Abstract
We have recently demonstrated that disruption of extracellular matrix (ECM)/integrin signaling via elimination of integrin-linked kinase (ILK) in hepatocytes interferes with signals leading to termination of liver regeneration. This study investigates the role of ILK in liver enlargement induced by phenobarbital (PB). Wild-type (WT) and ILK:liver−/− mice were given PB (0.1% in drinking water) for 10 days. Livers were harvested on 2, 5, and 10 days during PB administration. In the hepatocyte-specific ILK/liver−/− mice, the liver:body weight ratio was more than double as compared to 0 h at day 2 (2.5 times), while at days 5 and 10, it was enlarged three times. In the WT mice, the increase was as expected from previous literature (1.8 times) and seems to have leveled off after day 2. There were slightly increased proliferating cell nuclear antigen-positive cells in the ILK/liver−/− animals at day 2 as compared to WT after PB administration. In the WT animals, the proliferative response had come back to normal by days 5 and 10. Hepatocytes of the ILK/liver−/− mice continued to proliferate up until day 10. ILK/liver−/− mice also showed increased expression of key genes involved in hepatocyte proliferation at different time points during PB administration. In summary, ECM proteins communicate with the signaling machinery of dividing cells via ILK to regulate hepatocyte proliferation and termination of the proliferative response. Lack of ILK in the hepatocytes imparts prolonged proliferative response not only to stimuli related to liver regeneration but also to xenobiotic chemical mitogens, such as PB.
Keywords: integrin-linked kinase, phenobarbital, extracellular matrix, integrins, constitutive androstane receptor, liver
Extracellular matrix (ECM) is of great importance for the survival, differentiation, and normal function of cells within a tissue (Kim et al., 1997). This is particularly true for hepatocytes, the parenchymal cells of the liver. ECM is of key importance for determining differentiation and proliferation of hepatocytes in culture and in vivo (Block et al., 1996; Kim et al., 1997; Michalopoulos, 2007; Rudolph et al., 1999). ECM remodeling is an essential part of liver regeneration after partial hepatectomy (Kim et al., 1997). Signals from the ECM are transmitted to the interior of the cell via integrins (Hehlgans et al., 2007). Recently, there has been much progress in determining mechanisms by which integrins deliver their signals inside the cell. A major mediator of integrin signaling is integrin-linked kinase (ILK) (McDonald et al., 2008). ILK is a Ser/Thr kinase that is emerging as a key regulator of cell-ECM adhesions. Activation of ILK, either by integrin clustering or by growth factors, affects multiple cell signaling pathways that regulate different processes, such as survival, differentiation, proliferation, migration, and angiogenesis (Hehlgans et al., 2007; McDonald et al., 2008). Previous studies in our laboratory have shown that hepatocytes in primary culture lose their characteristic gene expression patterns (Block et al., 1996). They can be stimulated to proliferate under the influence of hepatocyte growth factor (HGF) and/or epidermal growth factor (EGF). Addition of artificial ECM to hepatocytes in culture (e.g., Matrigel, Type I collagen gels) restores full differentiation and inhibits hepatocyte proliferation (Block et al., 1996). Because it is practically impossible to eliminate ECM from an intact organ, elimination of the proteins responsible for transmission of the ECM signals to hepatocytes became a feasible alternative when ILK loxP/loxP mice became available. Integrin signaling involves multiple components and interactions with other receptors, etc. There are two proteins, however, primarily involved with transmission of the integrin signal, focal adhesion kinase and ILK (Hehlgans et al., 2007; van Nimwegen and van de Water, 2007). Thus, liver-targeted elimination of ILK disrupts in part the integrin signal.
Recently, we have been successful in eliminating the ILK gene specifically from hepatocytes (Gkretsi et al., 2008). Liver histology in the ILK/liver−/− mice is indistinguishable at birth from the wild type (WT) except for a decrease in the number of bile ductules. At 2–3 weeks after birth, hepatocyte plates in the ILK/liver−/− mice are irregular with clusters of multiple cells surrounded by irregular sinusoids. At 6 weeks and thereafter, there are multiple hepatocyte mitoses and apoptosis in the ILK/liver−/− mice. By the end of 30 weeks, the livers of ILK/liver−/− mice are almost 30% larger than the WT mice (Gkretsi et al., 2008). These 30-week-old ILK/liver−/− mice were subjected to 70% partial hepatectomy. Whereas the WT livers returned to exactly the same liver weight as prehepatectomy, the livers of ILK/liver−/− mice gained additional weight (59% increase). The increase in resting liver weight and the apparent “overgrowth” of the regenerating liver in the ILK/liver−/− mice shows that in absence of matrix signaling (as a result of removal of ILK), termination of liver regeneration does not function properly and liver grows to a much larger size (Apte et al., 2009). Thus, this study highlights essential role of ECM-mediated signaling via ILK in regulation of both liver regeneration and its termination.
