Abstract
The reference strains representing serotypes 1 to 12 of Actinobacillus pleuropneumoniae biotype 1 were examined for their ability to utilize porcine hemoglobin (Hb) or porcine hemin (Hm) as iron sources for growth. In a growth promotion assay, all of the reference strains were able to use porcine Hb, and all strains except 2 were able to use porcine Hm. Using a preliminary characterization procedure with Hm- or Hb-agarose, Hm- and Hb-binding outer membrane proteins (OMPs) of approximately 75 kDa were isolated from A. pleuropneumoniae serotype 1 strain 4074 grown under iron-restricted conditions. Matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF) analysis revealed a number of common tryptic peptides between the Hb-agarose- and Hm-agarose-purified 75 kDa OMPs, strongly suggesting that these peptides originate from the same protein. A database search of these peptide sequences revealed identities with proteins from various Gram-negative bacteria, including iron-regulated OMPs, transporter proteins, as well as TonB-dependent receptors. Taken together, our data suggest that A. pleuropneumoniae synthesizes potential Hm- and Hb-binding proteins that could be implicated in the iron uptake from porcine Hb and Hm.
Introduction
Iron is essential for growth of most bacteria (1,2). In the host, extracellular iron is bound to the iron-binding glycoproteins lactoferrin and transferrin in exocrine secretions, while most of the intracellular iron is sequestered as heme-containing proteins, such as hemoglobin (Hb). This sequestration limits the availability of free iron to levels below that required to support microbial growth (1,2). However, to survive in the host, bacterial pathogens have evolved different high-affinity iron-acquisition mechanisms designed to obtain iron. One such system comprises the elaboration of siderophores that chelate external iron followed by binding to their cognate receptor and subsequent internalization. Another system utilises a receptor-mediated mechanism to acquire iron from lactoferrin, transferrin, or heme-containing proteins (1,2,3,4). A large number of pathogenic bacterial species use heme compounds as a source of iron. Various outer membrane proteins (OMPs) which have been isolated and characterized can bind heme to the bacterial cell surface (2,3). In these systems the outer membrane receptor directly recognizes the heme compounds. Another more complex way to obtain heme involves an extracellular protein, named hemophore, that binds heme and shuttles it back to a specific outer membrane receptor (5).
Actinobacillus pleuropneumoniae, a member of the Pasteurellaceae family, is the agent of porcine pleuropneumonia. Among the 13 recognized NAD-dependant serotypes of biotype 1 (6), serotypes 1, 5, and 7 are most commonly found in North America while serotype 2 is predominant in many European countries (7). Several bacterial components, including RTX toxins (ApxI-ApxIV, 3 of which have hemolytic activity), lipopolysaccharides (LPS), capsular polysaccharides, and OMPs appear to contribute to the disease process (8,9). Potential iron sources for A. pleuropneumoniae include porcine transferrin (10) and heme compounds liberated from host cells (11). Our group has shown that A. pleuropneumoniae can use exogenous siderophores (12) as sole sources of iron for growth and has recently described the ferrichrome receptor FhuA (13). We and others have demonstrated that A. pleuropneumoniae serotypes 1 and 2 can utilize porcine Hb or hemin (Hm) as a sole source of iron for growth in vitro (11,14). We have previously reported the binding of porcine Hb to A. pleuropneumoniae LPS (14,15). Lipopolysaccharides might be implicated along with surface proteins in the iron uptake from porcine Hb. Using flow cytometry, comparison of the Hb-binding activity of A. pleuropneumoniae grown under iron-restricted conditions with cells grown under iron-sufficient conditions indicated that iron-restriction promoted the expression of Hb receptors, and that Hb-binding activity was, at least in part, iron-repressible (16).
The mechanism by which A. pleuropneumoniae utilizes Hm- and Hb-iron sources as well as the protein components involved have so far not been identified. Knowing that A. pleuropneumoniae expresses hemolysins, such a system could serve as an important mechanism for the in vivo iron acquisition by this organism. The aim of the present preliminary study was to identify potential proteins of A. pleuropneumoniae serotype 1 (strain 4074) with Hb- or Hm-binding activity.
