Abstract
This manuscript presents the results of a review of the effects of recombinant bovine somatotropin (rBST) on dairy cattle health, reproductive performance, and culling, that was carried out by an expert panel established by the Canadian Veterinary Medical Association (CVMA). The panel was established by the CVMA in response to a request from Health Canada in 1998 and their report was made public in 1999. A series of meta-analyses was used to combine data on health-related parameters that were extracted from all randomized clinical trials that had been published in peer-reviewed journals or which were provided by Health Canada from the submission by Monsanto for registration of rBST in Canada. A companion paper (1) presents the estimates of the effect of the drug on production parameters. Recombinant bovine somatotropin was found to increase the risk of clinical mastitis by approximately 25% during the treatment period but there was insufficient data to draw firm conclusions about the effects of the drug on the prevalence of subclinical intra-mammary infections. Use of rBST increased the risk of a cow failing to conceive by approximately 40%. For cows which did conceive, there was no effect on services per conception and only a small increase in average days open (5 days). Use of the drug had no effect on gestation length, but the information about a possible effect on the risk of twinning was equivocal. Cows treated with rBST had an estimated 55% increase in the risk of developing clinical signs of lameness. Few studies reported data on culling, but based on those that did, there appeared to be an increase risk of culling evident in multiparous cows. Use of the drug in 1 lactation period appeared to reduce the risk of metabolic diseases (particularly ketosis) in the early period of the subsequent lactation.
Introduction
Recombinant bovine somatotropin (rBST) is a synthetically derived hormone that may be identical to naturally occurring bovine growth hormone, or slightly modified by the addition of extra amino acids. In 1993, sometribove (Posilac; Monsanto Corporation, St. Louis, Missouri, USA) was approved for use in the United States but the product was not actually sold for commercial use until early in 1994. The product was approved for sale with a package insert which identified a number of possible adverse health effects including increased risk of adverse reproductive effects, clinical mastitis, foot and leg problems, injection site reactions, udder edema, and other general health effects. In 1991, Monsanto submitted an application to Health Canada, Bureau of Veterinary Drugs, for registration of sometribove in Canada, but this application was withdrawn in 1994 and replaced with a 2nd application for a product to be called Nutrilac. In late 1998, following considerable internal review, Health Canada decided to seek external assistance from 2 independent expert panels. The Royal College of Physicians and Surgeons was asked to establish an expert panel to review the human health implications of the use of rBST in Canadian dairy cattle. The Canadian Veterinary Medical Association (CVMA) was asked to establish an expert panel to review the data related to the efficacy of the product and potential effects on animal health. The full report of the panel was made available on the internet (2).
In a previous paper (1), the procedures used by the expert panel in the review and a general description of meta-analysis procedures was presented. Subsequently, summary estimates of the effects of rBST on milk production (3.5% fat-corrected milk), milk composition (% butterfat, % lactose, % protein), dry matter intake and body score were presented.
While the effects of rBST on milk production have been extensively studied and reported, less attention has been paid to the possible effects of the drug on target animal health. The objective of this paper is to report the results of meta-analyses of the effects of rBST treatment in lactating dairy cows on udder health, both clinical and subclinical mastitis; reproductive health and performance; other disease conditions; and culling. Given the large number of outcomes evaluated, a review and discussion of the possible mechanisms which might account for the observed effects was beyond the scope of this study.
Materials and methods
Descriptions of the literature review, the data extraction process and the statistical procedures used in the meta-analyses have been described (1). However, a few key points warrant repeating.
Outcome parameters evaluated and data extraction
The literature review process ultimately identified 53 manuscripts and reports (from Monsanto's submission to Health Canada) which provided data for use in 1 or more meta-analyses. From these 53 documents, a total of 546 outcome parameter estimates from 94 groups of cows were extracted and included in the database of production and health effects. The specific studies (references) contributing data to each of the meta-analyses are listed with each of the outcomes in the results and discussion section.
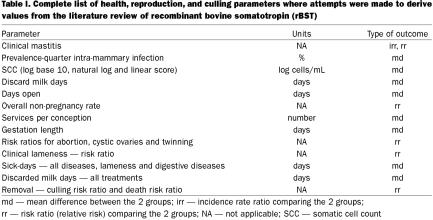
Table I lists all of the health, reproduction, and culling parameters identified as ones for which data would have been extracted, if available, from the reports identified above. Ultimately, the review focussed on measures of incidence or prevalence of health outcomes (risk of lameness), rather than measures of duration (treatment days) for 2 reasons. First, duration was not as commonly reported or not reported in a standardized manner. Second, duration measures may be more prone to bias since most studies were not blinded and knowledge of the study group (treated or control) may have influenced treatment decisions.
Table I.
For outcomes measured as risks or rates (clinical mastitis), the ratio of the 2 values (comparing treated and control groups) was recorded. Relative measures of association (ratios) are the standard methods of assessing the strength of an association between a factor and an outcome. They are less influenced by the level of disease, which varies across populations, than absolute measures (risk or rate differences). For outcomes measured on a continuous scale, days open, the mean difference between the 2 groups was recorded.
In many cases, measures of health effects were not specifically presented in the study report or paper. However, it was often possible to obtain the information needed to compute some of the key parameters. For example, a paper may not have reported the risk ratio (relative risk) of clinical mastitis, but it may have reported the number of cows affected and the number at risk of mastitis in each of the treatment groups. From these data, the risk ratio of mastitis and its confidence interval were computed and used in the meta-analysis.
Only data derived from randomized clinical trials were used in this study because this type of study provides the most objective assessment of effects and is less prone to bias than observational studies (3). If a study reported results separately for different age groups of cows or for different years (in a multi-year study), each of these sets of results were recorded separately. If a study contained data from multiple dosages of rBST, only the results from the dosage closest to the daily dose (500 mg/14 d = 35.7 mg/d) for the product currently approved in the United States (Posilac) were used.
Meta-analyses
Both fixed- and random-effects meta-analyses (1) were carried out, with the results from the latter being used if the test of heterogeneity was statistically significant (P < 0.05). Separate meta- analyses were carried out using results from all studies (regardless of the formulation of rBST), from studies that evaluated sometribove (Monsanto) and from studies based on formulations from other companies. Meta-regression analyses were used to evaluate the effects of product formulation (sometribove or other formulations), parity of cows (primiparous, multiparous, or all combined), study size (number of cows), study precision (standard error of estimate), publication source (peer-reviewed or company report), duration of treatment (days), and expected daily dosage (mg/d) on each outcome of interest. These analyses used a weighted regression to determine if there was any evidence of a linear relationship between the observed result and the factor being investigated (study size). The possibility of publication bias influencing the study results was evaluated using both Begg's (4) and Egger's (5) tests. The influence of individual studies on the overall results was evaluated using an influence plot. All analyses were carried out using a statistical program (Stata, Version 7; Stata Corporation, College Station, Texas, USA) (6).
