Abstract
This manuscript presents the results of a review of the effects of recombinant bovine somatotropin (rBST) on milk production, milk composition, dry matter intake, and body condition score that was carried out by an expert panel established by the Canadian Veterinary Medical Association (CVMA). The panel was established by the CVMA in response to a request from Health Canada in 1998 and their report was made public in 1999. A series of meta-analyses was used to combine data on production and nutrition related parameters that were extracted from all randomized clinical trials, which had been published in peer-reviewed journals or which were provided by Health Canada, from the submission by Monsanto for registration of rBST in Canada. A companion paper will present the results of the effects of the drug on measures of health, reproductive performance, and culling parameters. Recombinant bovine somatotropin was found to increase milk production by 11.3% in primiparous cows and 15.6% in multiparous cows; although there was considerable variation from study to study. While some statistically significant effects on milk composition (% butterfat, protein, and lactose) were found, they were all very small. Treatment increased dry matter intake by an average 1.5 kg/day during the treatment period and dry matter intake remained elevated on into the first 60 days of the subsequent lactation. Despite the increase in dry matter intake, treated animals had lower body condition scores at the end of the treatment period, and the reduced scores persisted through until the start of the subsequent lactation.
Introduction
Recombinant bovine somatotropin (rBST) is a synthetically derived hormone that may be identical to naturally occurring bovine growth hormone, or slightly modified by the addition of extra amino acids. In 1993, sometribove (Posilac; Monsanto Corporation, St. Louis, Missouri, USA) was approved for use in the United States, but the product was not actually sold for commercial use until early in 1994. The product was approved for sale with a package insert which identified a number of possible adverse health effects including increased risk of adverse reproductive effects, clinical mastitis, feet and leg problems, injections site reactions, udder edema, and other general health effects.
In 1991, Monsanto submitted an application to Health Canada, Bureau of Veterinary Drugs, for registration of sometribove in Canada, but this application was withdrawn in 1994 and replaced with a 2nd application for a product to be called Nutrilac. In late 1998, following considerable internal review, Health Canada decided to seek external assistance in the review process from 2 independent expert panels. The Royal College of Physicians and Surgeons was asked to establish an expert panel to review the human health implications of the use of rBST in Canadian dairy cattle. The Canadian Veterinary Medical Association (CVMA) was asked to establish an expert panel to review the data related to the efficacy of the product and potential effects on animal health. The specific mandate of this latter expert panel was as follows.
“1. Review the scientific data used by the Bureau of Veterinary Drugs to determine that Nutrilac (rBST) when used in accordance with its label directions will increase milk production without resulting in serious health problems which cannot be adequately controlled by current cattle management practices.
2. Make observations and recommendations regarding the adequacy of the scientific data submitted by the manufacturer of Nutrilac (rBST) or existing elsewhere to make sound scientific assessments regarding the product efficacy and animal health risks associated with the use of Nutrilac (rBST) in Canadian dairy cattle.”
In initial discussions involving the CVMA and Health Canada, it was made clear that issues related to animal welfare would be considered under the rubric of animal health.
Establishing the expert panel
The CVMA expert panel consisted of the authors of this manuscript and collectively they had expertise in epidemiology, dairy health management, dairy nutrition, animal welfare, and clinical pharmacology. All panel members were subjected to evaluation under Health Canada's conflict of interest guidelines and served without compensation, although panel expenses were covered by Health Canada. Once established, the panel operated completely independently of the CVMA and Health Canada. The panel commenced operation in May 1998 and submitted its final report (1) in November 1998.
Considerations in defining the process
A number of factors were considered in determining what review process would be followed. First, the panel recognized the need to review the impact of rBST on a wide variety of health and production parameters. Second, it was recognized that there was considerable evidence as to the effects of rBST in both the Monsanto submission to Health Canada and in the peer-reviewed published literature, and it would be important to consider both sources. Third, the peer- reviewed published literature includes studies based on sometribove (Monsanto) and on other rBST formulations from other companies. While the report submitted to Health Canada focussed on data obtained from studies involving sometribove, the results of other studies were also considered. Fourth, it was recognized that while studies that were reported in the submission or the published literature would each have a primary outcome (usually related to efficacy), most studies would have additional data on other outcomes (animal health effects). Finally, it was recognized that a process for combining information derived from multiple studies was needed, and this is discussed in more detail in the following section.
Combining data from multiple studies
There are 2 major reasons for combining information derived from multiple studies. The 1st is to derive an overall estimate of the magnitude of an effect along with an estimate of the variability of that effect across the studies. The 2nd is to identify important effects that may not be evident from individual studies. While many studies of rBST have been carried out, most of the studies had small or moderate sample sizes (less than 100 cows). While studies of this size were adequate to evaluate some of the major production effects of rBST, they had insufficient power to detect either beneficial or harmful health effects associated with the use of the drug. The power of a study is defined as the probability of finding a statistically significant effect if, in fact, a true effect of a defined magnitude is present. Studies with insufficient power may not detect important effects associated with the use of a drug. In general, much larger sample sizes are required to detect drug effects on parameters measured on a dichotomous scale (presence/absence of clinical mastitis) than outcomes measured on a continuous scale (milk production). The consequence of insufficient power in individual studies may be that a number of studies each report no significant effect on an outcome of interest, even though a real effect may exist and this effect may be detected by combining the data from the studies.
