scintillation counter. For unlabeled PFCA substrates, final concentrations in cell lysate were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The remaining cell lysates were used to determine the protein concentration using the bicinchoninic acid Protein Assay (Pierce Biotechnology, Rockford, IL). All transport activities were corrected by the total protein concentration. All experiments were done two to four times independently with triplicate determinations, that is, three wells per time point.
Kinetic analysis.
Kinetics for Oat1, Oat3, and Oatp1a1 were all determined within the initial linear time range. Transport of C7, C8, perfluorononanoate (C9), and C10 was measured from 10 to 300μM. Transporter-specific uptake was obtained by subtracting the uptake into empty vector–transfected HEK293 or wild-type CHO cells from the uptake into transporter-expressing cells. Michaelis-Menten type nonlinear curve fitting was carried out to obtain estimates of the maximal uptake rate (Vmax) and the apparent affinity constant (Km) (Graphpad Prism, GraphPad Software Inc., La Jolla, CA). All experiments were done two to four times independently with triplicate determinations, that is, three wells per time point.
LC-MS/MS analysis.
Two LC-MS/MS systems were employed in this study. For single concentration uptake and time dependency studies, Waters ACQUITY ultra performance LC System (Waters, Milford, MA) and Waters Quattro Premier XE triple quadrupole mass spectrometer with an electrospray ionization source (Waters) were used. The entire LC-MS system was controlled by MassLynx 4.1 software. Chromatographic separations were performed with an ACQUITY UPLC C18 column (1.7 μm, 50 × 2.1 mm inner diameter [i.d.]) (Waters). The mobile phase consisted of 35% H2O and 65% acetonitrile at a total flow rate of 0.300 ml/min. Multiple reaction monitoring transitions 363 → 319 amu, 413 → 369 amu, 463 → 419 amu, and 513 → 469 amu were chosen for C7, C8, C9, and C10 quantification. C7 was used as internal control for C8, C9, and C10 quantification, and C8 was used as internal control for C7 quantification. Samples were prepared by adding 60 μl cell lysate into 120 μl acetonitrile containing internal standard. The tubes were then vortexed vigorously for 5 s followed by centrifugation at 25,000 × g for 20 min. One hundred and fifty microliters of supernatant were then transferred to a new clean polypropylene tube and stored at − 20°C until analyzed. For transporter kinetic studies, the instrument used was the Sciex API 5000 mass spectrometer (Applied Biosystems/MDS-Sciex, Foster City, CA). The Turbo Ion Spray (pneumatically assisted electrospray ionization) in negative ion mode was used. Separation of the compounds were completed on a Mac-Mod ACE C-18, 5 μm, 100 × 2.1 mm i.d. high performance liquid chromatography (HPLC) column with a gradient flow rate of 0.250 ml/min. Mobile phase was acetonitrile and 2mM ammonium acetate solution made up in reagent grade water (MilliQ deionized water that has been further purified to remove residual traces of perfluorinated compounds). The initial mobile phase was 25% acetonitrile and 75% ammonium acetate solution. The concentration was held for 1.0 min. Between 1.0 and 4.0 min, the composition was changed to 10% 2mM ammonium acetate solution and 90% acetonitrile. The 90% acetonitrile composition was held until 7.0 min into the run. Between 7 and 7.80 min, the acetonitrile concentration was decreased to 25% and held until 10.5 min until re-equilibration of the HPLC columns was completed. All source parameters were optimized according to manufacturer's guidelines. Transition ions were monitored as follows: C7: 363 → 319 amu; C8: 413 → 369 amu; C9: 463 → 419 amu; C10: 513 → 469 amu; and Internal Standard—dual 13C-labeled C8: 415 → 370 amu. Samples were prepared by the addition of 25 μl of cell lysate to the polypropylene 1.8 ml conical tip tube containing the internal standard. Standards were introduced to similar tubes, and matrix-matched blank buffer (25 μl) of either CHO lysate or HEK293 lysate was pipetted as appropriate. To the tube containing the 25 μl of cell lysate, 175 μl of a solution containing 5% of 2mM ammonium acetate and 95% acetonitrile was then added (total volume 200 μl). The tube was then vortexed vigorously for 5 s followed by centrifugation at 2500 × g. An 80-μl aliquot of supernatant was transferred to a new clean 100-μl polypropylene vial; the vial was then capped with a polypropylene cap and placed on the autosampler for injection into the LC-MS/MS system. A 4-μl injection was used for the analysis.
Statistical analysis.
Statistical significance was analyzed using two-tailed, unpaired Student's t-test (GraphPad Prism, GraphPad Software Inc.). Data were considered statistically significant at p < 0.05.