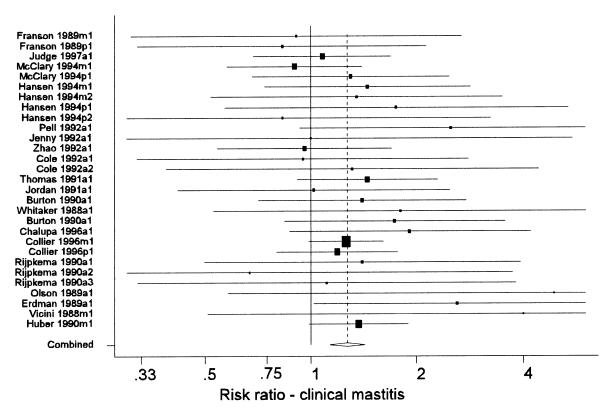
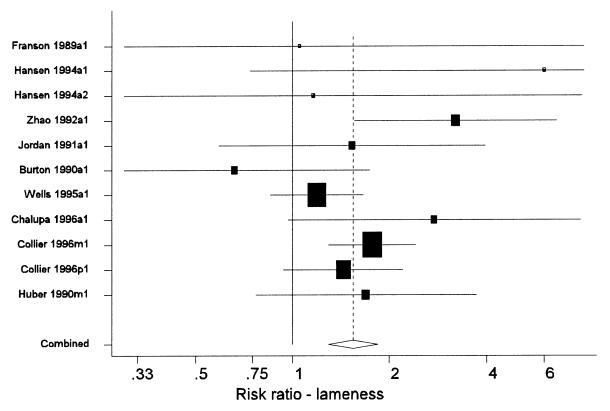
A primary method of presentation of results from a meta-analysis is a forest plot (Figure 1). In these plots, each line represents the results from a single study (or distinct group of cows within a study). Each line is labelled with a unique label that identifies the study and group of cows represented. The name of the first author and year of publication is followed by a single letter indicating the age of the cows in the study (p = primiparous, m = multiparous, a = all ages combined), and a final digit indicating the year of the study (this was only > 1 for multi-lactation studies that reported results separately for each successive lactation). The length of the line represents the 95% confidence interval for the parameter estimate from the study. The centre of the shaded box on each line marks the point estimate of the parameter from that study, and the area of the box is proportional to the weight assigned to the study in the meta-analysis. Studies with large boxes have had a strong influence on the overall estimate. The dashed vertical line marks the overall estimate of the effect. The <> at the bottom of the dashed line shows the 95% confidence interval for the overall effect estimation. The solid vertical line marks the value where rBST would have no effect (a mean difference of 0 or a risk ratio of 1).
Figure 1. Forest plot of the effects of recombinant bovine somatotropin (rBST) on the risk ratio for clinical mastitis. See the text for an explanation of the components of the graph. (Some confidence intervals truncated).
Udder health
The effects of rBST on udder health were divided into the effects on the frequency of clinical mastitis and the effects on subclinical mastitis (as measured by somatic cell counts, prevalence of intramammary infections, or both).
Clinical mastitis rate and risk — Two measures of clinical mastitis frequency (incidence rate and incidence risk) were examined. The incidence rate of clinical mastitis was computed by dividing the total number of clinical mastitis cases by the number of cow-days at risk. In many studies, the total number of clinical mastitis cases was presented for each study group (treated and control), but the total number of cow-days at risk was not presented. For these studies, the number of cow-days at risk was estimated based on the duration of treatment and the assumption that lactation lengths in the 2 groups were equal. The incidence rate ratio (irr) was the ratio of the incidence rate in the treated group divided by the incidence rate in the control group.
Clinical mastitis risk was computed by dividing the number of cows that were affected by 1 or more cases of mastitis during the treatment period by the number of cows at risk. As with incidence rate data, the risk ratio of clinical mastitis was often not presented per se, but the number of cows affected in each group could be determined from the tables in the report. From these data the risk ratio and its 95% confidence interval were calculated.
Subclinical mastitis — Somatic cell count (SCC) data were reported in various studies using any of the following scales: untransformed data, linear scores, log2 transformed data, log10 transformed data, or natural log (loge) transformed data. Previous research has shown that SCC data should be log transformed in some manner (log transformation or linear score) for appropriate analysis. Consequently, papers that reported SCCs as raw counts (untransformed data) were not included in the analyses.
Most studies evaluated the effects of rBST on SCC throughout the treatment period through the regular collection of milk samples for analysis. While it is appropriate to evaluate the effect of rBST on milk production throughout the treatment period because the response to the drug is very rapid, it can be argued that any effect of rBST on the SCC would be delayed in onset. This would likely arise from cows requiring a period of time on rBST before the prevalence of intramammary infections would rise or fall and, in turn, result in an increased or decreased SCC. Consequently, estimates of the effects of rBST on subclinical mastitis as measured by SCC may be biased towards the null (no effect) by the inclusion of data from the beginning of the treatment period. However, because this was the manner in which they were generally reported, these were the data used in the meta-analyses.
Reproduction
A number of measures of reproductive health and performance were evaluated using meta-analyses. Parameters that either affect or reflect the breeding performance included: incidence of cystic ovaries, number of services required per conception, average duration from calving to conception (days open), incidence of twinning (multiple births), and overall risk of a cow not becoming pregnant.
Subsequently, 3 parameters that reflect the state of the cow during her gestation period and at the subsequent calving, were evaluated and these included: the risk of abortion or fetal loss, effect of rBST on gestation length, and the incidence of retained placenta.
Many studies reported the proportion of cows that ultimately conceived during the treatment period (reported as a pregnancy rate). In order to be consistent with other health outcomes, the overall effect of rBST on pregnancy has been evaluated in this report as the risk of the cow failing to conceive (non-pregnancy). One difficulty in analyzing these data was the problem of identifying which pregnancies occurred before the onset of treatment and which ones occurred afterwards. Whenever possible, data from the 2 time periods were separated and only those from the treatment period were used in the analysis.