Materials and methods
Bacterial isolates and growth conditions
Actinobacillus pleuropneumoniae reference strains representing serotypes 1 to 12 were used in this study. Bacteria were grown in brain heart infusion liquid medium (BHI; Difco Laboratories, Detroit, Michigan, USA) supplemented with 5 μg of NAD mL−1. To obtain iron-restricted conditions for preparation of outer membranes, the culture medium was supplemented with 100 μM of deferrated ethylenediamine di-o-hydroxyphenylacetic acid (EDDHA; Sigma Chemical Company, St. Louis, Missouri, USA) (12). Cultures were incubated at 37°C for 18 to 24 h in a 5% CO2 atmosphere.
Growth promotion assay
The utilization of heme compounds by iron-restricted A. pleuropneumoniae was determined by a plate assay (14) with some modifications. Briefly, fresh overnight cultures of the reference strains of A. pleuropneumoniae were resuspended at a concentration of approximately 108 CFU mL−1 (A540 of 0.2) in phosphate-buffered saline (PBS 0.01 M, pH 7.4). Fifty microliters of the cells suspension were spread onto the surface of a BHI-NAD agar plate containing 200 μM of deferrated EDDHA, a concentration that inhibits the growth of A. pleuropneumoniae. Sterile filter disks (Becton Dickinson, Rutherford, New Jersey, USA), 6.25 mm in diameter, were then placed onto the agar plate and 10 μL of porcine Hb (10 mg mL−1, dissolved in PBS) or Hm (10 mg mL−1, dissolved in 30% NH4OH) were spotted onto the filter disks. This concentration of 100 μg disk−1 of porcine Hb or Hm was chosen after testing several dilutions (0.1 to 100 μg disk−1). Zones of growth around the disks were evaluated after incubation at 37°C under an atmosphere of 5% CO2 for 48 h. A solution of FeCl3 (10 mg mL−1, dissolved in PBS) and PBS buffer were used as controls.
Preparation of outer membranes
Outer membranes from A. pleuropneumoniae reference strain of serotype 1 (strain 4074) grown under iron-sufficient or iron-restricted conditions were extracted and isolated by using the same method as Elkins (17) with a small modification of using a French Press to disrupt whole cells of A. pleuropneumoniae. Protein concentrations were determined by using a protein assay (Bio-Rad, Mississauga, Ontario) with bovine serum albumin (BSA) as the standard. The pellets containing outer membranes were conserved at −20°C for further experiments.
Affinity purification with Hm- or Hb-agarose
Analytical purification was performed in microcentrifuge tubes as described by Elkins (17). Outer membranes were solubilized by using 1% Zwittergent 3·14 (Calbiochem, La Jolla, California, USA) in 50 mM Tris, 150 mM NaCl and 5 mM (EDTA) pH 7.5 with rocking at 37°C for 2 h. After centrifugation at 12 500 × g for 10 min, the soluble fraction containing OMPs was mixed with solid-phase bovine Hm- or bovine Hb-agarose (Sigma Chemical Company) and gently rocked for 2 h at room temperature. The agarose containing the ligand-receptor complex was washed in the above-mentioned buffer and with a high salt buffer (50 mM Tris-Cl pH 7.5, 1 M NaCl, 1% Zwittergent, 5 mM EDTA pH 7.5) to remove nonspecifically bound proteins. The complexes were resuspended in Laemmli sample buffer for analytical sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Electrophoresis
The SDS-PAGE was conducted by the discontinuous buffer system of Laemmli (18), with a 4.5% polyacrylamide stacking gel and a 12.5% polyacrylamide running gel (15). Samples were boiled for 10 min in solubilization buffer (62.5 mM Tris-HCl, pH 6.8, 2% (w/v) SDS, 10% (v/v) glycerol, 5% (w/v) β-mercaptoethanol, and 0.025% (w/v) bromophenol blue). Prestained low molecular mass protein markers were obtained (Bio-Rad). Gels were run in a Mini-PROTEAN II vertical slab electrophoresis cell (Bio-Rad) and then stained with Coomassie brilliant blue R-250.