RESULTS
Oat1- and Oat3-Mediated Transport of PFCAs
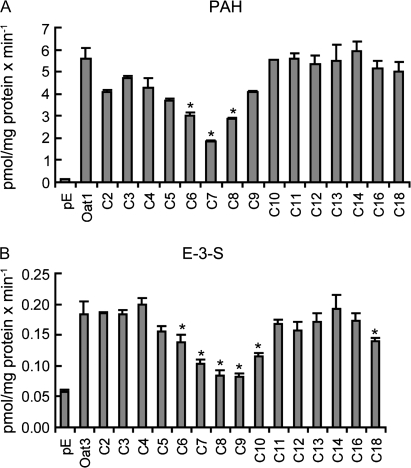
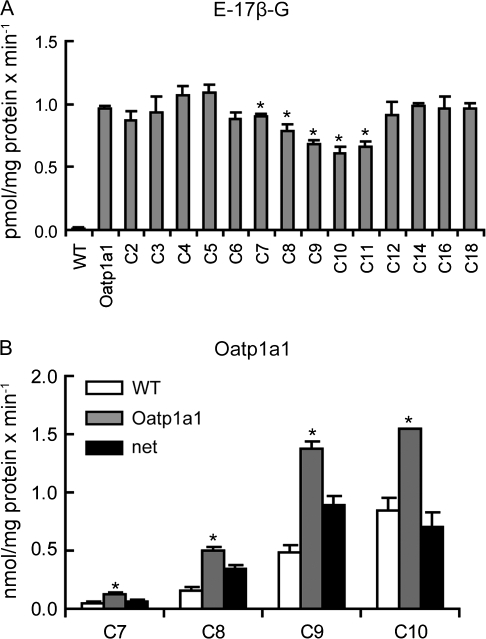
In HEK293 cells transiently transfected with rat Oat1, transport of the model substrate [3H]-PAH was strongly inhibited by 10μM of C7, followed by perfluorohexanoate (C6) and C8 (Fig. 2A) while other homologs did not show significant inhibition at this concentration. In HEK293 cells expressing Oat3, transport of the model substrate [3H]-E-3-S was strongly inhibited by 10μM of C8 and C9, followed by C7 and C10 (Fig. 2B), while other homologs did not show significant inhibition at this concentration. In both cases, uptake of model substrates into empty vector–transfected HEK293 cells was not affected by the presence of PFCAs (data not shown).
FIG. 2.
Inhibitory effect of PFCAs on Oat1- and Oat3-mediated transport. Oat1-mediated 88nM [3H]-PAH (A) or Oat3-mediated 7nM [3H]-E-3-S (B) uptake was measured at 37°C for 1 min in the absence or presence of 10μM PFCAs with chain lengths from 2 (C2) to 18 (C18) carbon atoms in Oat-expressing or empty vector pExpress-1 (pE)–transfected HEK293 cells. The results were corrected for total protein concentration in each well. Each bar is the mean ± SD of triplicates. *p < 0.05 compared to Oat-mediated uptake in the absence of PFCAs.
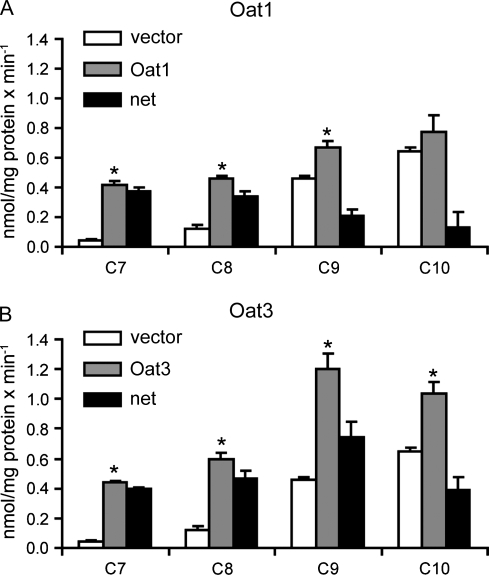
Based on the results of inhibition studies, Oat1- and Oat3-mediated uptake of C7, C8, C9, and C10 into Oat1- and Oat3-expressing HEK293 cells was measured using LC-MS/MS. These results were obtained at a single time point (1 min) and at a single concentration (10μM). As shown in Figure 3A, uptake by Oat1 was highest for C7 and C8 followed by C9, while uptake of C10 was the lowest. Uptake into Oat3-expressing cells was highest for C9 followed by C8, C7, and C10 (Fig. 3B).
FIG. 3.
Oat1- (A) and Oat3 (B)-mediated uptake of C7, C8, C9, and C10. Uptake of 10μM C7–C10 was measured at 37°C for 1 min with empty vector–transfected (white) or Oat-expressing (gray) HEK293 cells. Black bars indicate net uptake after subtracting the values of empty vector–transfected cells from Oat-expressing cells. Uptake values were corrected for total protein concentration in each well. Each bar represents the mean ± SD of triplicates. *p < 0.05 compared to vector control.