Other health conditions and culling
Other health conditions, including clinical lameness, were evaluated whenever there was sufficient information in the literature database to support an evaluation. Relatively few studies reported the effect of rBST on culling. The primary reason for this was that in pre-approval studies, the study design dictated that cows remained in the herd unless moribund or dead (7). However, data on culling were extracted from those studies in which they were reported.
Results and discussion
General results
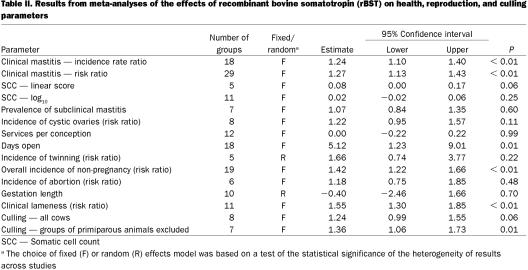
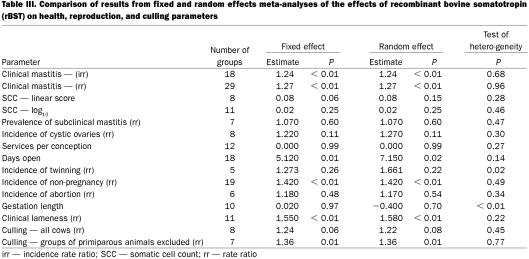
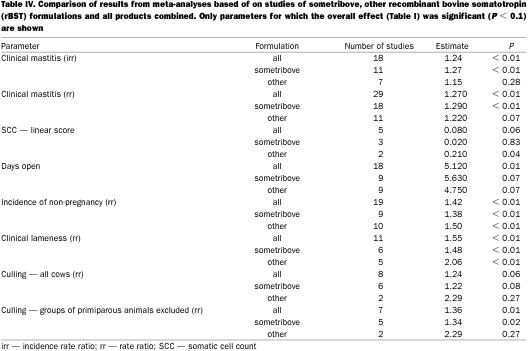
The overall summary results are presented in Table II. A comparison of results from fixed- and random-effects meta-analyses are presented in Table III. In general, the 2 approaches produced very similar results and in the following sections, the distinction between the 2 sets of results will only be noted if the test for heterogeneity was statistically significant. For parameters for which a statistically significant (P < 0.1) association with rBST use was observed, the comparison of results from analyses based on different formulations are presented in Table IV. The analyses of results from studies based on sometribove were generally very similar to those derived from all formulations. In some cases, but certainly not all, this was due to the fact that most of the data available came from studies using sometribove.
Table II.
Table III.
Table IV.
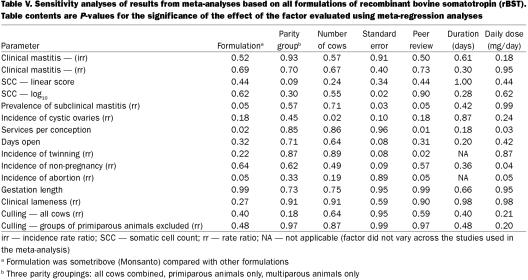
The results of the meta-regression analyses of factors that might explain variation in effects between studies are presented in Table V. For most parameters, it was not possible to identify any specific factors that were significantly associated with variation in effects of rBST between studies. This may have been due to the effect being relatively consistent across studies, or due to a lack of data available on the factors.
Table V.
Publication bias was not likely a serious concern in these meta-analyses for 3 reasons. First, both published and unpublished results (company reports) were included in the analyses. Second, most of the data on health-related parameters was derived from studies designed primarily to evaluate the effects of rBST on production parameters. Consequently, lack of a significant effect for a health outcome would not likely have influenced the decision about publication. Finally, lack of significance of a health effect resulting from treatment with rBST would probably have been considered a “good” result by most authors, so it would not have reduced the probability of publication. Nevertheless, publication bias was evaluated using standard techniques but, for the sake of brevity, results of this evaluation are only presented in subsequent sections if evidence of this bias was observed.
Udder health
Clinical mastitis — The summary estimates of both the irr and the risk ratio for clinical mastitis that were associated with use of rBST as compared with control cows are presented in Table II. These were based on 18 groups of cows from 9 studies (7,8,9,10,11,12,13,14,15) and 29 groups of cows from 20 studies (7,8,9,10,13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29), respectively. Figure 1 shows the range of estimates of the risk ratio for clinical mastitis. The summary estimate of the irr and the risk ratio were very similar (1.24 and 1.27, respectively), indicating a 24% to 27% increase in the risk of clinical mastitis. Most of the evidence about the effect of rBST on clinical mastitis frequency came from the Post Approval Monitoring Program (PAMP) study (7) and a large multi-location study (15) (these studies received the greatest weight in the meta-analyses). A separate analysis of just the PAMP study data (30) reported odds ratios of 1.31 and 1.39 for primiparous and multiparous cows, respectively.
Although the point estimates of the risk and rate ratios showed some variability (Figure 1), this variability was not beyond what would be expected due to chance as there was no evidence of significant heterogeneity of results across studies (Table III).
One recent study, designed specifically to evaluate the effect of rBST on clinical mastitis (16) reported an overall irr of 1.22, which would agree quite closely with the results of the meta-analyses. In that study, 1 farm had a statistically significant increased frequency of clinical mastitis while 3 other farms had non-significant increases or decreases. Because the standard error of the irr could not be determined from the report, these results were not included in the meta-analyses.
The available evidence suggests that rBST increases the frequency of clinical mastitis by approximately 25% during the treatment period. There has been some discussion in the literature as to whether the increased frequency of clinical mastitis associated with rBST is due to the indirect effects of increased milk production or if there is a direct increased risk associated with use of the product. It has been argued that this point is academic in that, even if the effect is indirect (mediated through increased milk production), it still represents an effect of administration of the drug (31). However, very few studies attempted to address this question directly by carrying out separate analyses that controlled or did not control for level of milk production using multi-variable models.
While it is generally accepted that there is genetic antagonism between milk production and risk of mastitis (higher risk with increasing production), the magnitude of the effect has not been well determined. In a review of the genetics of disease resistance, Shook (32) reported estimates of the genetic correlation between milk yield and clinical mastitis that ranged from −0.35 to +0.76. However, although milk production levels of cows are continually increasing over time, the overall incidence of clinical mastitis does not appear to be increasing as rapidly. The lactation incidence risk reported for cows in Southern Ontario was 16.8% in the early 1980s (2) and approximately 15 y later in the mid 1990s it was 19.8% (33). Over the same period, milk production per cow had risen approximately 40%. Although it was inevitable that there were some differences in definitions and recording procedures between the 2 studies, the lack of a substantial difference may indicate either that the expected increase in the frequency of clinical mastitis associated with genetic progress in milk production is not really large or that improvements in management practices have kept pace with the increased risk through genetic selection.