Studies from several investigators have shown, however, that the hepatic enlargement induced by chemical xenobiotic mitogens (such as phenobarbital [PB], dilantin, diazepam, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCBOPOP), peroxisome proliferators, etc.) (Columbano and Shinozuka, 1996) proceeds through very different signaling mechanisms in comparison to liver regeneration. Growth factors associated with liver regeneration are minimally involved, and many of the cell cycle–associated genes induced at the early stages of liver regeneration do not play a part in the hepatic enlargement induced by chemical mitogens. Thus, we wanted to explore whether the enhanced proliferative response and defective termination of proliferation seen in ILK/liver−/− mice in liver regeneration also occurs in hepatic enlargement induced by chemical mitogens. Given the dissimilarities of the two growth responses (Columbano and Shinozuka, 1996), we wanted to explore whether the enhanced proliferative response and defective termination of proliferation seen in ILK/liver−/− mice in liver regeneration also occurs in hepatic enlargement induced by chemical mitogens. The present study investigated the role of ILK in hepatocyte proliferation induced by PB, which is known to induce hyperplasia and hypertrophy of hepatocytes, resulting in hepatomegaly in mice and humans. Based on the previous studies in our laboratory on regeneration of liver in ILK/liver−/− mice, we hypothesized that ILK/liver−/− mice would also respond with increased and prolonged proliferative response to PB resulting in massive hepatomegaly. Our data indeed demonstrate that ECM proteins communicate with the signaling machinery of dividing cells in part via ILK to regulate hepatocyte proliferation and termination of the proliferative response not only in liver regeneration but also in response to hepatomegaly induced by xenobiotic chemical mitogens.
MATERIALS AND METHODS
Antibodies
The following primary antibodies were used in this study: rabbit anti-ILK, rabbit anti-yes-associated protein (YAP), rabbit anti-phosphorylated YAP, anti-cyclin D1 (1:1000 dilution; Cell Signaling Technologies, Danvers, MA), rabbit anti-c-Myc, rabbit anticonstitutive androstane receptor (CAR), mouse anti-Met (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-transforming growth factor (TGF)-β1 (Promega, Madison, WI), goat anti-HGF, mouse anti-hepatocyte nuclear factor (HNF)-4α (1:2000 dilution; R&D Systems, Minneapolis, MN), mouse anti-proliferating cell nuclear antigen (PCNA) (Dako, Carpinteria, CA), and mouse anti-β-actin (1:5000 dilution; Chemicon, Temecula, CA). Goat anti-mouse, donkey anti-goat, and donkey anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and were used at 1:50,000 dilution.
Generation of Liver-Specific ILK/Liver−/− Mice
ILK-floxed animals were generated as described previously (Terpstra et al., 2003) and donated by Drs René St. Arnaud (Shriners Hospital and McGill University, Montréal, Canada) and Shoukat Dedhar (British Columbia Cancer Agency and Vancouver Hospital, Jack Bell Research Center, Vancouver, Canada) and mated with alpha fetoprotein-enhancer, albumin-promoter, Cre-recombinase-expressing mice, which were kindly provided by Dr Klaus Kaestner (University of Pennsylvania). The offspring were genotyped as described previously (Terpstra et al., 2003), and the ILK-floxed/floxed Cre-positive mice were considered to be ILK knockout (ILK/liver−/−), while their Cre-negative siblings were used as controls or WT (Gkretsi et al., 2008).
PB Administration
Thirty-five-week-old WT and ILK/liver−/− mice were given PB at a concentration of 0.1% in their drinking water for 10 days. Livers were harvested on 2, 5, and 10 days during PB administration.
Protein Isolation and Western Blot
Total protein was isolated from the mouse liver using 1% SDS in radio-immunoprecipitation assay (RIPA) buffer (20mM Tris/Cl, pH 7.5; 150mM NaCl; 0.5% nonyl phenoxylpolyethoxylethanol (NP-40); 1% Triton X-100; 0.25% sodium deoxycholate; 0.6–2 μg/ml aprotinin; 10μM leupeptin; and 1μM pepstatin). Protein concentrations of all lysates were determined using the bicinchoninic acid protein assay reagents (BCA method) (Pierce Chemical Co., Rockford, IL). Nuclear proteins were prepared using the NE-PER nuclear and cytoplasmic protein isolation kit (Pierce Chemical Co.) according to the manufacturer's protocol.
Total cell lysates made in RIPA buffer (50 μg) or nuclear preps (20 μg) were separated by SDS-polyacrylamide gel electrophoresis in 4–12% NuPage Bis-Tris gels with one 3-(N-morpholino)propanesulfonic acid buffer (Invitrogen, Carlsbad, CA) and then transferred to Immobilon-P membranes (Millipore, Bedford, MA) in NuPAGE transfer buffer containing 20% methanol. Membranes were stained with Ponceau S to verify loading and transfer efficiency. Membranes were probed with primary and secondary antibodies in Tris-buffered saline Tween 20 containing 5% nonfat milk, then processed with SuperSignal West Pico chemiluminescence substrate (Pierce), and exposed to a x-ray film (Lab Product Sales, Rochester, NY).
Immunohistochemistry
Paraffin-embedded liver sections (4-μm thick) were used for immunohistochemical staining. Antigen retrieval was achieved by heating the slides in the microwave at high power in 1× citrate buffer for 10 min. The tissue sections were blocked in blue blocker for 20 min followed by incubation with mouse PCNA antibody overnight at 4°C. The primary antibody was then linked to biotinylated secondary antibody followed by routine avidin-biotin complex method. Diaminobenzidine was used as the chromogen, which resulted in a brown reaction product. Apoptotic nuclei were detected by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling staining using the ApopTag Peroxidase kit (Intergen Company, Purchase, NY). PCNA-positive cells, cells were counted in low-power fields (200×) in four sections from four different knockout or control livers. Mitotic and apoptotic cells were counted 10 different fields in four sections from four different knockout or control livers. While the apoptotic cells were counted at ×100 magnification, mitotic cells were counted at ×200 magnification.
RNA Isolation and Semiquatitative Reverse Transcription-PCR
RNA was extracted from frozen liver tissues with Trizol (Invitrogen) according to manufacturer’s instructions. RNA, 5 μg, was reverse transcribed to complementary DNA (cDNA) with Superscript III reverse transcriptase (Invitrogen). Standard PCR was performed with Taq polymerase (Qiagen, San Diego, CA). The primers for HGF were bought from SA Bioscience (Frederick, MD). PCR products were resolved on 2% agarose gels and visualized with ethidium bromide.