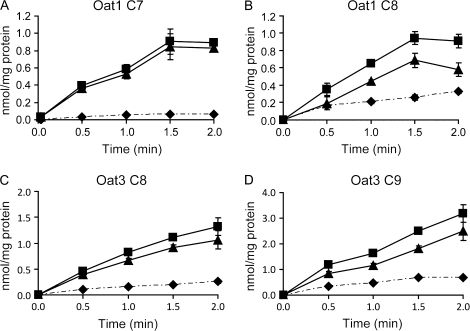
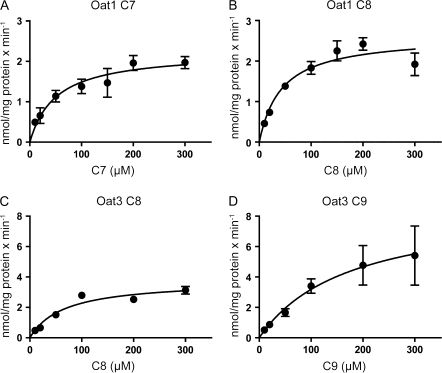
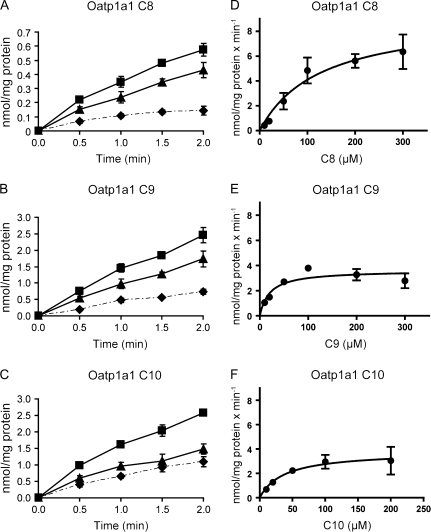
In order to further understand how well these compounds are transported by Oat1 and Oat3 as well as whether these two transporters are important in the renal elimination of C8 and C9, Oat1-mediated C7 and C8 as well as Oat3-mediated C8 and C9 transport was further characterized. First, time-dependent uptake at low (10μM) and high (300μM) substrate concentrations was determined. At the low concentration (10μM), uptake mediated by Oat1 appeared linear up to 1.5 min for both C7 and C8 (Figs. 4A and 4B), while uptake of C8 and C9 mediated by Oat3 appeared linear up to 2 min (Figs. 4C and 4D). At the high concentration (300μM), uptake of C7 and C8 by Oat1 as well as C8 and C9 by Oat3 appeared linear up to 1 min (data not shown). Therefore, for kinetic analysis, all concentration-dependent uptakes were measured at the 1 min time point. Concentration-dependent transport of PFCAs by Oat1 and Oat3 is shown in Figure 5. Kinetic parameters were calculated based on the Michaelis-Menten equation, and the Km and Vmax values are summarized in Table 1. The apparent Km values for Oat1-mediated transport of C7 (50.5μM) and C8 (43.2μM) were very similar. The Km value for Oat3-mediated C8 uptake (65.7μM) was lower than the one for C9 (174.5μM), but the Vmax value of C9 uptake (8.7 nmol/mg/min) was higher than that of C8 (3.8 nmol/mg/min). Therefore, although there were some differences in the affinities, both transporters had similar efficiencies (Vmax/Km) for both substrates that were measured.
FIG. 4.
Oat1- (A, B) and Oat3 (C, D)-mediated time-dependent transport of PFCAs. Uptake of 10μM of PFCAs was measured at 37°C at the indicated time points with empty vector–transfected (♦) or Oat-expressing (▪) HEK293 cells. Net uptake values (▴) were obtained by subtracting the uptake values of empty vector–transfected cells from Oat-expressing cells. Each point represents the mean ± SD of triplicates.
FIG. 5.
Kinetics of Oat1- (A, B) and Oat3 (C, D)-mediated transport of PFCAs. Uptake of increasing concentrations of PFCAs was measured under initial linear rate conditions at 37°C with Oat-expressing and empty vector–transfected HEK293 cells. After subtracting the values obtained with the empty vector–transfected HEK293 and corrected for total protein concentration, net Oat-mediated uptake was fitted to the Michaelis-Menten equation to obtain Km and Vmax values. Each point represents the mean ± standard error from a pool of two independent experiments with triplicates.
TABLE 1.