Labeling of cells with [3H]palmitate
[3H]palmitate (5 mCi mL−1) was added to exponentially growing cell colonies of A. pleuropneumoniae serotype 1 strain 4074, which were subjected to iron-restricted or iron-sufficient conditions, this was then added to a final concentration of 50 μCi mL−1 (19). Incubation was then continued for 2 h for the iron-sufficient and 8 h for iron-restricted cultures. Labeling was discontinued by precipitation with trichloroacetic acid (10%, w/v) for 30 min on ice. Proteins were pelleted by centrifugation at 15 000 × g for 20 min, and the pellets were washed twice with methanol to remove lipids. The dried pellets were resuspended in sample buffer and then analyzed by using SDS-PAGE. The radiolabeled protein bands in the dried gel were detected by fluorography (EN3HANCE; Dupont NEN Research Products, Boston, Massachusetts, USA) according to the manufacturer's instructions.
N-terminal amino acid sequence
For N-terminal amino acid analysis, the 75-kDa OMPs purified by either Hm- or Hb-agarose were separated by SDS-PAGE, electroblotted to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) and stained with Ponceau. The N-terminal amino acid sequence was determined by standard Edman degradation on a model ABI 477A microsequencer (Applied Biosystems, Foster City, California, USA).
Proteolytic digestion, peptide separation, and mass spectrometry
Protein bands from the 75-kDa OMPs, purified by either Hm- or Hb-agarose, were excised from gels; subjected to reduction, alkylation, or both using iodoacetamide; and in-gel digested by trypsin (sequencing grade; Promega, Madison, Wisconsin, USA) (20,21). After digestion, the bands were extracted with an aqueous solution of 5% acetic acid, 50% acetonitrile, and the tryptic peptide extracts were combined and evaporated to dryness on a Savant preconcentrator. The peptide extracts were separated (Brownlee HPLC microbore C18 column; Applied Biosystems) (OD-300, 7 μm, 2.1 × 30 mm, ABI) using a 130A HPLC with UV detection set at 220 nm (Applied Biosystems). Peptides were eluted at 200 μL min−1 with a linear gradient of 0 to 40 min (0% to 80% A) and 40 to 57 min (80% to 100% B) with solvant A containing 0.1% trifluoacetic acid (TFA) in water and solvant B made up of 70% aqueous acetonitrile (0.08% TFA). Fractions isolated were evaporated on a Savant preconcentrator and subjected to mass spectral analyses.
Matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF) mass spectra were conducted (PerSeptive biosystems Elite-STR; Framingham, Massachusetts, USA) using linear mode. The matrix a-cyano cinnaminic acid (Aldrich) was used for all MALDI-TOF analyses. Mass assignment was made using external calibration and mass accuracy was found to be within ± 0.3 Da of the predicted molecular weight for analyte less than 4000 Da. Typically 20% of the HPLC fraction was used to obtain the MALDI-TOF mass spectrum. Nanoelectrospray mass spectra were obtained using a hybrid quadrupole time-of-flight (Q-TOF) instrument (Micromass, Manchester, United Kingdom) for high resolution and on-line tandem mass spectrometric experiments (22). An equivalent of 25% of each HPLC fraction dissolved in 1 μL of 50% methanol and 1% acetic acid was loaded through the open end of the nanoelectrospray emitter (Micromass). Conventional mass spectra were obtained by operating the quadrupole in an RF-only mode while a pusher electrode was pulsed (approximately 16 kHz frequency) to transfer all ions to the time-of-flight analyzer. For tandem mass spectrometry experiments (MS-MS) precursor ions identified in a preliminary survey scan were selected by the first quadrupole while a pusher electrode was pulsed (approximately 16 kHz frequency) to transfer fragment ions formed in the RF-only hexapole cell to the time-of-flight analyzer. The detector is a dual-stage microchannel plate and acquisition is made through a time-to-digital convertor (TDC) operating at 1 GHz (Precision Instruments, Knoxville, Tennessee, USA). Mass spectral resolution was typically 4000 to 5000. A scan duration of 1 s and 2 s was set for conventional and MS-MS mass spectral acquisition, respectively. Collisional activation was performed using argon collision gas with a 25 V offset between the DC voltage of the entrance quadrupole and the RF-only hexapole cell. Data were acquired and processed in a data system (Mass Lynx Window NT; Micromass, Beverly, Massachusetts, USA).