There were inadequate data on mastitis in the carryover period (first 60 d of subsequent lactation) to determine if the increased risk of mastitis persisted through to the following lactation. However, given the apparent limited impact of treatment on the prevalence of subclinical mastitis (discussed below) this assumption may be reasonable. In this case, an increased risk of 25% during the treatment period does not equate to an overall increase of 25% in the total number of cases of clinical mastitis. In the control groups of the studies evaluated, 78% (345/441) of the cases of mastitis occurred during the treatment period (day 60 to 305 of lactation). Taking this factor into account, use of rBST would be expected to produce an increase of approximately 19.4% in the total number of cases of mastitis per cow.
Subclinical mastitis — Somatic cells counts were most commonly reported as linear scores (7,34,35) or log10 transformed SCC (8,14,15,17,19,36,37,38,39) (Table II). In general, the meta-analyses of SCC data did not show much evidence of an effect of rBST. The fixed effect analysis of the SCC-linear score using data from all companies' products achieved borderline statistical significance (P = 0.06), but the result of this meta-analysis was substantially driven by a single, small 2-lactation study involving 30 cows (34). The PAMP linear score data (7) was based only on linear scores determined between treatment days 110 and 200.
The log10 SCC results were heavily influenced by a single, 12-week study (14) where no effect on SCC was observed, but which had very precise estimates of the average SCC. If this study was omitted from the meta-analysis, the overall effect increased to 0.049 (P = 0.095).
Overall, it was concluded that although there was an apparent trend toward slightly increased SCC during the treatment period, no firm conclusion could be drawn that such an effect was present. Even if the effect were present, it was relatively small. An increase of 0.05 units in the log10 SCC over the baseline level observed in control cows in the studies evaluated would only correspond to an increase from 38 900 cells/mL to 43 600 cells/mL.
While several studies reported culture results from samples collected throughout the treatment period, only data from the last sample collection, in which most of the cows were still milking, were used in the meta-analyses (8,9,40,41). Consequently, the risk ratio for the prevalence of subclinical mastitis (occurrence of a pathogen) was based on only 1 sampling period per cow. This avoided the statistical problem of dealing with repeated measures, because this problem could not be readily handled without the original individual cow data being available for analysis. Table II presents the results of the meta-analysis of the effects of rBST on the prevalence of subclinical intra-mammary infections at the end of the treatment period.
When the prevalence data were examined, it was evident that the point estimates of the risk ratio for the prevalence of subclinical mastitis varied quite widely (range of 0.88 to 3.69). The results were also based on all organisms combined, because there were too few isolates of individual organisms to support meaningful analyses. While the point estimate for the risk ratio was slightly greater than 1 (1.07), it was not statistically significantly different from 1 (95% confidence interval for this estimate was 0.84 to 1.35).
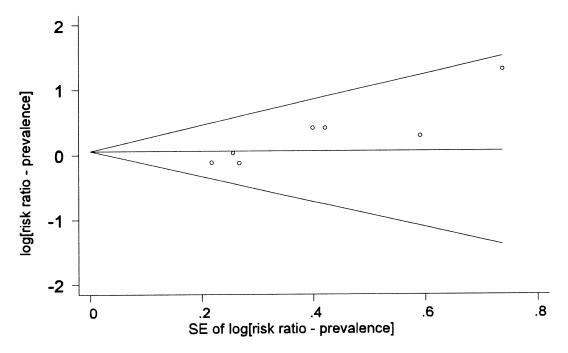
There was some evidence of publication bias affecting the meta-analyses of both log10 SCC and the prevalence of subclinical mastitis. For example, the funnel plot in Figure 2 shows that the point estimate of the risk ratio tended to increase as the standard error of the estimate increased. This was likely a coincidental finding as the 4 studies with the largest point estimates happened to be 4 unpublished reports from Monsanto. This also explained the meta-regression findings of an association between the estimated risk ratio and the formulation of the product, the standard error of the estimate and whether or not the study had been peer reviewed.
Figure 2. Funnel plot (with pseudo 95% confidence limits) of the point estimates of the risk ratio for the prevalence of subclinical infections versus the standard error (SE) of those estimates.
When the evidence from the analyses of SCCs and milk sample cultures were taken together, the data available did not allow strong conclusions to be drawn about the potential effects of rBST on subclinical mastitis. In general, subclinical mastitis is difficult to quantify and it is even more difficult to get a good evaluation of the etiological agents involved. Most of the trials conducted were directed at evaluating the effect of rBST on milk production and were not designed to delve into the potential problem of subclinical mastitis in any depth.
Reproduction
Parameters related to breeding and conception — Parameters related to breeding and conception that were evaluated included cystic ovaries, services per conception, calving to conception interval, incidence of twinning, and overall risk of a cow not becoming pregnant.
Most studies reported the incidence of cystic ovaries in terms of the risk of this condition (the number of cows affected divided by the number of cows at risk) (7,8,25,42,43). Table III presents the meta-analysis results for this condition. With the exception of the multiparous cows in the PAMP study (7), all studies reported an increased risk of cystic ovaries associated with rBST treatment, although only 1 of the irr estimates was statistically significant. This 1 significant result was derived from a study in which rBST had been administered intramuscularly (42). Overall, it appeared that treatment increased the risk by approximately 25%, although this apparent increase was not statistically significant (P = 0.11).
Services per conception reflects the number of times that cows, which ultimately conceived, had to be bred in order to conceive. The parameter does not take into account cows that were bred but did not conceive. Based on the studies included in the meta-analysis (8,10,11,12,15,17,28), there did not appear to be any effect of rBST on the number of services per conception required in cows that did conceive (Table III).
“Days open” is the number of days from calving until a cow is bred again and conceives. The summary effect on days open is presented in Table III. When the data from 18 groups studied (7,8,10,11,12,28,42,44,45) were evaluated, there was a small (5 d), but statistically significant (P = 0.01), increase in average days open. It was concluded that there was evidence that the average days open would be increased slightly by the use of rBST. However, as with services per conception, days open can only be computed for cows that conceived.