Kinetic Parameters of Oat1- and Oat3-Mediated PFCA Transport
| Transporter | PFCA | Km (μM) | Vmax (nmol/mg protein per min) | Vmax/Km (ml/mg protein per min) |
| Oat1 | C7 | 50.5 ± 13.9 | 2.2 ± 0.2 | 0.04 ± 0.01 |
| C8 | 43.2 ± 15.5 | 2.6 ± 0.3 | 0.06 ± 0.02 | |
| Oat3 | C8 | 65.7 ± 12.1 | 3.8 ± 0.5 | 0.06 ± 0.01 |
| C9 | 174.5 ± 32.4 | 8.7 ± 0.8 | 0.05 ± 0.01 |
Oatp1a1-Mediated Transport of PFCAs
In the CHO cell line stably expressing rat Oatp1a1, transport of the model substrate [3H]-E-17β-G was inhibited by 10μM of C9, C10, and perflouroundecanoate (C11) by 20–40% (Fig. 6A), while uptake of model substrate into CHO-wild-type cells was not affected by the presence of PFCAs (data not shown). Next, uptake of 10μM C7, C8, C9, and C10 into CHO-Oatp1a1–expressing cells was compared. Uptake of C9 and C10 was highest followed by C8 with minimal uptake of C7 (Fig. 6B). Therefore, saturation kinetics for Oatp1a1-mediated transport was determined for C8, C9, and C10. Figures 7A–C show that transport of C8, C9, and C10 mediated by Oatp1a1 appeared linear up to 2 min at the 10μM concentration, while at the high concentration (300μM), uptake was only linear up to 1 min (data not shown). As a result, all concentration-dependent uptakes for Oatp1a1 were measured at 1 min. Figures 7D–F show saturation kinetics for the three substrates, and the calculated parameters are summarized in Table 2. The Km value of 126.4μM for Oatp1a1-mediated C8 transport was about sixfold higher than the value for C9 (20.5μM) and fourfold higher than the value for C10 (28.5μM). Taking the Vmax values into account, the overall transport efficiency for C8 uptake was about twofold lower than that for C9 and C10 transport.
FIG. 6.
Inhibition of Oatp1a1-mediated transport and uptake of selected PFCAs by Oatp1a1. (A) Uptake of 10nM E-17β-G was measured at 37°C for 1 min with Oatp1a1-expressing or wild-type CHO cells (WT) in the absence or presence of 10μM PFCAs with chain lengths from 2 (C2) to 18 (C18) carbon atoms. Each bar represents the mean ± SD of triplicate samples *p < 0.05 compared to Oatp1a1-mediated uptake in the absence of PFCAs. (B) Uptake of 10μM C7–C10 was measured at 37°C for 1 min with wild-type (open bars) or Oatp1a1-expressing (gray bars) CHO cells. Black bars indicate net uptake after subtracting the uptake values of wild-type from Oatp1a1-expressing cells. Each bar represents the mean ± SD of triplicates. *p < 0.05 compared to vector control.
FIG. 7.
Oatp1a1-mediated time- and concentration-dependent transport of PFCAs. Uptake of 10μM of C8 (A), C9 (B), and C10 (C) was measured at 37°C at the indicated time points with wild-type (♦) or Oap1a1-expressing (▪) CHO cells. Net uptake values (▴) were obtained by subtracting the uptake values of wild-type from Oatp1a1-expressing cells. Each point represents the mean ± SD of triplicates. Uptake of increasing concentrations of C8 (D), C9 (E), and C10 (F) was measured under initial linear rate conditions at 37°C. Net Oatp1a1-mediated uptake was fitted to the Michaelis-Menten equation to obtain Km and Vmax values. Each point represents the mean ± standard error from a pool of two independent experiments with triplicates.
TABLE 2.
Kinetic Parameters of Oatp1a1-Mediated PFCA Transport
| Transporter | PFCA | Km (μM) | Vmax (nmol/mg protein per min) | Vmax/Km (ml/mg protein per min) |
| Oatp1a1 | C8 | 126.4 ± 23.9 | 9.3 ± 1.4 | 0.07 ± 0.02 |
| C9 | 20.5 ± 6.8 | 3.6 ± 0.5 | 0.18 ± 0.06 | |
| C10 | 28.5 ± 5.6 | 3.8 ± 0.3 | 0.13 ± 0.03 |
Oat2- and Urat1-Mediated PFCA Transport
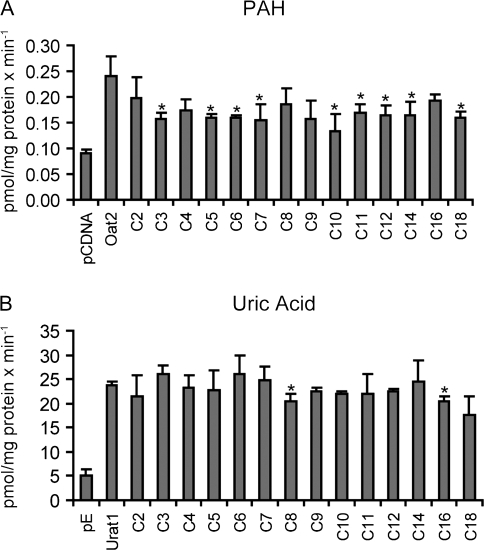
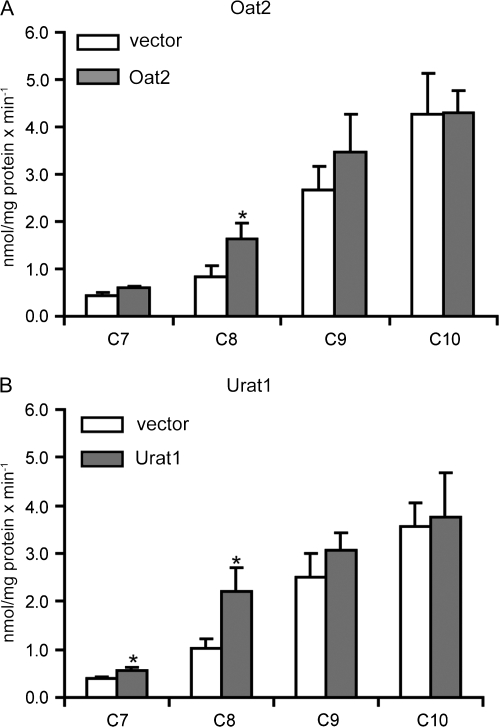