Results
Growth promotion assays
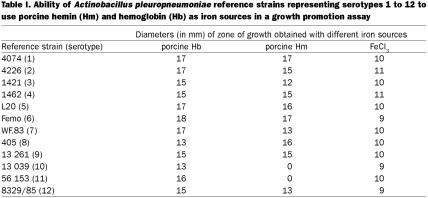
To determine whether A. pleuropneumoniae reference strains representing serotypes 1 to 12 could utilize heme compounds as the sole source of iron, we carried out plate assays in where the abilities of heme compounds to overcome EDDHA-induced iron restriction were evaluated. All the strains were able to use porcine Hb as an iron source for growth (Table I). In addition, all the strains, except strains 13 039 of serotype 10 and 56 153 of serotype 11, were also able to use porcine Hm as an iron source for growth. A brown pigmentation in colonies growing around the disks containing Hm or Hb was observed. All the reference strains were able to use the control iron source FeCl3.
Table I.
Identification of Hm- and Hb-binding OMPs
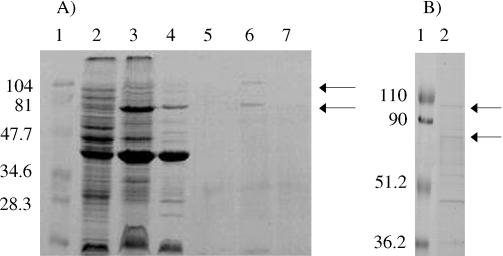
To determine whether A. pleuropneumoniae serotype 1 strain 4074 produces iron-regulated Hm- and Hb-binding OMPs, outer membranes were prepared from cells grown in both iron-sufficient and iron-restricted conditions. Solubilized outer membranes grown in both iron-sufficient (Figure 1a, lane 4) and iron-restricted (Figure 1a, lane 3) conditions were then subjected to affinity purification with bovine Hm (Figure 1b, lane 2) or Hb (Figure 1a, lanes 6 and 7) immobilized on agarose. This procedure yielded a major Hm- and Hb-binding OMP of approximately 75 kDa and another of approximately 104 kDa isolated from A. pleuropneumoniae grown under iron-restricted conditions (Figure 1a and b, lane 6 and 2, respectively). These OMPs were not observed under iron-sufficient conditions (Figure 1a, lane 7). The significance of additional bands in the Hm affinity purified fractions (Figure 1b, lane 2) are unknown at the moment. Hemoglobin-agarose (Figure 1a, lane 5) and Hm-agarose (not shown) were used as controls and no proteins over 34.6 kDa were observed by using an SDS-PAGE.
Figure 1. (A) Identification of Hb-binding outer membrane proteins (OMP) of Actinobacillus pleuropneumoniae strain 4074 using affinity chromatography. A sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel stained with Coomassie blue is shown. Molecular mass protein markers in kDa (lane 1); A. pleuropneumoniae grown under iron-sufficient conditions (lane 2); OMPs from A. pleuropneumoniae grown under iron-restricted conditions (lane 3) or grown under iron-sufficient conditions (lane 4); hemoglobin (Hb)-agarose control (lane 5); Hb-agarose affinity-purified OMPs from A. pleuropneumoniae grown under iron-restricted conditions (lane 6); Hb-agarose affinity-purified OMPs from A. pleuropneumoniae grown under iron-sufficient conditions (lane 7); Arrows indicate the position of the 75- and 104-kDa Hb-binding OMPs.