There are 3 general approaches to combining data from multiple studies: traditional narrative reviews, pooling of raw data from multiple studies, and meta-analyses. A traditional narrative review incorporating a qualitative assessment of the studies and subjective “pooling” of the results is most appropriate if there are a very limited number of studies and considerable detail about each of those studies is available. This approach has the advantage that the unique circumstances of each study can be taken into account. However, although many excellent reviews are carried out in this manner, they are subjective in nature and prone to reviewer bias (2). This type of review may also fail to detect meaningful effects which were not statistically significant in any individual studies. Finally, there is a tendency when subjectively combining data from multiple studies to assign roughly equal weights to each of the studies. As will be seen later in this report, it is clear that some studies should be assigned more weight than other studies. An rBST review of this nature was published in 1994 (3).
Directly pooling raw data from multiple studies and repeating an analysis based on a larger number of cows is one effective way of increasing the power of a group of studies to detect effects. The major limitation to this approach is that these analyses can only be carried out if all of the original data are available to the reviewers and have been recorded in a consistent manner that will allow them to be pooled. This approach is the most resource intensive and time- consuming approach to carrying out a systematic review (2) and was not feasible given the large number of rBST studies to be considered.
The 3rd approach to combining data from multiple studies is to use meta-analysis, which has been defined as “the statistical analysis of a large collection of analysis results from individual studies for the purpose of integrating the findings” (4). It is a formal statistical process which starts with reported results from multiple studies and produces 3 main outputs: an overall estimate of the effect (effect of rBST on risk of clinical mastitis), an estimate of the heterogeneity (variability) of results among studies, and a visual presentation of the results to enable the reviewer to easily assess the evidence. It provides an objective assessment of the available evidence and can also identify gaps in knowledge about a subject. However, there are a number of important issues to be considered in carrying out a meta-analysis, including: the criteria for selecting studies for inclusion in the analysis, the statistical method used to compute the overall estimate and its standard error, an evaluation of potential reasons for discrepancies in results among studies, and the potential effect of publication bias on the results. Each of these is discussed in more detail in the discussion section and the reader is referred to other literature for more complete descriptions of the meta-analysis process (2,5).
Objectives
The objective of this study was to summarize information available in the literature on the effects of rBST on both measures of productivity and health in dairy cattle. Specifically, the technique of meta-analysis was used to combine results from multiple studies in order to estimate an overall effect and to evaluate possible sources of variability of the results among studies. Given the broad range of effects considered, a review and discussion of the potential biological mechanisms underlying each of these effects was beyond the scope of this study. This first article presents the review of the effects of rBST on milk production, milk composition, dry matter intake, and body condition score (BCS). A companion article will present the results of the effects of rBST on health, reproductive performance, and culling parameters.
Materials and methods
Literature review
A literature review based on Medline Express (1991 to May 1998); Agricola (1984 to March 1998); and CABWeb Databases, including Index Veterinarius and Veterinary Bulletin (up to May 1998), identified a total of 1777 references related to rBST. Details of the literature search strategy were published in the initial panel report (1). References were removed if the title indicated that the study pertained to a species other than dairy cattle, pertained to the use of the drug in ages other than lactating cows, were specifically related to the use of the product in tropical environments, were related to the mechanism of action of the drug or to the potential human health effects, were not published in peer-reviewed publications, or if they were not written in English. The resulting list of 242 potentially relevant articles was reviewed by all of the panel members and a subset consisting of previous review papers plus all articles that any panel member felt was likely to contain results from randomized clinical trials was identified. These manuscripts were then combined with the set of study reports submitted by Monsanto to Health Canada as part of the drug approval process, to create the literature base upon which the review was carried out.
Outcome parameters evaluated/data extraction
Table I lists all of the parameters for which data were extracted, if available, from the selected literature. Data were only extracted from reports of randomized clinical trials although the studies need not have been conducted in a blind manner.
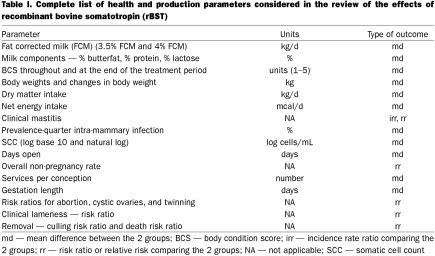
Table I.
If a study reported quantitative data for any of the parameters identified, the following information was recorded: the parameter of interest and its standard error or confidence interval, the P-value from the test of significance of the treatment effect, and whether or not the parameter estimate had been adjusted for level of milk production. In many cases, measures of health effects were not specifically presented in the study report or paper. However, it was often possible to obtain the information needed to compute some of the key parameters. For example, a paper may not have reported the relative risk of clinical mastitis, but it may have reported the number of cows affected and the number at risk of mastitis in each of the treatment groups. From these data, the relative risk of mastitis and its confidence interval were computed and used in the meta-analysis.
In addition to data on the outcome of interest, the following key features of the study were recorded: the manufacturer of the product, dose, interval between doses, route of administration (intra- muscular or subcutaneous), parity of the cows in the study, stage of lactation at start of treatment and duration of treatment, and whether the study was reported in peer-reviewed literature or only in a Monsanto study report. Some additional considerations in the data extraction process were as follows.
· Many of the studies were dose titration trials designed to determine the dose-efficacy relationship. For these multi-dose studies, data from the dose of rBST which was closest to the daily dose (500 mg/14d = 35.7 mg/d) for the product currently registered in the United States (Posilac) were used. If a study reported results from both sustained release and daily injection protocols, only the data from the dosage closest to 35.7 mg/d were used. Data that had already been pooled across doses in multiple dose studies and that did not provide individual dose data were not used.
· If data were reported separately by parity (usually primiparous versus multiparous), they were recorded as such and consequently, one study may have contributed more than 1 set of observations to the meta-analysis.