In FlpIn CHO cells stably transfected with Oat2, transport of the model substrate [3H]-PAH (Fig. 8A) was inhibited by most of the PFCAs at 10μM by 40–60%. However, the chain length–dependent inhibition pattern observed for Oat1-, Oat3-, and Oatp1a1-mediated transport was not seen for Oat2. Very similar results were obtained when the concentrations of the PFCAs were increased to 100μM (data not shown). In HEK293 cells transiently transfected with rat Urat1, [14C]-uric acid transport was not inhibited strongly by any of the tested PFCAs at 10μM (Fig. 8B). After increasing the inhibitor concentration to 100μM, C8 showed about 30% inhibition, while there was no difference for C7, C9, and C10 (data not shown). Direct uptake of 100μM C7, C8, C9, and C10 was determined in Oat2-expressing CHO cells and compared to wild-type CHO cells. No significant net Oat2-mediated uptake could be seen (Fig. 9A). Similarly, direct uptake of 100μM C7, C9, and C10 into Urat1-expressing HEK293 cells (Fig. 9B) was very similar to empty vector–transfected HEK293 cells, while uptake of C8 was approximately a third higher for Urat1-expressing cells, consistent with the inhibition in [14C]-uric acid observed at 100μM C8.
FIG. 8.
Effect of PFCAs on Oat2- and Urat1-mediated transport. Uptake of Oat2-mediated 88nM [3H]-PAH (A) or Urate1-mediated 10μM [14C]-uric acid (B) was measured at 37°C for 1 min in the absence or presence of 10μM PFCAs with chain lengths from 2 (C2) to 18 (C18) carbon atoms. Each bar represents the mean ± SD of triplicates. *p < 0.05 compared to Oat2- or Urat1-mediated uptake in the absence of PFCAs.
FIG. 9.
Oat2- (A) and Urat1 (B)-mediated uptake of C7, C8, C9, and C10. Uptake of 100μM C7–C10 was measured at 37°C for 1 min with empty vector–transfected (white bars) or transporter-expressing (gray bars) HEK293 cells. Concentrations of PFCAs were corrected for total protein concentration in each well. Each bar represents the mean ± SD of triplicates. *p < 0.05 compared to vector control.
DISCUSSION
A gender-dependent elimination pattern of PFCAs with different chain lengths has been reported in rats in vivo (Kudo et al., 2001). In the present study, we have demonstrated that, depending on the chain length, PFCAs from C7–C10 are substrates of three renal Oats: Oat1, Oat3, and Oatp1a1. We have also demonstrated that C7 and C8 have the highest affinities for Oat1 and Oat3, while C9 and C10 have the highest affinities for Oatp1a1.
Both Oat1 and Oat3 have been reported to be expressed at the basolateral membrane (Kojima et al., 2002; Tojo et al., 1999) and function as organic anion/dicarboxylate exchangers (Bakhiya et al., 2003; Sekine et al., 1997; Sweet et al., 1997, 2003; Wolff et al., 1997). Based on the estimation that greater than 90% of C8 would be bound to serum albumin (Han et al., 2003), Oat1- and Oat3-mediated excretion may play an important role in renal elimination of PFCAs. This is further supported by a study that showed that pretreatment with probenecid, which inhibits Oat1 and Oat3, significantly reduces renal clearance of C8 (Kudo et al., 2002). Katakura et al. (2007) showed that Oat3-transfected Xenopus laevis oocytes transported C8. The apparent Km values of Oat1- and Oat3-mediated C8 transport in our study are very close to previously published apparent Km values (51.0μM for Oat1 and 80.2μM for Oat3, respectively) (Nakagawa et al., 2008). We also found that Oat1 has very similar transport efficiencies for C7 and C8. Oat3 has similar overall transport efficiency for C8 and C9. Moreover, our results indicate that compared to Oat1, Oat3 is better at transporting PFCAs with longer carbon chains (C9 and C10). Although gender-dependent expression has been reported for both Oat1 and Oat3 (Cerrutti et al., 2002; Ljubojevic et al., 2004), the rats used in the C8 renal elimination study done by Kudo et al. (2002) did not show marked differences at the mRNA level for either Oat1 or Oat3. Thus, available data suggest that Oat1 and Oat3 may play an important role in the secretion of PFCAs from plasma into proximal tubule cells and that additional transporters must be involved in the observed gender-dependent renal excretion pattern.