Labelling of cells with [3H]palmitate
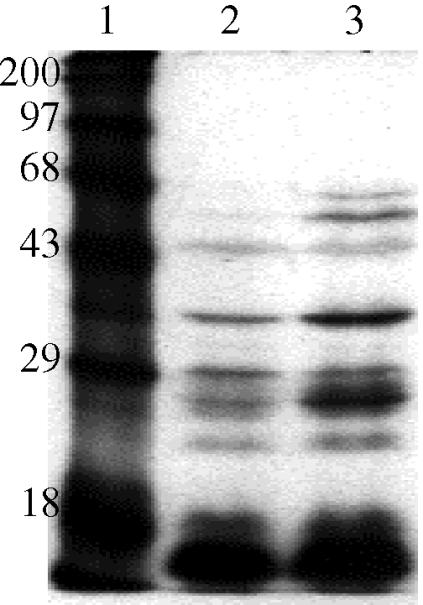
Actinobacillus pleuropneumoniae was metabolically labelled with [3H]-palmitic acid to determine whether the 75-kDa Hm- and Hb-binding OMP was a lipoprotein. The SDS-PAGE and fluorographic analysis of labelled A. pleuropneumoniae grown in the presence (Figure 2, lane 3) and absence of deferrated EDDHA (Figure 2, lane 2) indicated that the 75-kDa Hm- and Hb-binding OMP was not a lipoprotein since no band could be observed between 68 and 97 kDa on the film (Figure 2, lanes 2 and 3). When A. pleuropneumoniae was grown under iron-restricted conditions, several lipoproteins were observed, all of which were below 68 kDa (Figure 2, lane 3).
Figure 2. Fluorographic analysis of Actinobacillus pleuropneumoniae strain 4074 metabolically labelled with [3H]palmitic acid. Molecular mass protein markers in kDa (lane 1); A. pleuropneumoniae grown under iron-sufficient conditions (lane 2) or iron-restricted conditions (lane 3).
Amino acid sequence of the 75-kDa Hm- and Hb-binding OMP
The 75-kDa OMPs, purified by either Hm-agarose or Hb-agarose, were submitted to N-terminal amino acid sequencing. These OMPs were separated by SDS-PAGE, electroblotted to a polyvinylidene difluoride membrane, and stained with Ponceau. Despite many attempts and different enzymatic, non enzymatic, or limited acid hydrolysis treatments, the N-terminal amino acid sequence could not be determined by standard Edman degradation.
Mass spectral analyses of tryptic peptides
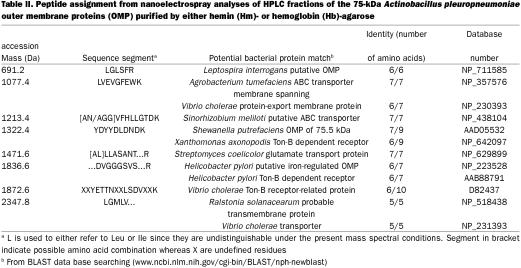
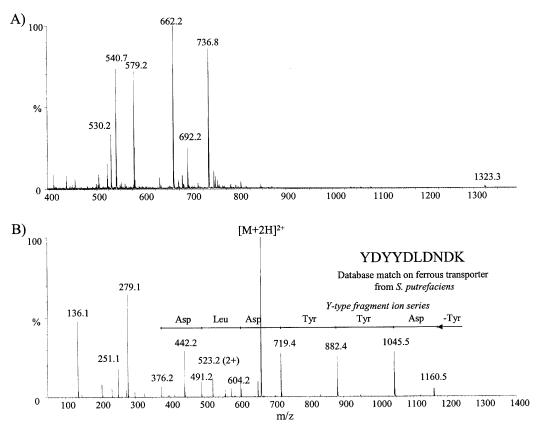
Since the N-terminal amino acid was blocked, the 75-kDa OMPs purified by Hm-agarose and Hb-agarose was selected for mass spectral analyses. The protein was excised and subjected to in-gel digestion with trypsin. Proteolytic fragments separated on a microbore HPLC column were subsequently analyzed by MALDI-TOF to identify potential protein candidates. These analyses indicated common tryptic peptides between the fractions from the Hb-agarose and Hm-agarose purified OMPs thus suggesting that these peptides originate from the same protein. However, no meaningful protein candidate was revealed by the peptide mass fingerprinting and database searching approach. Rather, sequencing of tryptic peptide was obtained using nanoelectrospray and MS-MS. A number of potential precursor ions were identified in a preliminary survey scan of the HPLC fractions and were subjected in turn to MS-MS sequencing (Table II). An example of this is shown in Figure 3 for the nanoelectrospray analysis of fraction 11 from the Hm- and Hb-binding OMP of 75 kDa. The conventional mass spectrum (Figure 3a) showed 3 abundant doubly-protonated ions at m/z 540.7 (1077.4 Da), 662.2 (1322.4 Da), and 736.8 (1471.6 Da). The fragment ions observed in the MS-MS spectrum of the first component