Serum HGF Levels
Serum levels of HGF were measured by ELISA using a commercially available kit for R&D Systems. Equal volumes of serum from three different animals were polled together for the analysis.
Microarray Analysis
cRNA preparation.
Total RNA was extracted and purified with Qiagen RNeasy kit (Qiagen) from whole livers harvested from ILK/liver−/− and WT at various time points after partial hepatectomy. Five micrograms of total RNA was used in the first-strand cDNA synthesis with T7-d(T)24 primer (GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGG-(dT)24) by SuperscriptTM II (GIBCO-BRL, Rockville, MD). The second-strand cDNA synthesis was carried out at 16°C by adding Escherichia coli DNA ligase, E. coli DNA polymerase I and RnaseH in the reaction. This was followed by the addition of T4 DNA polymerase to blunt the ends of newly synthesized cDNA. The cDNA was purified through phenol/chloroform and ethanol precipitation. The purified cDNA were then incubated at 37°C for 4 h in an in vitro transcription reaction to produce complementary RNA (cRNA) labeled with biotin using MEGAscriptTM system (Ambion, Inc., Austin, TX).
Affymetrix chip hybridization.
Fifteen to 20 μg of cRNA was fragmented by incubation in a buffer containing 200mM Tris-acetate, pH 8.1; 500mM KOAc; 150mM MgOAc at 95°C for 35 min. The fragmented cRNA was then hybridized with a pre-equilibrated Affymetrix chip (R430 2.0) at 45°C for 14–16 h. After the hybridization cocktails were removed, the chips were then washed in a fluidic station with low-stringency buffer (6× 1 M NaCl, 0.05 M phosphate, 5 mM EDTA pH 7.0, 0.01% Tween 20, and 0.005% antifoam) for 10 cycles (2 mixes per cycle) and stringent buffer (100mM 2-(N-morpholino)ethanesulfonic acid, 0.1M NaCl, and 0.01% Tween 20) for 4 cycles (15 mixes per cycle) and stained with strepto-avidin phycoerythrin (SAPE). This was followed by incubation with biotinylated mouse anti-avidin antibody and restained with SAPE. The chips were scanned in a HP ChipScanner (Affymetrix Inc., Santa Clara, CA) to detect hybridization signals. For quality assurance, all samples were run on Affymetrix test-3 chips to evaluate the integrity of RNA. Samples with RNA 3′/5′ ratios less than 2.5 were accepted for further analysis.
Statistics
Data are expressed as the mean ± SE. Group comparison at the same time point was made using the Student’s t-test using JMP software (SAS Institute, Inc., Cary, NC). A p value of less than 0.05 was considered significant.
RESULTS
Increased Liver to Body Weight Ratio and Cell Proliferation in ILK/Liver−/− mice
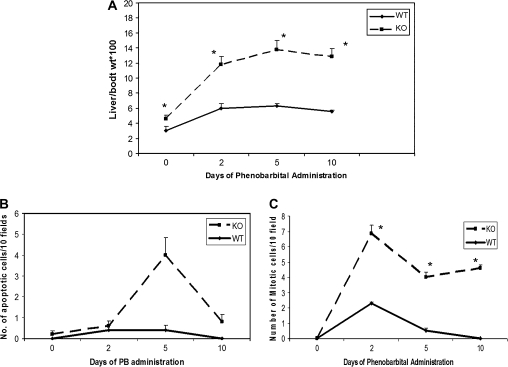
The liver to body weight ratios were measured in WT and knock out (KO) mice at days 2, 5, and 10 during PB administration (Fig. 1A). On day 2, ILK/liver−/− mice had a 2.5-fold increase in liver to body weight ratio as compared to 0 day. By days 5 and 10, there was almost a threefold increase as compared to day 0. In the WT time mice, the increase was close to twofold at all time points. These data show that liver in ILK/liver−/− mice seems to have an increased proliferative response to PB. PB is also known to induce hypertrophy. The increased liver weight seen in the WT and the ILK/liver−/− is also in part due to hypertrophy of the cells.
FIG. 1.
Quantitative assessment of hepatocyte proliferation, liver enlargement, and apoptosis. (A) Liver to body weight ratios of WT and ILK/liver−/− mice at days 0, 2 5, and 10 during PB administration. Each data point is the mean ± SE from more than three measurements per point. (B) Mitotic index as a marker of hepatocyte proliferation (C) apoptosis measured by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling assay was estimated in WT and ILK KO mice at various days during PB. Mitotic and apoptotic cells were counted 10 different fields in four sections from four different knockout or control livers. While the apoptotic cells were counted at ×100 magnification, mitotic cells were counted at ×200 magnification. *Significantly different from the WT mice at that same time point (p ≤ 0.05).