Oatp1a1 is an important transporter expressed on the apical membrane of proximal tubule cells with a male-dominant expression pattern (Bergwerk et al., 1996; Kato et al., 2002; Kudo et al., 2002). It mediates reabsorption of organic anions in exchange for intracellular reduced glutathione (Li et al., 1998). Katakura et al. (2007) demonstrated that Oatp1a1-expressing X. laevis oocytes transported C8, and Yang et al. (2009) extended these studies and determined the apparent Km value for Oatp1a1-mediated C8 transport as 162.2μM. Furthermore, they calculated apparent inhibition constants for inhibition of E-3-S uptake by C6, C7, C8, C9, and C10. Based on these inhibition constants and assuming that beside C8 other PFCAs are also substrates of Oatp1a1, they predicted that PFCAs with longer chain lengths would have higher affinities (apparent Ki values for C6: 1857.8μM; C7: 398.9μM; C8: 83.8μM; C9: 44.6μM; C10: 26.8μM). In this present study, we directly determined the apparent Km values for C8 (126.4μM), C9 (20.5μM), and C10 (28.5μM). The apparent Km value for C8 of 126.4μM determined in this study is comparable to the apparent Km value of 162.2μM described by Yang et al. (2009). Furthermore, the apparent Km value of 28.5μM for C10 determined in this study is similar to the apparent Ki value of 28.5μM (Yang et al., 2009). However, the predicted affinity by Yang et al. (2009) for C8 (83.8μM) is much lower than the values measured by Yang et al. (2009) (162.2μM) and by us (126.4μM), and the predicted affinity for C9 (44.6μM) is higher than the values measured by us (20.5μM).
The significant differences in the Km values that we measured in this study for Oatp1a1-mediated C8, C9, and C10 uptake suggest that Oatp1a1 transports C10 and C9 with higher efficiency (Vmax/Km) than C8. Since Oatp substrates are mainly anionic amphipathic molecules with high molecular weight (> 450) that under normal physiological conditions are bound to albumin (Hagenbuch and Meier, 2003), it is not surprising that C10 (MW 514) and C9 (MW 464) are better substrates for Oatp1a1 than C8 (MW 414). Furthermore, the strong reabsorption mediated by Oatp1a1 for PFCAs with greater chain length may also explain the fact that the longer the carbon chain the less PFCA is excreted in urine (Kudo et al., 2001). Since male rats have more Oatp1a1 present at the apical membrane, it is reasonable that in male rats less C8 and C9 are excreted in urine than in female rats as was shown previously (Kudo et al., 2001).
In this study, we also investigated the role of two other apical Oats, Oat2 and Urat1. Oat2 has a female-dominant expression pattern in the rat at the apical membrane of proximal tubule cells. However, in humans, this transporter is localized to the basolateral membrane (Enomoto et al., 2002; Kojima et al., 2002; Ljubojevic et al., 2007). Although most PFCA tested in this study inhibited Oat2-mediated PAH uptake by 40–60%, the chain length–dependent inhibition pattern as shown for Oat1-, Oat3-, and Oatp1a1-mediated transport was not seen. The general inhibition by PFCAs observed could be due to the low signal to noise ratio since the amount of PAH transported into Oat2-expressing cells is only about twice as much as that of wild-type cells. Furthermore, direct transport of C7–C10 into Oat2-expressing CHO cells did not show any difference from wild-type CHO cells. The same result was obtained when we tested the direct transport of C7–C10 in HEK cells transiently transfected with rat Oat2 (data not shown). Thus, we conclude that in our experimental systems, Oat2 does not transport C7, C8, C9, or C10, which for C8 is consistent with the previous finding by Nakagawa et al. (2008) that Oat2 does not transport C8 in transiently transfected HEK cells. Similar to Oat2, Urat1 did not show any chain length–dependent inhibition pattern. Although C8 transport into Urat1-expressing HEK293 cells was slightly higher than empty vector–transfected cells, the signal to noise ratio was not big enough for further characterization. Among the three transporters expressed at the apical membrane, Oatp1a1 is the major player in the reabsorption of PFCAs.