· If data were reported separately by study year, such as year 1, year 2, etc. in multi-lactation studies, they were recorded as such.
· The early period, for example the first 60 d, of a lactation that followed a lactation where rBST had been used was defined as the “carry-over period” and some parameters were recorded for this period.
Each entry (record) in the database created from the data extraction process represented one outcome of interest (parameter) in one group of cows in a study. For example, one entry might represent the effect of rBST on the somatic cell count (log transformed) in primiparous cows in one study. Since not all studies reported outcomes using measures listed in Table I, other relevant outcomes were recorded, as deemed appropriate, and were considered subjectively in the evaluation process.
Meta-analyses
For each outcome parameter for which there were several valid estimates available from the literature, a minimum of 4 meta- analyses were carried out. First, a fixed effect meta-analysis (with each study being weighted by the inverse of the variance of the parameter estimate) was carried out using data only from studies in which sometribove had been the product used. A fixed effects meta- analysis assumes a constant (or “fixed”) treatment effect with variability in observed effects only being due to random variation. This assumption of homogeneity of treatment effects was tested with “Q” statistic (2). Subsequently, a random effects analysis based on the method of DerSimonian and Laird (6) was carried out. This method assumes the study estimates have a normal distribution with an overall mean and a between-study variance, which is estimated from the data. A 2nd pair of analyses (fixed and random effects) was subsequently carried out using all of the studies (regardless of rBST formulation) in which the parameter had been reported. A 3rd set of analyses was carried out using studies based on formulations other than sometribove. In addition, separate meta-analyses were carried out for the different age groups of cows (primiparous versus multiparous), where warranted.
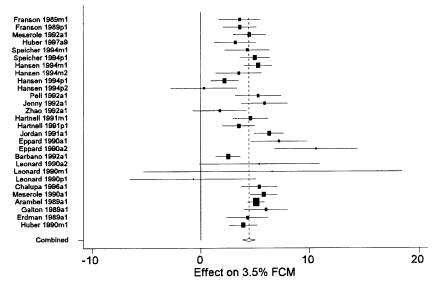
Whether the results presented were based on the random effects model or the fixed effects model was determined by examining a statistical test of the heterogeneity of the results across the studies. If significant heterogeneity was observed (P < 0.05) the random effects estimator was used and included in the forest plot (Figure 1) that was generated for each outcome parameter evaluated.
Figure 1. Forest plot of effects of recombinant bovine somatotropin (rBST) on 3.5% fat corrected milk (FCM) (kg/d). The overall estimate was derived from the random effects meta-analysis. See text for explanation of components of the graph.
Meta-regression analyses (7) were used to evaluate the effects of product formulation (sometribove or other formulations), parity of cows (primiparous, multiparous, or all combined), study size (number of cows), study precision (standard error of estimate), publication source (peer-reviewed or company report), duration of treatment (days), and expected daily dosage (mg/d) on each outcome of interest. These analyses used a weighted regression to determine if there was any evidence of a linear relationship between the observed result and the factor being investigated (study size). Each factor was investigated separately as there were too few studies for any of the outcomes of interest to attempt multivariable analyses. For comparability with the methods used for the meta-analyses, a moments estimator of the between-study variance was used in the meta-regressions.
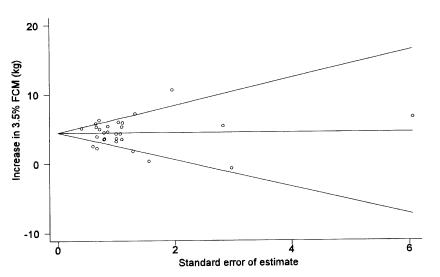
The possibility of publication bias affecting the meta-analyses was investigated in several ways. First, the effects of study size or precision (as determined above) on observed effects were evaluated. Second, both Begg's (8) and Egger's (9) tests for publication bias were computed. Finally, “funnel plots” (Figure 2) were used to graphically assess whether publication bias was a problem (2). All analyses were carried out using a statistical program (Stata, Version 7; Stata Corporation, College Station, Texas, USA) (10).
Figure 2. Funnel plot of the point estimates of the effect of recombinant bovine somatotropin (rBST) on 3.5% fat corrected milk (FCM) versus the standard errors of those estimates. Evidence of publication bias would be the presence of numerous points in the upper right quadrant (large effect, large standard error) with few being present in the lower right quadrant (small or negative effect, large standard error).
Results and discussion
Literature review
From the original 1777 articles identified by the literature review, 242 were considered potentially relevant and were examined by all panel members. Of these, 60 were identified as useful review articles or reports likely to contain data from a randomized controlled clinical trial. These manuscripts were combined with 26 study reports submitted by Monsanto as part of their submission to Health Canada to form the literature base for this review. Of these 86 reports, 53 provided data for use in 1 or more meta-analyses. The other 33 articles were excluded because they did not contain data on any of the relevant outcomes (n = 17), were review articles with no original data (n = 6), were based on studies of less than 10 cows or the treatment duration lasted less than 1 mo (n = 4), presented data in a manner that were not usable in the meta-analyses (n = 4), or were duplicates of other reports (n = 2). If data from a study were reported in both a Monsanto report and a peer-reviewed manuscript, the latter has been cited. From the 53 reports providing data useful for meta-analyses, a total of 546 outcome parameter estimates from 94 groups of cows were extracted and included in the database.
All data included in the meta-analyses were derived from randomized clinical trials with random assignment of cows to treatment groups. Such studies are much less subject to the forms of bias that affect non-randomized studies of treatment effects (11).