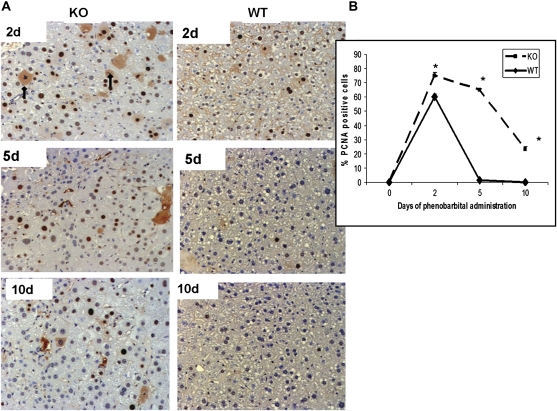
We monitored the cell kinetics in WT and ILK/liver−/− mice at 2, 5, and 10 days of PB administration. The PCNA-positive cells and the number of mitotic cells were significantly higher in the ILK/liver−/− at all time points (Figs. 1C and 2A and 2B). In the WT livers, the percent of PCNA-positive cells and mitotic cells increased at day 2 but came back to baseline levels at days 5 and 10. On the other hand, in the livers of ILK/liver−/− mice, the percentage of PCNA-positive cells and mitotic cells remained elevated at all time points. Even though the number of PCNA-positive cells and mitotic cells declined after day 2, it always remained elevated in the ILK/liver−/− livers as compared to 0 day, suggesting a sustained and a prolonged proliferative response. The numbers of hepatocytes in apoptosis (Fig. 1B) were higher at days 5 and 10 in the ILK/liver−/− mice as compared to the WT mice, but overall, the percent of hepatocytes in apoptosis was very small.
FIG. 2.
(A) Representative photomicrographs of PCNA-stained liver section of ILK/liver−/− and WT mice at days 2, 5, and 10 during PB administration. Arrows show mitotic cells; magnification: ×200. (B) Hepatocyte proliferation was quantitated by counting the PCNA-positive cells. PCNA-positive cells were counted in low-power fields (×200) in four sections from four different knockout or control livers. Each data point is the mean ± SE from more than three measurements per point. *Significantly different from the WT mice at that same time point (p ≤ 0.05).
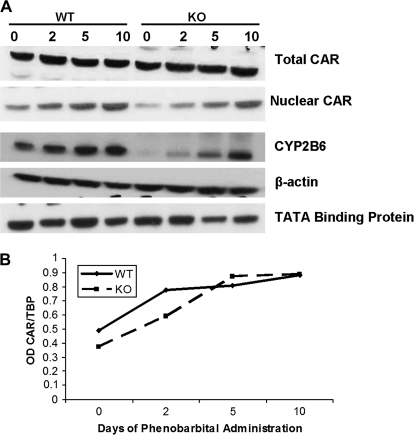
It is well documented that PB causes nuclear translocation of CAR (Ross et al., 2009). We looked at CAR protein levels in total cell lysates and nuclear fractions at days 0, 2, 5, and 10 during PB administration (Figs. 3A and 3B). In the ILK/liver−/− mice and WT mice, we did not observe any change in CAR expression in the total cell lysate at days 2, 5, and 10 as compared to 0 day. In the nuclear fraction, we saw an induction in the expression of CAR at days 2, 5, and 10 as compared to 0 day both in the WT and in the ILK/liver−/− mice. We also looked at the expression of CYP2B6 (Fig. 3), a CAR target before and after PB treatment. In both WT and ILK/liver−/− animals, there was an induction of CYP2B6 after PB administration. Interestingly, ILK/liver−/− mice had low levels of CYP2B6 as compared to WT at 0 day. Several recent studies using HNF-4α−/− mice and antisense-HNF-4α adenovirus also found that many CAR- and pregnane X receptor (PXR)-regulated genes require HNF-4α for efficient induction by PB and other drugs or xenobiotics. Studies in our laboratory have shown that increased nuclear expression of HNF-4α is a distinct part of the early (0–4 h) PB response in liver and a likely mediator of PB-induced gene regulation in concert with CAR. Thus, we looked at the expression of HNF-4α in the ILK/liver−/− and WT mice. In the WT animals, HNF-4α expression at 24 h decreased after PB administration. This complements our earlier studies (Bell and Michalopoulos, 2006) and shows that the effect of PB in HNF-4α is biphasic, with an earlier stage of increased expression (0–4 h) and a secondary stage of decrease at 24 h and beyond. In the ILK/liver−/− animals, HNF-4α expression was unchanged as compared to 0 day but was higher as compared to the WT animals at days 2, 5, and 10 during PB administration (Fig. 5A). HNF-4α, in the liver, is also a hepatocyte-associated transcription factor (Lazarevich et al., 2004). We have shown previously that hepatocytes in primary culture supplemented with HGF and EGF proliferate and lose their characteristic gene expression patterns (Block et al., 1996). HNF-4α is (for the liver) a hepatocyte-associated transcription factor. Short-term administration of PB causes an increase in nuclear levels of HNF-4α. There are no data for what happens, however, for long-term administration of PB, and this is the first evidence that this protocol in WT mice is associated with eventual decline in the nuclear HNF-4α levels. On the other hand, we have shown in our previous paper (Gkretsi et al., 2008) that the ILK−/− livers have enhanced differentiation with increased expression of most of the hepatocyte-associated genes, most of which in turn depend on HNF-4α. Though we had not reported, we did have evidence for overall increase in HNF-4α nuclear levels in the ILK−/− livers. As we stated in the above reference, at 35 weeks of age, the ILK−/− livers are expressing altered levels and new components of both ECM proteins and integrins, and we have in the past provided evidence that ECM does regulate levels and processing of HNF-4α (Runge et al., 1999). Thus, the altered response of the two types of mice to PB may well reflect the fact that the ECM and integrins of the two liver types are quite different.
FIG. 3.
CAR activation in ILK/liver−/− and WT mice after PB administration. (A) Protein levels of CAR (nuclear and total cell extract) and CYP2B6 (total cell extract). Pooled liver samples from at least three mice were used for protein analysis. β-Actin- and TATA-binding protein were used as a loading control for total cell extract and nuclear extract, respectively. (B) Graphical representation of nuclear CAR expression following PB treatment. Expression levels were normalized to TATA-binding protein.