could not be matched to any amino acid residue and was presumed to be a gel contaminant. The product ion spectrum of m/z 662.2 (Figure 3b) shows a series of y-type fragment ions consistent with consecutive cleavage of the peptide bonds. From the spacing between adjacent fragment ions it was possible to deduce the peptide sequence YDYYDLDNDK. A database search using this peptide segment revealed a potential match with an OMP of 75.5 kDa from Shewanella putrefaciens (23) which is required for Fe(III) and Mn(IV) reduction (7 out of 9 amino acid matched: YDYYDRDNN). Similarly, the MS-MS spectrum of m/z 736.8 enabled the identification of a peptide segment comprising the sequence LLASANT. The results from these different MS-MS experiments on HPLC fractions are summarized in Table II. A database search of these peptide sequences revealed identities with proteins from various Gram-negative bacteria, including iron-regulated OMPs, transporter proteins, as well as TonB-dependent receptors.
Table II.
Figure 3. Mass spectral analysis of tryptic peptides of the Actinobacillus pleuropneumoniae 75-kDa outer membrane proteins (OMP) purified by either hemin (Hm)- or hemoglobin (Hb)-agarose. Nanoelectrospray mass spectral analysis of HPLC fraction 11. Conventional mass spectrum (A) showing 3 abundant doubly-protonated ions at m/z 540.7 (1077.4 Da), 662.2 (1322.4 Da), and 736.8 (1471.6 Da) and tandem mass spectrum of m/z 662.2 (1322.4 Da), (B) indicating a series of y-type fragment ions consistent with consecutive cleavage of the peptide bonds. Spacing between adjacent fragment ions allowed to deduce the peptide sequence YDYYDLDNDK.
Discussion
The mechanisms by which A. pleuropneumoniae utilizes Hm- or Hb-iron sources, as well as the protein components involved, are presently unknown. We previously described the binding of porcine Hb to A. pleuropneumoniae LPS (14,15). More recently, using flow cytometry and fluorescein-labeled porcine Hb, we observed that iron restriction promoted the expression of Hb receptors. Surface proteins could be involved in the iron uptake from porcine Hb (16).
In the present study, we report that all A. pleuropneumoniae reference strains tested were able to use porcine Hb and that all strains, except strains 13 039 of serotype 10 and 56 153 of serotype 11, were able to use porcine Hm. These results confirmed the previously published findings (11,14) that most strains of A. pleuropneumoniae serotypes 1 and 2 could utilize Hm or Hb as a sole source of iron for growth. Interestingly, we observed the presence of a brown pigmentation in colonies growing around the disks containing Hm or Hb. We believe that those brown colonies were caused by accumulation of Hm or Hb at the surface of the cells. In Porphyromonas gingivalis black pigmentation of colonies by heme accumulation is thought to be related to virulence (24).