“.... the randomised controlled trial is at present the unchallenged source of the highest standard of evidence used to guide clinical decision making.” (12)
Observational studies of the effects of treatments can potentially suffer from a serious selection bias, in that producers choosing to use the treatment may be very different from those that do not. In addition, only those producers continuing to use the treatment for a reasonable period of time are generally considered in the “treatment group” and these are presumably self-selected producers who observed a favourable response to the treatment with few side effects.
The use of a placebo in the control cows was common, but it was not universal across studies. There is some debate about the value of placebo treatments in rBST studies given that the product tends to produce a fairly obvious increase in production that negates the masking effect of the placebo. Both placebo and non-placebo based studies were included in the meta-analyses. There was some variability in how withdrawal of animals from studies was handled across studies. However, most studies were relatively short-term in nature and few animals were removed from the study, so no adjustment was made on this basis.
Meta-analysis methods
A primary method of presentation of results from a meta-analysis is a forest plot (Figure 1). In these plots, each line represents the results from a single study (or distinct group of cows within a study). Each line is labelled with a unique label which identifies the study and group of cows represented. The name of the first author and year of publication is followed by a single letter indicating the age of the cows in the study (p = primiparous, m = multiparous, a = all ages combined) and a final digit indicating the year of the study (this was only >1 for multi-lactation studies that reported results separately for each successive lactation). The length of the line represents the 95% confidence interval for the parameter estimate from the study. The centre of the shaded box on each line marks the point estimate of the parameter from that study, and the area of the box is proportional to the weight assigned to the study in the meta-analysis. Studies with large boxes have had a strong influence on the overall estimate. The dashed vertical line marks the overall estimate of the effect. The <> at the bottom of the dashed line shows the confidence interval for the overall effect estimation. The solid vertical line marks the value where rBST would have no effect (a mean difference of 0 or a relative risk of 1).
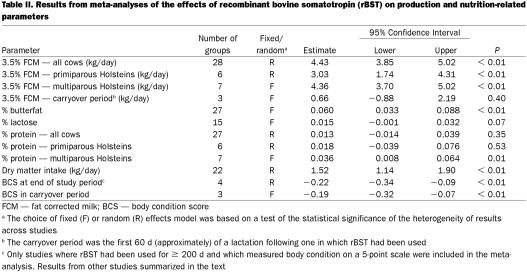
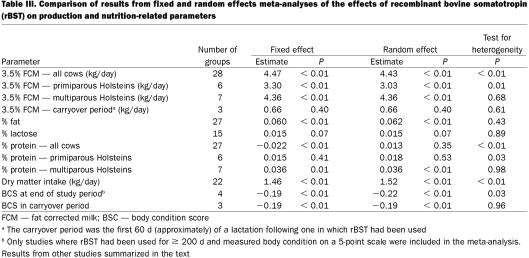
One important consideration in a meta-analysis is whether to assume a fixed effect (the treatment effect is constant across all studies) or a random effect (analysis does not make that assumption although it still computes an overall estimate of the effect). Both types of analysis were carried out in this study and the results from the fixed effect model presented as the primary result (Table II) if the statistical test of heterogeneity was non-significant. However, tests for heterogeneity have relatively low power in situations where only a small number of studies have been included, so a complete set of results from both types of analysis have been presented (Table III). With the exception of % protein when all age groups of cows were considered together (and in which there was clear evidence of heterogeneity), the estimates of the overall effect from the 2 sets of analyses were very close.
Table II.
Table III.
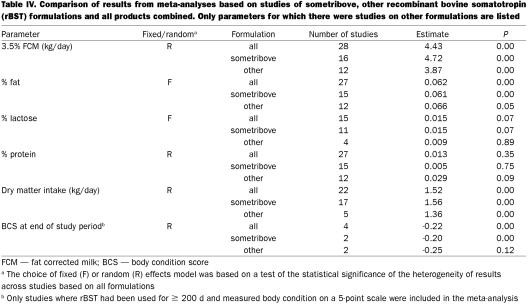
One of the concerns that was raised following the publication of the panel's report (1) was that the analyses combined data from studies based on different formulations of rBST (from different manufacturers) and from studies with different protocols (different dosages, durations of treatment, etc.) (13). This concern was presumably based on an incorrect assumption that meta-analyses should only be carried out on a series of very similar studies. In fact, the finding of consistent results across a range of studies based on varying methodologies adds strength to the conclusion of an overall effect (5). For example, a recent meta-analysis of the ability of β-blockers to reduce the short-term risk of myocardial infarction included results from studies using 12 different drugs (14). Similarly, a review of homeopathic treatments was based on a wide range of different treatments (15). Nevertheless, it was considered important that the results from a meta-analysis based only on sometribove (produced by Monsanto) be compared with those obtained from studies based on other formulations or on all studies combined (Table IV). The results from the 3 sets of analyses were very similar.
Table IV.
Publication bias is a concern in any form of review that combines data from multiple studies. The primary concern is that studies with positive results are more likely to be published, resulting in an estimate of effect that is biased away from the null (apparent effects of rBST would be inflated). Since smaller studies (or more generally, those studies with large standard errors of the estimated effect) are less likely to find a positive result, they may be more prone to not being published. In the presence of publication bias, a plot (funnel plot) of effect estimates against their standard errors should show a lack of studies with small effects but large standard errors (bottom right quadrant in Figure 2). In addition to visually assessing funnel plots for each parameter, 2 statistical tests (Begg's and Egger's) based on the same principle of identifying an association between parameter estimates and their standard error were computed. None of the funnel plots provided any strong evidence of publication bias and, with the exception of Egger's test when applied to % protein, none of the statistical tests were significant at P = 0.05. However, it should be noted that these procedures have relatively low power to detect bias when they are based on fewer than 20 studies. Nevertheless, the fact that there was little evidence of publication bias and that this review did include results from studies that had not been published in the peer-reviewed literature, suggests that publication bias was not a major problem for this meta-analysis.