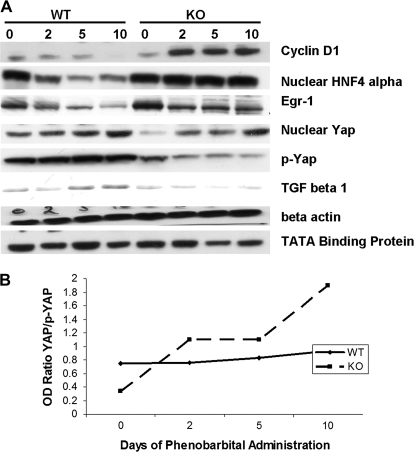
FIG. 5.
(A) Changes in cell cycle-related proteins in ILK/liver−/− and WT mice after PB administration. Pooled liver samples from at least three mice were used for protein. β-Actin and TATA-binding protein were used as loading controls for total cell extracts and nuclear extracts, respectively. (B) Graphical representation of YAP/p-YAP expression following PB treatment.
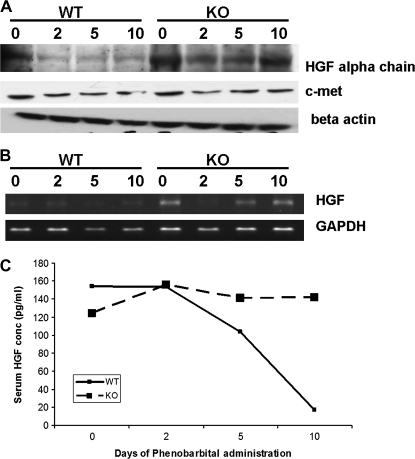
To further analyze signaling pathways involved in increased and prolonged proliferative response to PB in the ILK/liver−/− mice, we investigated the levels of growth factors and cell cycle genes. HGF protein levels were higher in the ILK/liver−/− mice on 0 as compared to WT mice. The protein levels go down after PB administration in WT mice (Fig. 4A). On the other hand, in the ILK/liver/−/− mice, the levels go down at days 2 and 5 but come back to the basal levels by day 10. Though the protein levels of HGF go down in both the groups after PB administration, the levels were higher in the ILK/liver−/− mice at all time point as compared to WT mice before and after PB administration. At the messenger RNA (mRNA) level, the HGF levels were very low in the WT animals before and after PB administration (Fig. 4B). In the ILK/liver−/− mice, the levels go down at day 2 but come back to the basal levels at days 5 and 10. The HGF mRNA levels were higher in the ILK/liver−/− mice as compared to the WT mice at all the points before and after PB treatment. We also measured the serum HGF levels by ELISA (Fig. 4C). In the WT mice, the levels remained steady till day 2 after which there was a dramatic decrease at days 5 and 10. In the ILK/liver−/− mice, the HGF levels increased at day 2 after which they came back to the basal levels. Again, the serum levels of HGF were always higher in the ILK/liver−/− mice at all time points except day 2 when they were about equal. There was no difference in the protein levels of its receptor MET between the WT and the ILK/liver−/− mice (Fig. 5A). There was an induction of cyclin D1 at days 2, 5, and 10 after PB administration in the ILK/liver−/− mice. There was no change in cyclin D1 levels in WT mice. In fact, the levels of cyclin D1 go down at day 10 in WT mice. These findings are later discussed in the Discussion section. Egr-1 protein levels (5A) go down in the WT and ILK/liver−/− mice after PB administration but were always higher in the ILK/liver−/− mice as compared to the WT at all time points (Fig. 5A).
FIG. 4.
(A) HGF and its receptor cMet protein expression after PB administration in WT and ILK/liver−/− mice. Pooled liver samples from at least three mice were used for protein. β-Actin was used as the loading control. (B) Semiquantitative PCR for HGF in WT and ILK/liver−/− mice after PB administration. Pooled samples from at least three mice were for RNA isolation. (C) Serum HGF levels in WT and ILK/liver−/− mice before and after PB administration. Equal volumes of serum from three different animals were polled together for the analysis.
TGF-β1 is another molecule implicated in termination of liver regeneration due to its antiproliferative activity (Michalopoulos, 2007; Michalopoulos and DeFrances, 2005; Zhao et al., 2009). In the WT mice, TGF-β1 expression was increased at days 5 and 10 during PB administration (Fig. 5A). On the other hand, the ILK/liver−/− mice showed lower expression of TGF-β1 at all time points as compared to the WT mice.
Recently, the role of Hippo Kinase pathway in regulation of organ size in Drosophila as well as mammals has been reported (Zhao et al., 2008). The mammalian Hippo Kinase pathway converges on YAP, which plays a role in liver size regulation and cancer development (Saucedo and Edgar, 2007; Zhao et al., 2008). YAP is a nuclear protein whose phosphorylation results in its nuclear export and degradation, which correlates with a decrease in cell proliferation. We investigated whether ILK regulates YAP expression during PB-induced liver enlargement. In the WT animal, we found no change in the nuclear YAP levels before and after PB administration. But in the ILK/liver−/− mice, there was an induction of YAP after PB administration (Fig. 5A). The phospho-Yes Associated Protein (p-YAP) levels in the WT mice remained elevated at all time points, while the levels go down in the ILK/liver−/− mice after PB administration (5A). The YAP/p-YAP ratio (Fig. 5B) increases dramatically in the ILK/liver−/− mice after PB administration, while it remains unchanged in the WT mice.