In the context of this review, twinning signifies the birth of 2 calves at the parturition following the lactation in which rBST was used. The meta-analysis was based on 5 groups of cows from 3 studies (7,10,42) and the results are presented in Table III. Most of the evidence for, or against, an increased risk of twinning came from the PAMP study (7). The results from that study are equivocal. There appeared to be a decreased risk in primiparous cows and an increased risk of twinning in multiparous cows (although neither were statistically significant). One other study (42) reported large increases in the risk of twinning associated with rBST, risk ratios of 7.1 and 11.7 in primiparous and multiparous cows, respectively, although only the latter was statistically significant. However, it should be noted that cows in this latter study were injected intramuscularly. The problem with assessing the impact of rBST on twinning was the limited number of studies that followed cows through to calving following treatment with the drug. Although the 2 main studies providing data on the risk of twinning had data from a total of 791 cows, one would require data from 2000 cows (1000 cows in each treatment group) to be relatively certain of detecting a doubling (from 2.5% to 5%) of the risk of twinning.
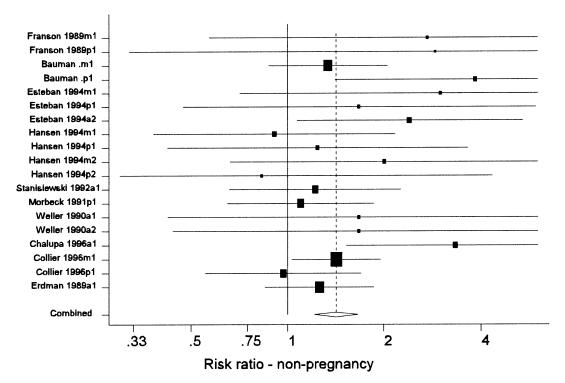
Summary results from the meta-analysis of the overall risk of non-pregnancy (failure to conceive) are presented in Table III and the distribution of effect estimates can be seen in Figure 3. The definition of failure to conceive varied from study to study (7,8,10,11,26,28,42,0,46), depending on the length of the follow-up period, during which pregnancy could have been observed. The range in observation periods was from 130 to 400 d (or the end of the lactation). However, most studies followed cows at least until 200 d and within each study, the observation period of the treated and control cows was the same. Although the point estimates of the risk ratio of non-pregnancy varied widely across studies, they were consistently greater than 1. Overall, the risk ratio of non-pregnancy was approximately 1.4 (equivalent to a 40% increase in the risk of non-pregnancy). One study (44) reported conception data in terms of the hazard ratio for pregnancy, which estimates the risk of a treated cow getting pregnant at a given point in time compared to the risk of a control cow. That study reported significantly reduced hazard ratios (0.38), indicating that treated cows were less likely to conceive.
Figure 3. Forest plot of the effects of recombinant bovine somatotropin (rBST) on the risk ratio for non-pregnancy. See the text for an explanation of the components of the graph. (Some confidence intervals truncated).
In conclusion, the use of rBST in non-pregnant cows increases the risk of a cow not becoming pregnant by approximately 40%. In commercial dairy operations, failure to conceive would normally result in the cow being culled although rBST treatment may extend the productive length of the lactation. A recently published study of the effects of the timing of onset of rBST supplementation found that starting treatment at 9 to 10 wk (compared to 17 to 18 wk) increased the overall risk of non-pregnancy by 35% in primiparous cows and 10% in multiparous cows, but neither effect was statistically significant (47).
Parameters related to gestation — Three parameters related to the gestation period were evaluated: risk of abortion or fetal loss, gestation length, and incidence of retained placenta.
The definition of abortion and how it was determined varied considerably across studies (7,8,48). However, most of the evidence about the effect of rBST on the risk of abortion was derived from the PAMP study (7,44) in which abortion was simply defined as “abortion indicated by dairyman.” When all studies were evaluated (Table III), the point estimate of the risk ratio of abortion was greater than 1, but it was not statistically significant. Fetal loss was also reported in 2 studies (7,15). Although not clearly defined, this was presumably based on the loss of rectally confirmed pregnancies. Risk ratios of 1.2 and 1.11 were reported for primiparous and multiparous cows, respectively, in the 1st study and 1.78 for all cows in the 2nd study. However, none of the individual estimates were significantly greater than one. The overall conclusion was that while there was some evidence of an increased risk of abortion or fetal loss associated with use of rBST, there was inadequate data to draw a firm conclusion.
Gestation length was the time (number of days) from breeding and conception to calving. There was no consistent evidence of an effect of rBST on gestation length (Table III) in the 10 groups of cows from 5 studies used in the meta-analysis (7,8,10,12,15).
There was little information about the effect of rBSt on the risk of a retained placenta following the subsequent calving. One study (15) which recorded the frequency of retained placentas following treatment with rBST reported a risk ratio of 1.6 (P = 0.1). It was concluded that while there appeared to be some evidence of increased risk of retained placenta, there was insufficient data on which to base a firm conclusion.
Subsequent to the publication of the panel's report, results from 1 additional randomized clinical trial carried out in 4 Michigan dairy herds were published (49). The authors of that study found no significant effects of rBST on any reproductive performance parameters except for a statistically significant increase in the risk of twinning.
Lameness
There was considerable variation across studies in how clinical lameness was defined, diagnosed, and recorded (7,8,10,15,19,22,25,26,50). For the purpose of the meta-analyses, all causes of clinical lameness were combined and the overall effect of the drug on the risk of clinical lameness was examined. Results are presented in Table III and Figure 4. The summary estimate of the risk ratio was 1.55 and virtually all studies that reported the incidence of clinical lameness in the treatment and control groups had point estimates of the risk ratio greater than 1. The panel concluded that the risk of clinical lameness was increased approximately 50% in cows treated with rBST.
Figure 4. Forest plot of the effects of recombinant bovine somatotropin (rBST) on the risk ratio for lameness. See the text for an explanation of the components of the graph. (Some confidence intervals truncated).
While details of the diagnoses of the lameness were often not available, the 2 main studies contributing to the meta-analysis were: 1 study carried out in 8 commercial herds (50) and the PAMP study (7). In the former, lesions of the carpus and tarsus, followed by interdigital swelling were the most commonly reported lesions. In the latter, lesions of the fetlock and hoof were most commonly reported, but lesions of the hock contributed the most to the number of days on treatment.
Other health problems
A wide variety of health conditions could have been considered by the panel. These include problems such as abomasal displacement, hypocalcemia, diarrhea, bloat, etc. However, in general, there were insufficient data in the literature to draw any conclusions for many possible health outcomes other than mastitis, lameness, and reproductive diseases. First, there were not many studies that reported health-related outcomes. Second, there was no consistency in the method of reporting health outcomes across studies. Third, even if a health outcome was reported, the number of animals affected in the treated and control groups was so small that it was impossible to draw any meaningful conclusions. The PAMP study (7) reported the most extensive health data. While there were insufficient data in many categories to draw any firm conclusions, there was some evidence of increased episodes of cows being off feed in the treated group. However, care needs to be taken in drawing conclusions from a single study.