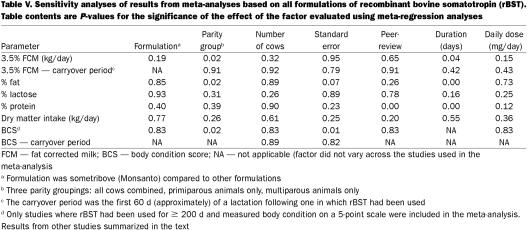
In any meta-analysis it is important to determine what factors may be contributing to variation in study results. Meta-regression was used to evaluate the effects of several factors on each outcome of interest. The limited sample size precluded the simultaneous evaluation of multiple factors so that each factor was evaluated individually in its own meta-regression (Table V). Drug formulation (sometribove versus others), study size (number of cows), and estimated daily dose did not significantly affect any of the outcome parameters studied. Other factors: standard error of the estimate, parity group (primiparous versus multiparous), publication in peer-reviewed journals (versus a company report), and duration of treatment appeared to be related to selected outcome parameters. These associations are discussed below.
Table V.
Effects on milk production
A meta-analysis based on 28 groups of cows from 19 studies (16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34) determined that the overall effect of rBST was to produce an increase in 3.5% fat-corrected milk of approximately 4.4 kg per day (Table II and Figure 1). It was noted that there was considerable variability among studies and that parity group was a significant contributor to this variation (Table V). Consequently, separate analyses for primiparous and multiparous Holstein cows were carried out (Table II). This substantially reduced the variability among studies and suggested that, on average, primiparous Holsteins produced an extra 3.0 kg per day, while multiparous Holsteins produced an extra 4.3 kg per day when treated with rBST. The average production levels in primiparous and multiparous cows in the control groups of these studies were 26.6 and 27.9 kg per day, respectively, so the average percentage increase in milk production was 11.3% for primiparous cows and 15.6% for multiparous cows. The duration of the treatment period was marginally significantly associated with the treatment effect with an apparent decrease of 0.008 kg/d in the magnitude of the treatment response for each additional day of treatment.
The meta-analysis of milk production during the “carry-over” period (Table II) suggested that there was no significant effect of rBST on production in the early lactation period in a lactation subsequent to one in which rBST had been used.
While most North American studies reported milk production in terms of 3.5% fat-corrected-milk (3.5% FCM), a second measure of overall efficacy recorded in the database was 4% FCM derived from studies carried out in the United Kingdom (35,36), France (37) and Germany (38). The first 2 studies reported increased yields similar to those observed in North American studies. However, the German study reported reduced yields in each of the 3 study years, although none of the individual year reductions were statistically significant.
Most of the studies had been carried out in institutional herds (university or pharmaceutical company), and may not reflect results obtained under commercial conditions. However, the results of these meta-analyses were consistent with the 894 kg increase over a 305-day lactation period observed in an observational study of 340 dairy herds from the northeastern United States (39).
Effects on milk composition
Eighteen studies contained usable data on milk composition (16,17,19,20,21,22,23,24,25,27,28,29,30,31,32,33,34,40). From these studies, there was evidence of a very small, but statistically significant, increase in the level of butterfat in the milk from treated cows (Table II). However, most of the evidence for this increase came from 2 short-term (12 wk) studies carried out in New York (32) and Michigan/New York (30). There was evidence from the meta-regressions that as the duration of treatment lengthened, the increase in butterfat percentage dropped by 0.0005% per day. In relative terms, an increase of 0.06 percentage points in the butterfat level would represent only a 1.5 to 2.0% increase. It was concluded that, even if consistently obtained, an increase of this magnitude would not be of any substantial consequence to the dairy industry.
When looking at all of the studies together, there was no consistent evidence of an overall effect on the protein content of milk produced by treated cows (Table II). While there were 3 specific studies (30,31,32) which reported statistically significant reductions, there were many other studies which reported increases. Given that protein demands differ substantially between primiparous and multiparous animals, separate meta-analyses for these 2 groups were carried out. These results suggested that there was no effect of rBST on % protein in primiparous cows but a small positive effect in multiparous cows of 0.036 percentage points (approximately a 1% increase). Meta- regression analyses also suggested that results from non peer-reviewed studies were more likely to report an increase in protein levels. This was likely a spurious finding. It was felt that the small positive effect in multiparous cows was too small to be of any practical consequence to the dairy industry.
Although there appeared to be a very small effect on the lactose concentration in milk (Table II), this effect was not statistically significant (P = 0.07). Even if the apparent effect was real, it was too small to be of any practical consequence.
Effects on dry matter intake
The effects of rBST on several nutritional factors, including dry matter intake, gross feed efficiency and a net energy intake were considered. Of these, only dry matter intake was evaluated in detail and summarized for 2 reasons. First, the other parameters were infrequently reported and second, even if reported, the methods of reporting were highly variable across studies, precluding computation of any summary effects.