There are also efflux transporters expressed at the apical membrane, including Mdr1a/1b and Mrps, which may play a role in active secretion of PFCAs into urine. Mdr1b is more highly expressed in female rats at the mRNA level (Lu and Klaassen, 2008), therefore, potentially affecting secretion of PFCAs across the apical membrane in female rats. Given the complexity of transporter systems expressed in the kidney, further studies are needed to elucidate PFCA excretion from proximal tubule cells into the tubular lumen.
The roles of several human transporters in renal clearance of C8 have been studied. It has been shown that human OAT1 and OAT3 have similar C8 transport capacity as their rodent homologues (Nakagawa et al., 2008). The same group also showed that the human-specific transporter hOAT4 can transport C8 as well (Nakagawa et al., 2009). Our functional studies with rat transporters together with the previously published data (Yang et al., 2009) combined with the available in vivo toxicokinetics data should allow us to identify additional human transporter candidates that may play important roles in PFCA disposition. For example, Oatp1a1 has been shown in current and previous studies to be important in the reabsorption of C8, C9, and C10 in rats. Therefore, we are currently investigating the role of human OATP1A2, which is the orthologue of rat Oatp1a1, in transporting PFCAs with different chain lengths. Such studies should also provide valuable experimental kinetic parameters for toxicokinetic modeling of PFCAs in humans.
In conclusion, our studies reveal that PFCAs with different chain lengths are substrates of the two basolateral transporters, Oat1 and Oat3, as well as of the apical transporter, Oatp1a1, in rat proximal tubule cells. The male-dominant expression level of Oatp1a1 in the kidney as well as the differences in transport efficiencies for PFCAs with different chain lengths suggest a role of Oatp1a1 in the gender-specific renal elimination pattern in rats. Further research is required to understand the basis of potential species differences in Oat-mediated transport of PFCAs and how these may relate to species differences in elimination kinetics that have been observed.
FUNDING
National Institute of Health grants (RR021940, GM077336); 3M Company.
References
- Andersen ME, Butenhoff JL, Chang SC, Farrar DG, Kennedy GL, Jr, Lau C, Olsen GW, Seed J, Wallace KB. Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicol. Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Clewell HJ, III, Tan YM, Butenhoff JL, Olsen GW. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys—probing the determinants of long plasma half-lives. Toxicology. 2006;227:156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, et al. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J. Pharmacol. Exp. Ther. 2005;315:534–544. doi: 10.1124/jpet.105.088583. [DOI] [PubMed] [Google Scholar]
- Bakhiya A, Bahn A, Burckhardt G, Wolff N. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol. Biochem. 2003;13:249–256. doi: 10.1159/000074539. [DOI] [PubMed] [Google Scholar]
- Bergwerk AJ, Shi X, Ford AC, Kanai N, Jacquemin E, Burk RD, Bai S, Novikoff PM, Stieger B, Meier PJ, et al. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am. J. Physiol. 1996;271:G231–G238. doi: 10.1152/ajpgi.1996.271.2.G231. [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. J. Pharmacol. Exp. Ther. 2002;301:145–151. doi: 10.1124/jpet.301.1.145. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL, Jr, Hinderliter PM, Lieder PH, Jung R, Hansen KJ, Gorman GS, Noker PE, Thomford PJ. Pharmacokinetics of perfluorooctanoate in cynomolgus monkeys. Toxicol. Sci. 2004;82:394–406. doi: 10.1093/toxsci/kfh302. [DOI] [PubMed] [Google Scholar]
- Cerrutti JA, Brandoni A, Quaglia NB, Torres AM. Sex differences in p-aminohippuric acid transport in rat kidney: Role of membrane fluidity and expression of OAT1. Mol. Cell. Biochem. 2002;233:175–179. doi: 10.1023/a:1015563021602. [DOI] [PubMed] [Google Scholar]
- Chang S, Das K, Ehresman DJ, Ellefson ME, Gorman GS, Hart JA, Noker PE, Tan YM, Lieder PH, Lau C, et al. Comparative pharmacokinetics of perfluorobutyrate (PFBA) in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol. Sci. 2008;104:40–53. doi: 10.1093/toxsci/kfn057. [DOI] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Myers NR, Shinohara M, Stetson PL, Sved DW. Comparison of the toxicokinetic behavior of perfluorohexanoic acid (PFHxA) and nonafluorobutane-1-sulfonic acid (PFBS) in cynomolgus monkeys and rats. Reprod. Toxicol. 2009;27:400–406. doi: 10.1016/j.reprotox.2009.01.013. [DOI] [PubMed] [Google Scholar]
- D'Eon JC, Hurley MD, Wallington TJ, Mabury SA. Atmospheric chemistry of N-methyl perfluorobutane sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and mechanism of reaction with OH. Environ. Sci. Technol. 2006;40:1862–1868. doi: 10.1021/es0520767. [DOI] [PubMed] [Google Scholar]