The data presented here reports for the first time the identification of Hm- and Hb-binding proteins in A. pleuropneumoniae. These proteins were affinity purified with bovine Hm or Hb immobilized on agarose as the ligand. A major Hm- and Hb-binding OMP of 75 kDa was identified in A. pleuropneumoniae. The synthesis of this protein is likely to be iron-regulated, as it was not observed when purification was performed with cells obtained under iron-sufficient conditions. Metabolic labeling with [3H]palmitic acid of A. pleuropneumoniae grown in the presence and absence of EDDHA indicated that the major 75-kDa Hm- and Hb-binding OMP was not a lipoprotein. A 76-kDa protein of A. pleuropneumoniae strain 79–9 of serotype 1 identified by Deneer and Potter (11) displayed an ability to bind Congo red and hemin. We suggest that this protein might be related to the 75-kDa Hm- and Hb-binding OMP identified in the present study. Interestingly, a TonB-dependent heme receptor of 75 kDa was identified in Haemophilus ducreyi (25).
To determine potential homology and to investigate whether the 75-kDa OMPs purified by either Hm-agarose or Hb-agarose were identical MALDI-TOF analyses were undertaken since the N-terminal amino acid sequence of these OMPs could not be determined by standard Edman degradation. The analyses revealed common tryptic peptides between fractions from the Hb-agarose- and Hm-agarose- purified OMPs strongly suggesting that these peptides originate from the same protein. A database search of these peptide sequences revealed identities with iron-regulated OMPs, transporter proteins, as well as TonB-dependent receptors from various Gram-negative bacteria.
Negrete-Abascal et al (26) have reported that culture supernatants of A. pleuropneumoniae displayed protease activities of different molecular weights. These proteases were able to degrade porcine immunoglobulin (Ig)A and porcine Hb. This may suggest that proteolysis at the proximity of the outer membrane of A. pleuropneumoniae could be important for removal of the transported ligand (Hm) from the bound macromolecular ligand (Hb) to allow transport accross the outer membrane. How heme crosses the outer membrane of A. pleuropneumoniae is not presently known. Studies in H. ducreyi and H. influenzae have determined that this process is dependent on the activity of the TonB protein (25,27).
The Hm- and Hb-binding proteins of 75 kDa and also of 104 kDa identified in this study are found in outer membrane preparations; hence, they are likely potential candidates for Hm and Hb receptors. Although the preliminary isolation of these proteins by Hm- and Hb-agarose suggests an implication in a Hm- and Hb-receptor- mediated iron-acquisition process, the precise role of these components awaits definitive experiments with isogenic mutants in the genes coding for these proteins. The pathways by which A. pleuropneumoniae utilize Hm- and Hb-iron sources are of interest in an attempt to understand the role of surface molecules in pathogenesis and evaluate their potential as vaccine candidates.
Footnotes
Acknowledgments
This work was supported in part by grants from Natural Sciences and Engineering Research Council of Canada (RGPIN003428 to M.J.) and from Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (FCAR; 99-ER-0214). M.A. is the recipient of a studentship from FCAR. The authors thank Julie Couture for her technical assistance in the palmitate labeling of whole cells of A. pleuropneumoniae. We are also grateful to Micromass for the access to the Q-TOF instrument and for their generous support and assistance during this study.
Dr. Archambault's current address is University of Guelph, Laboratory Services Division, Animal Health Laboratory, P.O. Box 3612, Guelph, Ontario N1H 6R8; Dr. Rioux's current address is ID Biomedical Corporation, 7150 Frederick Banting, Ville Saint-Laurent, Québec H4S 2A1.
Address all correspondence and reprint requests to Dr. Mario Jacques; telephone (450) 773-8521 ext. 8348; fax: (450) 778-8108; e-mail: mario.jacques@umontreal.ca
Received November 15, 2002. Accepted March 18, 2003.