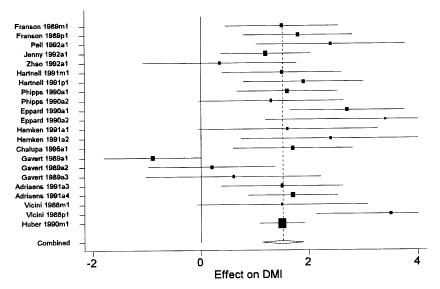
There was considerable variability among studies (16,21,22,23,24,26,29,34,36,38,40,41,42) with respect to the observed effect of rBST on dry matter intake in dairy cows. However, overall, the dry matter intake of treated cows was increased, on average, by approximately 1.5 kg/d (Table IV and Figure 3). None of the factors investigated in the meta-regressions were found to have a significant effect on the DMI estimates, but these analyses would have had very limited power given the small number of studies available.
Figure 3. Forest plot of effects of recombinant bovine somatotropin (rBST) on dry matter intake (DMI) (kg/d). The overall estimate was derived from the random effects meta-analysis. See text for explanation of components of the graph. Upper ends of confidence intervals truncated at 4 kg/d.
There was also evidence that the increased dry matter intake carried over to the early lactation period in the subsequent lactation. Two studies (40,43) reported dry matter intakes during the carry-over period and both found significantly increased dry matter intake during this period.
Effects on body condition
Studies of rBST used multiple ways of measuring the effects of the drug on body condition. The database established for the review of rBST included values for each of the following parameters: BCS throughout the treatment period (the average scores when assessed at regular intervals throughout the treatment period), BCS at the end of the treatment period (recorded separately for treatment periods > 200 d and < 200 d), change in BCS (change in BCS over the treatment period), body weight (at end of treatment period), and daily weight change (throughout the treatment period). All BCS measurements were on the standard scale of 1 to 5 units.
Although all of these parameters were included in the database, BCS at the end of a treatment period lasting more than 200 d was the parameter most commonly extracted from the studies reviewed and was the only parameter subjected to meta-analysis. However, although this parameter was reported in many studies, many of them did not report standard errors of the estimates and consequently these results could not be included in the meta-analyses.
The overall estimate of the effect of rBST on body condition score at the end of the treatment period (> 200 d) was a reduction of approximately 0.2 units (Tables II and IV). However, only 2 studies with 4 groups of cows (16,20) reported adequate data (point estimates and standard errors) to allow them to be included in the meta-analysis. The influence of individual studies on the overall estimate was determined by sequentially leaving out individual studies and repeating the analysis. Doing so resulted in a range of estimates from -0.15 to -0.25 units. Four other studies (18,22,40,44) with 10 groups of cows, which reported this parameter but did not include standard errors of the estimate, reported an average reduction of 0.15 units (based on a simple arithmetic average).
There was some evidence of publication bias in the meta-analysis of BCS. Both Begg's and Egger's tests for bias were marginally significant (P = 0.09 and 0.07, respectively). The meta-regression of the effect on BCS on the standard errors of the subjects confirmed that studies with large standard errors tended to have larger (more negative) effects. Since monitoring body condition was not the primary focus of any of these trials, it is difficult to determine how such a publication bias might arise.
Two studies (19,37) involving 4 groups of cows reported BCS throughout the treatment period. On average they reported scores approximately 0.4 units lower in treated cows than in control cows. Statistical significance was only assessed in the former study and in that study the reductions were significant.
Of the 5 studies (17,31,32,45,46) that reported BCS at the end of a treatment period, which had been less than 200 d in length, 4 of them reported a reduction in body condition. Body weights at the end of a treatment period were recorded from 5 groups of cows in 4 studies (41,47,48,49) with lower body weights in treated cows being reported in 3 groups.
Change in BCS values were reported from 4 groups of cows in 2 studies (20,50) with treated cows gaining less body condition than control cows in all groups. Two studies (23,29) reported changes in body weight as daily weight changes and once again, treated cows gained less weight than control cows.
There was evidence that this difference in body condition carried over into the first 60 d of the subsequent lactation (the carryover period). Although this was recorded from relatively few studies, the meta-analysis of 2 studies (3 groups of cows) (34,42), which did report this parameter, found a significant reduction in body condition of approximately 0.2 units in the early lactation period of the subsequent lactation (Table II).
The effect of rBST on body condition was reported in many different ways across many studies. Overall, it was evident that treatment with rBST resulted in lower BCS among treated cows at the end of the treatment period. It was also evident that this reduction in body condition persists into the early period of the subsequent lactation. Although it appeared that most studies reported paying careful attention to the nutritional requirements of the cows, these results may have arisen from treated cows being underfed or control cows being overfed. Despite the increase in dry matter intake associated with treatment and the high level of nutritional management, treated cows entered the subsequent lactation in a lower state of body condition than control cows. Depending on the level of body condition in these cows, this effect may have been beneficial or detrimental. The ability to adequately manage the nutrition of cows treated with rBST may also have improved in the, approximately, 10 y since most of the studies used in the meta-analyses were conducted.
In conclusion, rBST increases average daily milk by 3.0 kg (11.3%) in primiparous Holsteins and 4.3 kg (15.6%) in multiparous Holsteins. However, these are average values and actual responses varied considerably from study to study. There was a very small increase in the butterfat content of milk produced and in the protein content of milk produced by multiparous cows. However, the magnitudes of these increases were very small and not likely of much consequence to the dairy industry.
Dry matter intake was increased by, on average, 1.5 kg/d and there was evidence that this increased consumption carried over through the dry period and into the first 60 d of the subsequent lactation. Despite this increase in dry matter intake, treatment with rBST reduced the body condition of cows, compared to control cows. The increased dry matter intake did not appear to be adequate to compensate for the increased energy output associated with the increased milk yield. This BCS reduction appeared to carry over into the early portion of the next lactation.