Two other specific health issues, which were considered in more detail, were the occurrence of injection site reactions and the effect of rBST on metabolic diseases. One Vermont study (17) reported a high frequency (50% to 60%) of injection site reactions scored 2 or 3 on a scale of 0 to 3 following subcutaneous injections (a score of 2 represented moderate swelling while 3 represented severe swelling). One other study (15) reported a low frequency (2% to 7%) of reactions following intramuscular injections. Another study (40) reported much higher average injection site scores following subcutaneous injections compared to intramuscular injections (1.1 versus 0.2 to 0.5). The evidence suggests more problems with injections site reactions following subcutaneous administration but the reason for the high frequency in the Vermont study is unknown. An Adverse Drug Experience report for the period of February 1994 to February 1998 that was filed by Monsanto reports 212 injections site reactions, of which 176 were classified as “probably” caused by the injection of rBST. Without any information about the frequency of use of rBST or the proportion of reactions that are reported, it is impossible to estimate the overall frequency of reactions. Overall, it was concluded that problems with injection site reactions do occur but there was insufficient data to adequately assess the frequency or severity of these reactions or to determine what factors might influence their occurrence (breed dispositions).
One study reported a significant reduction in metabolic diseases (ketosis and parturient paresis) in the carry-over period in the lactation after rBST treatment (15) (risk ratio = 0.25, P = 0.01). In addition, one single herd study (51) specifically designed to look at the effect of rBST on clinical ketosis reported a substantial, but not statistically significant, reduction in the risk of clinical ketosis (risk ratio = 0.08; 95% confidence interval = 0.005, 1.3) in the carry-over period. It was concluded that use of rBST did reduce the risk of metabolic diseases during the carry-over period.
Culling
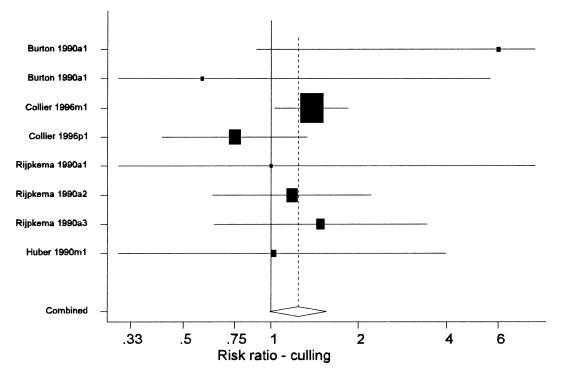
Relatively few studies reported the effect of rBST on culling because the study design in all pre-approval studies dictated that cows remain in the herd unless moribund or dead (7). The results from 8 groups of cows in the 5 studies (7,13,15,23,25), which did present culling data, are shown in Table IV and Figure 5. Overall, there appeared to be approximately a 20% to 25% increase in the risk of culling that was associated with the use of rBST. However, this effect did not achieve statistical significance (P = 0.06) in the meta-analyses. Much of the data about culling was derived from the PAMP study (7), which reported a statistically significant increased risk of culling in multiparous cows (risk ratio = 1.38), but a non-significant reduction in primiparous cows. It may be that the effects are different in primiparous and multiparous cows. Primiparous cows that receive rBST may be less likely to be culled as they have higher milk production levels. Older cows may experience more adverse health effects and may experience a true increased risk of culling. When the single group of entirely primiparous cows were excluded from the meta-analysis, the risk ratio of culling associated with the use of rBST rose from 1.24 to 1.36 and was statistically significant (P = 0.01).
Figure 5. Forest plot of the effects of recombinant bovine somatotropin (rBST) on the risk ratio for culling. See the text for an explanation of the components of the graph. (Some confidence intervals truncated).
One problem with interpreting culling data relates to the inclusion criteria for culling in the study. In one multi-year study (13), reproductive culls were included and reproductive failure was the most common reason for culling. In that study, 4 cows were removed (culled) from the control group when they were transferred to another study. This would have reduced any apparent treatment effect on culling. In 2 other studies (7,15), reproductive culls were not included. Because rBST increases the risk of non-pregnancy and cows that do not conceive are invariably culled, the overall increased risk of culling associated with rBST would be underestimated by the meta-analyses shown in Table IV and Figure 5. On the other hand, use of rBST in cows that had failed to conceive may delay culling by prolonging the productive length of the lactation.
In conclusion, the use of rBST increased the risk of culling, particularly in multiparous cows. A separate analysis of just the PAMP data (30) concluded there was no effect on culling by evaluating each specific reason (mastitis, lameness) individually rather than evaluating overall culling. However, given the small numbers of animals culled for each individual reason, the study had virtually no power to detect differences within each of these categories so a lack of significant findings was not surprising.
Two large observational studies that evaluated culling (52) or average age of cows (as a surrogate measure of longevity) (53) reported that rBST use did not affect survivability of dairy cattle. Data from these studies were not included in the meta-analyses because they were not derived from randomized clinical trials. Observational studies, such as these, are not a reliable indicator of the effect of a treatment because they are prone to selection bias (54).
In conclusion, the study found that there was approximately a 25% increase in the risk of clinical mastitis in rBST-treated cows. It appeared as though there may also have been a slight increase in the prevalence of subclinical intramammary infections, but the data relating to subclinical mastitis was limited.
There were a number of effects on reproductive performance that were associated with the use of rBST. These included a substantial increase in the risk of non-pregnancy and a slight increase in days open in cows that do conceive. There was also inconclusive evidence of an increased risk of cystic ovaries and twinning. All of these adverse effects could be controlled by delaying use of the drug until cows were confirmed pregnant. There was some limited evidence of an increased risk of retained placenta and abortion or fetal loss in treated cows but there was insufficient data to draw a firm conclusion about these possible effects.
It was concluded that there was approximately a 50% increase in the risk of clinical lameness associated with the use of rBST. There was not very much information about other potential health effects of rBST, however, use of the product may reduce the risk of metabolic diseases (ketosis in particular) in the subsequent lactation.
In general, there was an increased risk of culling associated with the use of rBST, particularly in multiparous cows. When considered along with the increased risk of non-pregnancy, it was concluded that the use of rBST would likely reduce the lifespan of dairy cattle.