- D'Eon JC, Mabury SA. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): Exploring routes of human contamination. Environ. Sci. Technol. 2007;41:4799–4805. doi: 10.1021/es070126x. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Benskin JP, Martin LJ, Arsenault G, McCrindle R, Riddell N, Martin JW, Mabury SA. Disposition of perfluorinated acid isomers in Sprague-Dawley rats; part 2: Subchronic dose. Environ. Toxicol. Chem. 2009;28:555–567. doi: 10.1897/08-254.1. [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Schroeder A, Stieger B, Hochli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am. J. Physiol. 1999;276:G1037–G1042. doi: 10.1152/ajpgi.1999.276.4.G1037. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, et al. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J. Pharmacol. Exp. Ther. 2002;301:797–802. doi: 10.1124/jpet.301.3.797. [DOI] [PubMed] [Google Scholar]
- Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, Buck RC, Korzeniowski SH, Hinderliter PM, Kemper RA. Absorption, distribution, metabolism, and elimination of 8-2 fluorotelomer alcohol in the rat. Toxicol. Sci. 2006;91:341–355. doi: 10.1093/toxsci/kfj160. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y. Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J. Health Sci. 2007;53:77–83. [Google Scholar]
- Kato Y, Kuge K, Kusuhara H, Meier PJ, Sugiyama Y. Gender difference in the urinary excretion of organic anions in rats. J. Pharmacol. Exp. Ther. 2002;302:483–489. doi: 10.1124/jpet.102.033878. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J. Am. Soc. Nephrol. 2002;13:848–857. doi: 10.1681/ASN.V134848. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem. Biol. Interact. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem. Biol. Interact. 2001;134:203–216. doi: 10.1016/s0009-2797(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lemal DM. Perspective on fluorocarbon chemistry. J. Org. Chem. 2004;69:1–11. doi: 10.1021/jo0302556. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J. Biol. Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, Burckhardt G, Sabolic I. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am. J. Physiol. 2007;292:F361–F372. doi: 10.1152/ajprenal.00207.2006. [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am. J. Physiol. 2004;287:F124–F138. doi: 10.1152/ajprenal.00029.2004. [DOI] [PubMed] [Google Scholar]
- Lu H, Klaassen C. Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metab. Dispos. 2008;36:16–23. doi: 10.1124/dmd.107.014845. [DOI] [PubMed] [Google Scholar]
- Lu R, Kanai N, Bao Y, Wolkoff AW, Schuster VL. Regulation of renal oatp mRNA expression by testosterone. Am. J. Physiol. 1996;270:F332–F337. doi: 10.1152/ajprenal.1996.270.2.F332. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc. Natl. Acad. Sci. USA. 2004;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Jutabha P, Miura D, Harada KH, Inoue K, Anzai N, Endou H, Inui K, et al. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin. Pharmacol. Toxicol. 2008;103:1–8. doi: 10.1111/j.1742-7843.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Terada T, Harada KH, Hitomi T, Inoue K, Inui K, Koizumi A. Human organic anion transporter hOAT4 is a transporter of perfluorooctanoic acid. Basic Clin. Pharmacol. Toxicol. 2009;105:136–138. doi: 10.1111/j.1742-7843.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009;27:212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: Biopharmaceutical, physiological, and pathological roles. Pharm. Res. 2007;24:450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J. Am. Soc. Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am. J. Physiol. 2006;290:F251–F261. doi: 10.1152/ajprenal.00439.2004. [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J. Biol. Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am. J. Physiol. 2003;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J. Biol. Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- Tan YM, Clewell HJ, III, Andersen ME. Time dependencies in perfluorooctylacids disposition in rat and monkeys: A kinetic analysis. Toxicol. Lett. 2008;177:38–47. doi: 10.1016/j.toxlet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, Endou H. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J. Am. Soc. Nephrol. 1999;10:464–471. doi: 10.1681/ASN.V103464. [DOI] [PubMed] [Google Scholar]
- van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: Putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Wallington TJ, Hurley MD, Xia J, Wuebbles DJ, Sillman S, Ito A, Penner JE, Ellis DA, Martin J, Mabury SA, et al. Formation of C7F15COOH (PFOA) and other perfluorocarboxylic acids during the atmospheric oxidation of 8:2 fluorotelomer alcohol. Environ. Sci. Technol. 2006;40:924–930. doi: 10.1021/es051858x. [DOI] [PubMed] [Google Scholar]
- Wolff NA, Werner A, Burkhardt S, Burckhardt G. Expression cloning and characterization of a renal organic anion transporter from winter flounder. FEBS Lett. 1997;417:287–291. doi: 10.1016/s0014-5793(97)01304-5. [DOI] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicol. Lett. 2009;190:163–171. doi: 10.1016/j.toxlet.2009.07.011. [DOI] [PubMed] [Google Scholar]