Footnotes
Acknowledgments
The panel thanks Nicky Schaeffer for all of her assistance with the organization of the material used in this review and Health Canada for their timely assistance in locating all necessary resource material.
Address all correspondence and reprint requests to Dr. Ian Dohoo; telephone: (902) 566-0640; fax: (902) 566-0823; e-mail: dohoo@upei.ca
Received February 13, 2003. Accepted April 29, 2003.
References
- 1.Dohoo IR, DesCôteaux L, Dowling P, et al. Report of the Canadian Veterinary Medical Association Expert Panel on rBST. http://www.hc-sc.gc.ca/english/archives/rbst/animals/ [PubMed]
- 2.Egger M, Davey Smith G, Altman DG, eds. Systematic Reviews in Health Care. Meta-analysis in context. London: BMJ Books, 2001.
- 3.Burton JL, McBride BW, Block E, Glimm DR, Kennelly JJ. A review of bovine growth hormone. Can J Anim Sci 1994;74:167–201.
- 4.Glass GV. Primary, secondary and meta-analysis of research. Educ Res 1976;5:3–8.
- 5.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epi Rev 1992;14:154–176. [DOI] [PubMed]
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177–188. [DOI] [PubMed]
- 7.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat in Med 1999;18: 2693–2708. [DOI] [PubMed]
- 8.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50: 1088–1101. [PubMed]
- 9.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed]
- 10.StataCorp. Stata Statistical Software. Release 7. College Station, Texas, USA: Stata Corporation, 2001.
- 11.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott-Raven, 1998.
- 12.Lavori PW, Kelsey J. Introduction and overview (Clinical Trials). Epidemiologic Reviews 2002;24:1–3.
- 13.Baker, DE. Response to citizen petition. http://www.fda.gov/ohrms/dockets/dailys/00/jun00/062700/pnd0001.pdf
- 14.Freemantle N, Cleland J, Young P, Mason J, Harrison J. β Blockade after myocardial infarction: systematic review and meta regression analysis. British Medical Journal 2002;318:1730–1737. [DOI] [PMC free article] [PubMed]
- 15.Linde K, Clausius N, Ramirez G, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo- controlled trials. Lancet 2002;350:834–843. [DOI] [PubMed]
- 16.Franson SE, Cole WJ, Madsen KS, et al. Response of cows throughout lactation to Sometribove in a prolonged system — a dose titration study conducted at four U.S. sites (#87-023, #87-034, #87-029, #87-024). Monsanto Study Report. 10–12–1989. St. Louis, Missouri, USA.
- 17.Meserole VK, Duque JA, Hintz RL, Peel CJ. Evaluation of the galactopoietic response of bovine somatotropin (Sometribove (CP115099-F), 500 mg and CP115400-P, 260 mg) when administered subcutaneously to lactating Jersy cows in a commercial dairy herd (#89-075, #88-192). Monsanto Study Report. 8–9–1992. St. Louis, Missouri, USA.
- 18.Huber JT, Wu Z, Fontes C, Sullivan JL, Hoffman RG, Hartnell GF. Administration of recombinant bovine somatotropin to dairy cows for four consecutive lactations. J Dairy Sci 1997;80: 2355–2360. [DOI] [PubMed]
- 19.Speicher JA, Tucker HA, Ashley RW, Stanisiewski EP, Boucher JF, Sniffen CJ. Production responses of cows to recombinantly derived bovine somatotropin and to frequency of milking. J Dairy Sci 1994;77:2509–2517. [DOI] [PubMed]
- 20.Hansen WP, Otterby DE, Linn JG, Anderson JF, Eggert RG. Multi-farm use of bovine somatotropin for two consecutive lactations and its effects on lactational performance, health, and reproduction. J Dairy Sci 1994;77:94–110. [DOI] [PubMed]
- 21.Pell AN, Tsang DS, Howlett BA, et al. Effects of a prolonged-release formulation of Sometribove (n-methionyl bovine somatotropin) on Jersey cows. J Dairy Sci 1992;75:3416–3431. [DOI] [PubMed]
- 22.Jenny BF, Grimes LW, Pardue FE, Rock DW, Patterson DL. Lactational response of Jersey cows to bovine somatotropin administered daily or in a sustained-release formulation. J Dairy Sci 1992;75:3402–3407. [DOI] [PubMed]
- 23.Zhao X, Burton JH, McBride BW. Lactation, health, and reproduction of dairy cows receiving daily injectable or sustained-release somatotropin. J Dairy Sci 1992;75:3122–3130. [DOI] [PubMed]
- 24.Hartnell GF, Franson SE, Bauman DE, et al. Evaluation of Sometribove in a prolonged-release system in lactating dairy cows — production responses. J Dairy Sci 1991;74:2645–2663. [DOI] [PubMed]
- 25.Jordan DC, Aguilar AA, Olson JD, Bailey C, Hartnell GF, Madsen KS. Effects of recombinant methionyl bovine somatotropin (Sometribove) in high producing cows milked three times daily. J Dairy Sci 1991;74:220–226. [DOI] [PubMed]
- 26.Eppard PJ, Hudson S, Cole WJ, et al. Response of dairy cows to high doses of a sustained-release bovine somatotropin administered during two lactations. 1. Production response. J Dairy Sci 1991;74:3807–3821. [DOI] [PubMed]
- 27.Barbano DM, Lynch JM, Bauman DE, Hartnell GF, Hintz RL, Nemeth MA. Effect of a prolonged-release formulation of N-methionyl bovine somatotropin (Sometribove) on milk composition. J Dairy Sci 1992;75:1775–1793. [DOI] [PubMed]
- 28.Leonard M, Gallo G, Gallo M, Block E. Effects of a 28-day sustained-release formulation of recombinant bovine somatotropin (rbST) administered to cows over two consecutive lactations. Can J Anim Sci 1990;70:795–809.