A detailed microarray analysis (Affymetrix Inc.) was performed to investigate mechanisms involved in the termination defect in the ILK/liver−/− mice. We tabulated the top 20 upregulated and downregulated genes following administration of PB in the WT and the ILK/liver−/− mice (Tables 1 and 2). The tabulation was done based on the ratio of the expression value of the gene at days 10 to 0. Most of the genes upregulated and downregulated in the WT and the ILK/liver−/− mice were metabolic enzymes and drug transporters. This was not surprising since CAR activation is known to affect expression of several metabolic enzymes and drug transporters. We also tabulated the top 5 cytochrome P450's (CYP's) upregulated in WT mice. Except for CYP2B6 (marker for CAR activation), all the other CYP's showed lower ratios in the ILK/liver−/− mice, suggesting that the ILK−/− liver has not restored complete differentiation as compared to WT after 10 days of PB expression (Table 3).
TABLE 1.
Top 20 Upregulated and Downregulated Genes in KO Mice. Pooled Liver Samples from At least Three Mice Were Used for Microarray Analysis. WT Ratio D10/D0 and KO Ratio D10/D0 Represent the Ratio of the Actual Expression Values of the Gene at Day 10 of PB Expression to Day 0
| Genes | WT ratio D10/D0 | KO ratio D10/D0 |
| Top 20 upregulated genes in KO mice after PB administration | ||
| Folate hydrolase | 1.70 | 309.22 |
| Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | 1.27 | 217.41 |
| Transforming growth factor, beta 2 | 0.18 | 177.00 |
| CXC chemokine MIP-2gamma | 0.96 | 172.15 |
| GDP-mannose 4, 6-dehydratase | 0.94 | 111.02 |
| Alpha fetoprotein | 1.14 | 69.45 |
| cyp2b6 | 40.92 | 68.09 |
| CYP2c55 | 80.68 | 46.02 |
| Lipoprotein lipase | 1.94 | 45.27 |
| Protocadherin beta 9 | 0.52 | 38.47 |
| Stearoyl-Coenzyme A desaturase 2 | 0.73 | 37.71 |
| Coronin, actin-binding protein 1A | 0.72 | 33.77 |
| Sphingomyelin phosphodiesterase 3 | 13.73 | 32.66 |
| Sulfotransferase family 1E, member 1 | 0.20 | 30.23 |
| ect2 oncogene | 1.28 | 29.90 |
| Myotubularin-related protein 11 | 2.11 | 28.77 |
| Chemokine (C-X3-C) receptor 1 | 0.87 | 11.93 |
| Organic solute transporter beta | 0.44 | 17.17 |
| Reduced expression 3 | 0.86 | 7.14 |
| Amino levulinate synthase | 8.73 | 0.60 |
| Top 20 downregulated genes in KO mice after PB administration | ||
| Hydroxysteroid dehydrogenase-5 | 277.36 | 0.00 |
| Odorant-binding protein 2B | 84.03 | 0.01 |
| Cytosine methyltransferase | 28.00 | 0.01 |
| Hepcidin antimicrobial peptide 2 | 0.18 | 0.04 |
| Retinoic acid early transcript 1, alpha | 0.46 | 0.05 |
| Thioesterase 2 | 0.80 | 0.07 |
| Uridine phosphorylase 2 | 0.69 | 0.07 |
| Peroxisomal acyl-CoA thioesterase 2A | 0.03 | 0.09 |
| Cholesterol 24-hydroxylase | 0.98 | 0.09 |
| Cyp7b1 | 3.72 | 0.10 |
| Caspase 9 | 0.94 | 0.11 |
| 0-6-methylguanine-DNA methyltransferase | 1.09 | 0.11 |
| Glutamate transporter | 0.29 | 0.13 |
| Aquaporin 4 | 1.60 | 0.14 |
| Ubiquitin-specific protease 2 | 0.60 | 0.16 |
| Uridine phosphorylase 2 | 0.65 | 0.17 |
| Monocyte to macrophage differentiation associated 2 (Mmd2) | 0.57 | 0.17 |
| Growth factor receptor-bound protein 2 | 0.88 | 0.17 |
| Leukemia inhibitory factor receptor | 1.21 | 0.17 |
| UDP glucuronosyltransferase 2 | 3.40 | 0.17 |
Note. GDP, guanyl-diphosphate; MIP, major intrinsic protein; UDP, uridyl diphosphate.
TABLE 2.