Footnotes
Acknowledgments
The panel thanks Nicky Schaeffer for all of her assistance with the organization of the material used in this review and Health Canada for their timely assistance in locating all necessary background material.
Address all correspondence and reprint requests to Dr. Ian Dohoo; telephone: (902) 566-0640; fax: (902) 566-0823; e-mail:dohoo@upei.ca
Received February 13, 2003. Accepted April 29, 2003.
References
- 1.Dohoo IR, Leslie KE, DesCôteaux L, et al. A meta-analysis review of the effects of rBST. 1. Methodology and effects on production and nutrition related parameters. Can J Vet Res 2003;67:241–251. [PMC free article] [PubMed]
- 2.Dohoo IR, DesCôteaux L, Dowling P, et al. Report of the Canadian Veterinary Medical Association Expert Panel on rBST. http://www.hc-sc.gc.ca/english/archives/rbst/animals/ [PubMed]
- 3.Lavori PW, Kelsey J. Introduction and overview (clinical trials). Epidemiologic Reviews 2002;24:1–3.
- 4.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed]
- 5.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed]
- 6.StataCorp. Stata Statistical Software. Release 5. College Station, Texas, USA: Stata Corporation, 1998.
- 7.Collier RJ. Post-approval evaluation of Prosilac bovine somatotropin in commercial dairy herds (#93-051). Monsanto Study Report. 1996. St. Louis, Missouri, USA.
- 8.Franson SE, Cole WJ, Madsen KS, et al. Response of cows throughout lactation to Sometribove in a prolonged system — a dose titration study conducted at four U.S. sites (#87-023, #87-034, #87-029, #87-024). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 9.Mcclary DG, Green HB, Basson RP, Nickerson SC. The effects of a sustained-release recombinant bovine somatotropin (Somidobove) on udder health for a full lactation. J Dairy Sci 1994;77:2261–2271. [DOI] [PubMed]
- 10.Hansen WP, Otterby DE, Linn JG, Anderson JF, Eggert RG. Multi-farm use of bovine somatotropin for two consecutive lactations and its effects on lactational performance, health, and reproduction. J Dairy Sci 1994;77:94–110. [DOI] [PubMed]
- 11.Weller RF, Phipps RH, Craven N, Peel CJ. Use of prolonged-release bovine somatotropin for milk production in British Friesian dairy cows. 2. Effect on health and reproduction in two consecutive lactations of treatment. J Agric Sci 1990;115:105–112.
- 12.Leonard M, Gallo G, Gallo M, Block E. Effects of a 28-day sustained-release formulation of recombinant bovine somatotropin (rBST) administered to cows over two consecutive lactations. Can J Anim Sci 1990;70:795–809.
- 13.Rijpkema YS, vanReeuwijk L, Hard DL. Responses of dairy cows to treatment with Sometribove (r-BST) during three consecutive years. Livestock Production Science 1990;26:193–216.
- 14.Meserole VK, Madsen KS, Hartnell GF, et al. Response of cows to biweekly administration of sometribove (N-Methionyl Bovine Somatotropin) in a prolonged release system (CP115099-F) in commercial dairy herds in Michigan and New York (#87-065, #87-067). Monsanto Study Report. 1987. St. Louis, Missouri, USA.
- 15.Huber JT, Bauman DE, Samuels WA, Lamb RC, Hard DL. Long term evaluation of zinc methionyl bovine somatotropin treatment in a prolonged release system for lactating multiparous cows at four U.S. clinical trial sites (85-039, 85-038, 85-021, 85-003). Monsanto Study Report. 1990. St. Louis, Missouri, USA.
- 16.Judge LJ, Erskine RJ, Bartlett PC. Recombinant bovine somatotropin and clinical mastitis: incidence, discarded milk following therapy, and culling. J Dairy Sci 1997;80:3212–3218. [DOI] [PubMed]
- 17.Pell AN, Tsang DS, Howlett BA, et al. Effects of a prolonged-release formulation of Sometribove (n-methionyl bovine somatotropin) on Jersey cows. J Dairy Sci 1992;75:3416–3431. [DOI] [PubMed]
- 18.Jenny BF, Grimes LW, Pardue FE, Rock DW, Patterson DL. Lactational response of Jersey cows to bovine somatotropin administered daily or in a sustained-release formulation. J Dairy Sci 1992;75:3402–3407. [DOI] [PubMed]
- 19.Zhao X, Burton JH, McBride BW. Lactation, health, and reproduction of dairy cows receiving daily injectable or sustained-release somatotropin. J Dairy Sci 1992;75:3122–3130. [DOI] [PubMed]
- 20.Cole WJ, Eppard PJ, Boysen BG, et al. Response of dairy cows to high doses of a sustained-release bovine somatotropin administered during two lactations. 2. Health and reproduction. J Dairy Sci 1992;75:111–123. [DOI] [PubMed]
- 21.Thomas JW, Erdman RA, Galton DM, et al. Responses by lactating cows in commercial dairy herds to recombinant bovine somatotropin. J Dairy Sci 1991;74:945–964. [DOI] [PubMed]
- 22.Jordan DC, Aguilar AA, Olson JD, Bailey C, Hartnell GF, Madsen KS. Effects of recombinant methionyl bovine somatotropin (Sometribove) in high producing cows milked three times daily. J Dairy Sci 1991;74:220–226. [DOI] [PubMed]
- 23.Burton JH, MacLeod GK, McBride BW, et al. Overall efficacy of chronically administered recombinant bovine somatotropin to lactating dairy cows. J Dairy Sci 1990;73:2157–2167. [DOI] [PubMed]
- 24.Whitaker DA, Smith EJ, Kelly JM, Hodgson-Jones LS. Health, welfare and fertility implications of the use of bovine somatotrophin in dairy cattle. Vet Rec 1988;122:503–505. [DOI] [PubMed]
- 25.Burton JL, McBride BW, Burton JH, Eggert RG. Health and reproductive performance of dairy cows treated for up to two consecutive lactations with bovine somatotropin. J Dairy Sci 1990;73:3258–3265. [DOI] [PubMed]
- 26.Chalupa W, Vecchiarelli B, Galligan DT, et al. Responses of dairy cows supplemented with somatotropin during weeks 5 through 43 of lactation. J Dairy Sci 1996;79:800–812. [DOI] [PubMed]
- 27.Olson JD, Green GA, Madsen KS. Farm trials in Colorado using Somatotropin (#87-057). Monsanto Study Report. 1-6-1989. St. Louis, Missouri, USA.