- 29.Chalupa W, Vecchiarelli B, Galligan DT, et al. Responses of dairy cows supplemented with somatotropin during weeks 5 through 43 of lactation. Journal of Dairy Science 1996;79:800–812. [DOI] [PubMed]
- 30.Meserole VK, Madsen KS, Hartnell GF, et al. Response of cows to biweekly administration of sometribove (N-Methionyl Bovine Somatotropin) in a prolonged release system (CP115099-F) in commercial dairy herds in Michigan and New York (#87-065, #87-067). Monsanto Study Report. 1987. St. Louis, Missouri, USA.
- 31.Arambel MJ, Lamb RC, Green GA, Madsen KS. Farm trials in Utah using bovine somatotropin (#87-066). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 32.Galton DM, Samuels WA, Madsen KS. Farm trials in New York using bovine somatotropin (#87-067). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 33.Erdman R, Samuels WA, Madsen KS. Farm trials in Maryland and Pennsylvania using bovine somatotropin (#88-063). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 34.Huber JT, Bauman DE, Samuels WA, Lamb RC, Hard DL. Long term evaluation of zinc methionyl bovine somatotropin treatment in a prolonged release system for lactating multiparous cows at four U.S. clinical trial sites (85-039, 85-038, 85-021, 85-003). Monsanto Study Report. 1990. St. Louis, Missouri, USA.
- 35.Adriaens FA, Craven N, Phipps RH, et al. Efficacy and safety of CP115099-F (Sometribove) in dairy cows through three consecutive lactations of treatment in U.K. (#85-009C). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 36.Adriaens FA, Phipps RH, Weller RF, et al. Efficacy and safety of CP115099-F in dairy cows treated for a fourth consecutive lactation in the U.K. (#85-009D). Monsanto Study Report. 1991. St. Louis, Missouri, USA.
- 37.Schockmel LR, Vedeau F, Peel CJ, deKerchove G, Madsen KS, Hartnell GF. Efficacy and safety of CP115099-F in dairy cows. Report on lactations 1 and 2 of the French clinical trials performed at Sanders Experimental Centre, Saint Symphorien. (#85-16B). Monsanto Study Report. 1988. St. Louis, Missouri, USA.
- 38.Gavert HO, Pabst K, Hard DL, et al. Safety and efficacy of CP115099-F. (Sometribove) in dairy cows through three consecutive lactations of treatment (#85-012A). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 39.Bauman DE, Everett RW, Weiland WH, Collier RJ. Production responses to bovine somatotropin in northeastern dairy herds. J Dairy Sci 1999;82:2564–2573. [DOI] [PubMed]
- 40.Phipps RH, Weller RF, Craven N, Peel CJ. Use of prolonged-release bovine somatotropin for milk production in British Friesian dairy cows. 1. Effect on intake, milk production and feed efficiency in two consecutive lactations of treatment. J Agric Sci 1990;115:95–104.
- 41.Hemken RW, Harmon RJ, Silvia WJ, Tucker WB, Heersche G, Eggert RG. Effect of dietary energy and previous bovine somatotropin on milk yield, mastitis, and reproduction in dairy cows. J Dairy Sci 1991;74:4265–4272. [DOI] [PubMed]
- 42.Vicini JL, Eppard PJ, Lanza GM, et al. Assessment of the effective range of CP115099-F in lactating primiparous and multiparous dairy cows (86-023). Monsanto Study Report. 1988. St. Louis, Missouri, USA.
- 43.Gallo L. Effects of recombinant bovine somatotropin on nutritional status and liver function of lactating dairy cows. J Dairy Sci 1990;73:3276–3286. [DOI] [PubMed]
- 44.White TC, Collier RJ, Hartnell GF, et al. Comparison of the effectiveness of intramuscular and subcutaneous administration of CP115099-F (#86-032). Monsanto Study Report. 1990. St. Louis, Missouri, USA.
- 45.Thomas JW, Erdman RA, Galton DM, et al. Responses by lactating cows in commercial dairy herds to recombinant bovine somatotropin. J Dairy Sci 1991;74:945–964. [DOI] [PubMed]
- 46.Stanisiewski EP, Krabill LF, Lauderdale JW. Milk yield, health, and reproduction of dairy cows given somatotropin (Somavubove) beginning early postpartum. J Dairy Sci 1992;75:2149–2164. [DOI] [PubMed]
- 47.Burton JH, MacLeod GK, McBride BW, et al. Overall efficacy of chronically administered recombinant bovine somatotropin to lactating dairy cows. J Dairy Sci 1990;73:2157–2167. [DOI] [PubMed]
- 48.McBride BW, Burton JL, Gibson JP, Burton JH, Eggert RG. Use of recombinant bovine somatotropin for up to two consecutive lactations on dairy production traits. J Dairy Sci 1990;73: 3248–3257. [DOI] [PubMed]
- 49.Waterman DF, Silvia WJ, Hemken RW, Heersche G, Swenson TS, Eggert RG. Effect of bovine somatotropin on reproductive function in lactating dairy cows. Theriogenology 1993;40: 1015–1028. [DOI] [PubMed]
- 50.Whitaker DA, Smith EJ, Kelly JM, Hodgson-Jones LS. Health, welfare and fertility implications of the use of bovine somatotrophin in dairy cattle. Vet Rec 1988;122:503–505. [DOI] [PubMed]