Top 20 Upregulated and Downregulated Genes in WT Mice. Pooled Liver Samples from At least Three Mice Were Used for Microarray Analysis. WT Ratio D10/D0 and KO Ratio D10/D0 Represent the Ratio of the Actual Expression Values of the Gene at Day 10 of PB Expression to Day 0
| Genes | WT ratio D10/D0 | KO ratio D10/D0 |
| Top 20 upregulated genes in WT mice after PB administration | ||
| Hydroxy-delta-5-steroid dehydrogenase | 277.36 | 0.00 |
| Calgranulin A8 | 107.25 | 17.01 |
| CYP2c55 | 80.68 | 46.02 |
| CYP4a12 | 48.27 | 0.58 |
| CYP2b6 | 40.92 | 68.09 |
| Eukaryotic translation initiation factor 2, subunit 3 | 39.68 | 1.00 |
| CYP2d9 | 35.63 | 0.44 |
| CYP2c65 | 28.80 | 2.90 |
| Osteomodulin | 22.22 | 0.99 |
| Keratin complex 2 | 20.55 | 4.26 |
| Alcohol dehydrogenase 4 | 15.56 | 0.32 |
| Sphingomyelin phosphodiesterase 3 | 13.73 | 32.66 |
| Complement component 6 | 13.63 | 0.85 |
| Miosis expressed gene 1 | 12.15 | 5.51 |
| N-myc downstream-regulated gene 1 | 10.90 | 6.20 |
| Protease, serine, 8 | 9.65 | 3.31 |
| Amino levulinate synthase | 9.24 | 1.48 |
| Sorting nexin 29 | 8.73 | 0.60 |
| BCL2-interacting killer | 8.59 | 0.39 |
| Top 20 downregulated genes in WT mice after PB administration | ||
| Flavin-containing monooxygenase 3 | 0.01 | 16.40 |
| ATP-binding cassette member 2 | 0.02 | 12.10 |
| Sulfotransferase 2A | 0.02 | 1.04 |
| Cyp3a | 0.02 | 0.70 |
| Peroxisomal acyl-CoA thioesterase 2A | 0.03 | 0.09 |
| Cyp17 | 0.03 | 0.21 |
| Cyp3a16 | 0.03 | 18.82 |
| Cyp3a41 | 0.05 | 3.03 |
| Methyltransferase 11 domain containing 1 | 0.06 | 4.71 |
| Procollagen, type V, alpha 2 | 0.07 | 3.06 |
| Acyl-CoA synthetase long-chain family member 6 | 0.08 | 0.83 |
| Putative integral membrane transport protein | 0.08 | 3.35 |
| Dual specificity phosphatase-like 15 | 0.08 | 1.61 |
| Chloride channel 5 | 0.08 | 1.47 |
| Cyp4a10 | 0.09 | 0.27 |
| Organic anion transporter 6 | 0.09 | 1.81 |
| cyp4a14 | 0.09 | 1.14 |
| Glutathione peroxidase 5 | 0.09 | 0.84 |
| Flavin-containing monooxygenase 2 | 0.10 | 3.11 |
| Ubinuclein 2 | 0.10 | 3.11 |
TABLE 3.
Top 5 Upregulated CYP's in WT Mice. Pooled Liver Samples from At least Three Mice Were Used for Microarray Analysis. WT Ratio D10/D0 and KO Ratio D10/D0 Represent the Ratio of the Actual Expression Values of the Gene at Day 10 of PB Expression to Day 0
| Genes | WT ratio D10/D0 | KO ratio D10/D0 |
| CYP2c55 | 80.6844086 | 46.01523297 |
| CYP4a12 | 48.27089497 | 0.579841095 |
| CYP2b6 | 40.92406589 | 68.09328684 |
| CYP2d9 | 35.62966138 | 0.43632564 |
| CYP2c65 | 28.80035336 | 2.900934579 |
DISCUSSION
The basic hypothesis of this study is that the main source of signals leading to control of initiation and termination of hepatocyte proliferation is in part the hepatic ECM. This hypothesis was well supported by the data shown in our recent previous study of extended liver regeneration after partial hepatectomy in ILK/liver−/− mice (Apte et al., 2009). Several studies have shown that chemical compounds can cause liver enlargement, by a combination of hepatocyte hyperplasia and hypertrophy. PB was one of the first chemicals connected with this response, and the cellular kinetics and signaling pathways associated with PB-induced hepatomegaly are well characterized (Burger and Herdson, 1966; Carthew et al., 1998; Columbano and Shinozuka, 1996; Coni et al., 1993). PB is known to interact with CAR and PXR transcription factors (Wei et al., 2000) and to also increase the very important HNF-4α transcription factor in hepatocyte nuclei (Bell and Michalopoulos, 2006) after a single dose. This single-dose approach, however, is not sufficient to cause the full hepatic response to PB. Thus, we used the well-established protocol of continuous administration of PB in the drinking water, well characterized from numerous studies associated with hepatic tumor promotion. This approach causes the massive hepatic enlargement associated with PB, and we used this model to make comparisons with the results we obtained in liver regeneration. In this chronic model of PB administration, PB was administered in drinking water for 10 days. Steady supply of PB in drinking water led to a modest nuclear translocation of CAR in the WT and ILK/liver−/− mice. CAR target-like CYP2B6 was also increased in both the groups. We then looked at the hyperplastic response of PB in both the groups. Our studies show that 10 days of PB administration led to a three times increased liver to body weight ratio as compared to 0 day in the ILK/liver−/− mice, while the WT mice show only a two times increase. ILK/liver−/− mice also have increased percent of PCNA-positive cells and mitotic cells even at days 5 and 10 of PB administration, suggesting a prolonged proliferative response. Taken together, our studies document that ECM-mediated signaling mediated through ILK is also a defining parameter in hepatocyte proliferation, initiation of hepatic enlargement, and stabilization of the liver weight to a new plateau, as induced by chemical xenobiotic mitogens.