- 28.Erdman R, Samuels WA, Madsen KS. Farm trials in Maryland and Pennsylvania using bovine somatotropin (#88-063). Monsanto Study Report. 1-6-1989. St. Louis, Missouri, USA.
- 29.Vicini JL, Eppard PJ, Lanza GM, et al. Assessment of the effective range of CP115099-F in lactating primiparous and multiparous dairy cows (86-023). Monsanto Study Report. 1988. St. Louis, Missouri, USA.
- 30.Collier RJ, Byatt JC, Denham SC, et al. Effects of sustained release bovine somatotropin (Sometribove) on animal health in commercial dairy herds. J Dairy Sci 2001;84:1098–1108. [DOI] [PubMed]
- 31.Willeberg P. An international perspective on bovine somatotropin and clinical mastitis. J Am Vet Med Assoc 1994;205:538–541. [PubMed]
- 32.Shook GE. Selection for disease resistance. J Dairy Sci 1989;72:1349–1362. [DOI] [PubMed]
- 33.Sargeant JM, Scott HM, Leslie KE, Ireland MJ, Bashiri A. Clinical mastitis in dairy cattle in Ontario: Frequency of occurrence and bacteriological isolates. Can Vet J 1998;39:33–38. [PMC free article] [PubMed]
- 34.Hemken RW, Harmon RJ, Silvia WJ, Tucker WB, Heersche G, Eggert RG. Effect of dietary energy and previous bovine somatotropin on milk yield, mastitis, and reproduction in dairy cows. J Dairy Sci 1991;74:4265–4272. [DOI] [PubMed]
- 35.Galton DM, Samuels WA, Madsen KS. Farm trials in New York using bovine somatotropin (#87-067). Monsanto Study Report. 1989. St. Louis, Missouri, USA.
- 36.Meserole VK, Duque JA, Hintz RL, Peel CJ. Evaluation of the galactopoietic response of bovine somatotropin (Sometribove (CP115099-F), 500 mg and CP115400-P, 260 mg) when administered subcutaneously to lactating Jersy cows in a commercial dairy herd (#89-075, #88-192). Monsanto Study Report. 1992. St. Louis, Missouri, USA.
- 37.Huber JT, Wu Z, Fontes C, Sullivan JL, Hoffman RG, Hartnell GF. Administration of recombinant bovine somatotropin to dairy cows for four consecutive lactations. J Dairy Sci 1997;80: 2355–2360. [DOI] [PubMed]
- 38.Hartnell GF, Franson SE, Bauman DE, et al. Evaluation of Sometribove in a prolonged-release system in lactating dairy cows — production responses. J Dairy Sci 1991;74:2645–2663. [DOI] [PubMed]
- 39.Barbano DM, Lynch JM, Bauman DE, Hartnell GF, Hintz RL, Nemeth MA. Effect of a prolonged-release formulation of N-methionyl bovine somatotropin (Sometribove) on milk composition. J Dairy Sci 1992;75:1775–1793. [DOI] [PubMed]
- 40.White TC, Collier RJ, Hartnell GF, et al. Comparison of the effectiveness of intramuscular and subcutaneous administration of CP115099-F (#86-032). Monsanto Study Report. 1990. St. Louis, Missouri, USA.
- 41.Lissemore KD, Leslie KE, McBride BW, Burton JH, Willan AR, Bateman KG. Observations on intramammary infection and somatic cell counts in cows treated with recombinant bovine somatotropin. Can J Vet Res 1991;55:196–198. [PMC free article] [PubMed]
- 42.Bauman DE, Huber JT, Lamb RC, Samuels WA. Multi-location intramuscular single dose study (single dose IM) (#85-039, #85-038, #85-021, #86-003). Monsanto Study Report. 1987. St. Louis, Missouri, USA.
- 43.Stanisiewski EP, Krabill LF, Lauderdale JW. Milk yield, health, and reproduction of dairy cows given somatotropin (Somavubove) beginning early postpartum. J Dairy Sci 1992;75:2149–2164. [DOI] [PubMed]
- 44.Esteban E, Kass PH, Weaver LD, et al. Interval from calving to conception in high producing dairy cows treated with recombinant bovine somatotropin. J Dairy Sci 1994;77:2549–2561. [DOI] [PubMed]
- 45.Morbeck DE, Britt JH, McDaniel BT. Relationships among milk yield, metabolism, and reproductive performance of primiparous Holstein cows treated with Somatotropin. J Dairy Sci 1991;74:2153–2164. [DOI] [PubMed]
- 46.Esteban E, Kass PH, Weaver LD, et al. Pregnancy incidence in high producing dairy cows treated with recombinant bovine somatotropin. J Dairy Sci 1994;77:468–481. [DOI] [PubMed]
- 47.Silvia WJ, Hemken RW, Hatler TB. Timing of onset of somatotropin supplementation on reproductive performance in dairy cows. J Dairy Sci 2002;85:384–389. [DOI] [PubMed]
- 48.Esteban E, Kass PH, Weaver LD, et al. Reproductive performance in high producing dairy cows treated with recombinant bovine somatotropin. J Dairy Sci 1994;77:3371–3381. [DOI] [PubMed]
- 49.Judge LJ, Bartlett PC, Lloyd JW, Erskine RJ. Recombinant bovine somatotropin: association with reproductive performance in dairy cows. Theriogenology 1999;52:481–496. [DOI] [PubMed]
- 50.Wells SJ, Trent AM, Collier RJ, Cole WJ. Effect of long-term administration of a prolonged release formulation of bovine somatotropin (Sometribove) on clinical lameness in dairy cows. Am J Vet Res 1995;56(8):992–996. [PubMed]
- 51.Lean IJ, Bruss ML, Troutt HF, et al. Bovine ketosis and somatotrophin: risk factors for ketosis and effects of ketosis on health and production. Bovine somatotropin (bST) and the dairy industry. Res Vet Sci 1991;57:200–209. [DOI] [PubMed]
- 52.Ruegg PL, Fabellar A, Hintz RL. Effect of the use of bovine somatotropin on culling practices in thirty-two dairy herds in Indiana, Michigan, and Ohio. J Dairy Sci 1998;81:1262–1266. [DOI] [PubMed]
- 53.Bauman DE, Everett RW, Weiland WH, Collier RJ. Production responses to bovine somatotropin in northeastern dairy herds. J Dairy Sci 1999;82:2564–2573. [DOI] [PubMed]
- 54.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott — Raven, 1998.