We investigated the mechanisms behind this prolonged proliferative response in the ILK/liver−/− mice. We looked into the key genes that are shown to be involved in hepatocyte proliferation. Cyclin D1 has been shown to play an important role in cell proliferation. While there was an induction of cyclin D1 in the ILK/liver −/− mice, there was no change in the WT. This was surprising since we see proliferation at day 2 in WT mice but no induction of cyclin D1. In a xenobiotic-based model of hyperplasia, induction of cyclin D1 is not a mandatory process to induce proliferation (Ledda-Columbano et al., 2002). This study shows that lack of cyclin D1, although causing a delay in the entry into S phase, does not inhibit mouse liver growth and hepatocyte proliferation induced by TCBOPOP, suggesting that cyclin D1 is not essential for hepatocyte proliferation induced by mitogenic stimuli. Even though there have been studies that have shown induction of cyclin D1 after a single dose of PB administration, we could not find any literature that has looked into the levels of cyclin D1 in a model where there is a steady supply of PB in drinking water. It is possible that the proliferative response induced by a steady supply of PB in drinking water is on a long term is cyclin D1 independent, while it is cyclin D1 dependent in ILK/liver−/− mice. We should also point out that previous studies have shown that (contrary to intuitive expectations) long-term administration of PB renders hepatocytes unresponsive to both EGF and HGF (Tsai et al., 1991). Thus, changes seen with PB and cyclin D1 shown in our manuscript suggest that the relationship between the two needs to be better understood, and we hope that our findings will set the stage for further investigations in this matter. Similarly, the YAP levels go up in the ILK/liver−/− mice after PB administration, while they remain unchanged in the WT mice. Even though the levels of YAP were lower in the ILK/liver−/− mice as compared to the WT animals at all times points (expect day 10 when they are about equal), we still see more proliferation in ILK/liver−/− mice as compared to the WT mice. The answer to this might lie in the levels of p-YAP. The WT mice have increased p-YAP levels at all time points as compared to ILK/liver−/− mice, suggesting that there is more YAP degradation in WT as compared to ILK/liver−/− mice. The ratio of YAP/p-YAP is more than 1 in ILK/liver−/− mice, suggesting less degradation of nuclear YAP. Also, the YAP/p-YAP ratio does not change before and after PB administration in the WT animals, suggesting that the proliferative response in the WT mice may not be YAP dependent. Egr-1 and HGF levels even though went down in both the groups after PB administration, they were always higher in the ILK/liver−/− mice at all time points after PB administration. Serum levels of HGF were also higher in the ILK/liver−/− mice after PB administration. Mitoinhibitory protein TGF-β1 was induced in the WT mice after PB administration, while it was downregulated in the ILK/liver−/− mice. This to some extent might explain why proliferative response does not stop in the ILK/liver−/− mice. Detailed investigation of the TGF-β1 signaling might yield verification of this possibility.
While detail mechanisms involved in the ILK-related signaling in relation to the hepatomegaly model need to be further understood, our studies do demonstrate that signaling events that are involved in the proliferative response of the ILK/liver/−/− mice are different from that of the WT mice. The results demonstrate that even though the initiating signals in regeneration and chemically induced hepatomegaly are different, the termination signals for both processes are equally affected by ECM acting in part via ILK.
FUNDING
National Institutes of Health Grant (CA035273).
References
- Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, Monga SP, Wu C, Michalopoulos GK. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–851. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AW, Michalopoulos GK. Phenobarbital regulates nuclear expression of HNF-4alpha in mouse and rat hepatocytes independent of CAR and PXR. Hepatology. 2006;44:186–194. doi: 10.1002/hep.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PC, Herdson PB. Phenobarbital-induced fine structural changes in rat liver. Am. J. Pathol. 1966;48:793–809. [PMC free article] [PubMed] [Google Scholar]
- Carthew P, Edwards RE, Nolan BM. The quantitative distinction of hyperplasia from hypertrophy in hepatomegaly induced in the rat liver by phenobarbital. Toxicol. Sci. 1998;44:46–51. doi: 10.1006/toxs.1998.2473. [DOI] [PubMed] [Google Scholar]
- Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- Coni P, Pichiri-Coni G, Curto M, Simbula G, Giacomini L, Sarma DS, Ledda-Columbano GM, Columbano A. Different effects of regenerative and direct mitogenic stimuli on the growth of initiated cells in the resistant hepatocyte model. Jpn. J. Cancer Res. 1993;84:501–507. doi: 10.1111/j.1349-7006.1993.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932–1941. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim. Biophys. Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI, Engelhardt NV, Duncan SA. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology. 2004;39:1038–1047. doi: 10.1002/hep.20155. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Concas D, Cossu C, Tripodi M, Columbano A. Loss of cyclin D1 does not inhibit the proliferative response of mouse liver to mitogenic stimuli. Hepatology. 2002;36:1098–1105. doi: 10.1053/jhep.2002.36159. [DOI] [PubMed] [Google Scholar]
- McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J. Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J. Cell. Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances M. Liver regeneration. Adv. Biochem. Eng. Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Ross PK, Woods CG, Bradford BU, Kosyk O, Gatti DM, Cunningham ML, Rusyn I. Time-course comparison of xenobiotic activators of CAR and PPARalpha in mouse liver. Toxicol. Appl. Pharmacol. 2009;235:199–207. doi: 10.1016/j.taap.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Trautwein C, Kubicka S, Rakemann T, Bahr MJ, Sedlaczek N, Schuppan D, Manns MP. Differential regulation of extracellular matrix synthesis during liver regeneration after partial hepatectomy in rats. Hepatology. 1999;30:1159–1166. doi: 10.1002/hep.510300502. [DOI] [PubMed] [Google Scholar]
- Runge D, Runge DM, Daskalakis N, Lubecki KA, Bowen WC, Michalopoulos GK. Matrix-mediated changes in the expression of HNF-4alpha isoforms and in DNA-binding activity of ARP-1 in primary cultures of rat hepatocytes. Biochem. Biophys. Res. Commun. 1999;259:651–655. doi: 10.1006/bbrc.1999.0848. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat. Rev. Mol. Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- Terpstra L, Prud'homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WH, Zarnegar R, Michalopoulos GK. Long-term treatment with hepatic tumor promoters inhibits mitogenic responses of hepatocytes to acidic fibroblast growth factor and hepatocyte growth factor. Cancer Lett. 1991;59:103–108. doi: 10.1016/0304-3835(91)90173-f. [DOI] [PubMed] [Google Scholar]
- van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem. Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr. Opin. Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JD, Jiang GL, Hu WG, Xu ZY, Wang CF. Hepatocyte regeneration after partial liver irradiation in rats. Exp. Toxicol. Pathol. 2009;61:511–518.. doi: 10.1016/j.etp.2009.02.114. [DOI] [PubMed] [Google Scholar]