References
- 1.Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin Microbiol Rev 1993;6:137–149. [DOI] [PMC free article] [PubMed]
- 2.Lee BC. Quelling the red menace: haem capture by bacteria. Mol Microbiol 1995;18:383–390. [DOI] [PubMed]
- 3.Otto BR, Verweij-van Vught JJ, MacLaren DM. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol 1992;18:217–233. [DOI] [PubMed]
- 4.Cornelissen CN, Sparling PF. Iron piracy: acquisition of transferrin- bound iron by bacterial pathogens. Mol Microbiol 1994;14:843–850. [DOI] [PubMed]
- 5.Ghigo JM, Létoffé S, Wandersman C. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J Bacteriol 1997;179: 3572–3579. [DOI] [PMC free article] [PubMed]
- 6.Blackall PJ, Klaasen HL, Van den Bosch H, Kuhnert P, Frey J. Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet Microbiol 2002;84:47–52. [DOI] [PubMed]
- 7.Dubreuil JD, Jacques M, Mittal KR, Gottschalk M. Actinobacillus pleuropneumoniae surface polysaccharides: their role in diagnosis and immunogenicity. Anim Health Res Rev 2000;1:73–93. [DOI] [PubMed]
- 8.Haesebrouck F , Chiers K, Van Overbeke I, Ducatelle R. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol 1997;58:239–249. [DOI] [PubMed]
- 9.Bosse JT, Janson H, Sheehan BJ, et al. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect 2002;4:225–235. [DOI] [PubMed]
- 10.Niven DF, Donga J, Archibald FS. Responses of Haemophilus pleuropneumoniae to iron restriction: changes in the outer membrane protein profile and the removal of iron from porcine transferrin. Mol Microbiol 1989;3:1083–1089. [DOI] [PubMed]
- 11.Deneer HG, Potter AA. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun 1989;57:798–804. [DOI] [PMC free article] [PubMed]
- 12.Diarra MS, Dolence JA, Dolence EK, et al. Growth of Actinobacillus pleuropneumoniae is promoted by exogenous hydroxamate and catechol siderophores. Appl Environ Microbiol 1996;62:853–859. [DOI] [PMC free article] [PubMed]
- 13.Mikael LG, Pawelek PD, Labrie J, Sirois M, Coulton JW, Jacques M. Molecular cloning and characterization of the ferric hydroxamate uptake (fhu) operon in Actinobacillus pleuropneumoniae. Microbiology 2002;148:2869–2882. [DOI] [PubMed]
- 14.Bélanger M, Bégin C, Jacques M. Lipopolysaccharides of Actinobacillus pleuropneumoniae bind pig hemoglobin. Infect Immun 1995;63:656–662. [DOI] [PMC free article] [PubMed]
- 15.Archambault M, Olivier M, Foiry B, Diarra MS, Paradis SÉ, Jacques M. Effects of pig hemoglobin binding on some physical and biological properties of Actinobacillus pleuropneumoniae lipopolysaccharides. J Endotoxin Res 1997;4:53–65.
- 16.Archambault M, Rioux S, Jacques M. Evaluation of the hemoglobin-binding activity of Actinobacillus pleuropneumoniae using fluorescein-labeled pig hemoglobin and flow cytometry. FEMS Microbiol Lett 1999;173:17–25. [DOI] [PubMed]
- 17.Elkins C. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect Immun 1995;63:1241–1245. [DOI] [PMC free article] [PubMed]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. [DOI] [PubMed]
- 19.Gerlach GF, Anderson C, Potter AA, Klashinsky S, Willson PJ. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun 1992;60:892–898. [DOI] [PMC free article] [PubMed]
- 20.William KR, LoPresti M, Stone K. Internal protein sequencing of SDS-PAGE separated proteins: Optimization of an in gel digest protocol. In: Marshak D, ed. Techniques VIII. San Diego: Academic Press, 1997:79–90.
- 21.Williams K, Hellman U, Kobayashi R, Lane W, Mische S, Speicher D. Internal protein sequencing of SDS-PAGE separated proteins: A collaborative ABRF study. In: Marshak D, ed. Techniques VIII. San Diego: Academic Press, 1997:99–109.
- 22.Morris HR, Paxton T, Dell A, et al. High sensitivity collisionally activated decomposition tandem mass spectrometry on a novel quadrupole/orthogonal-acceleration time-of-flight mass spectrometer. Rapid Commun Mass Spectrom 1996;10:889–997. [DOI] [PubMed]
- 23.Beliaev AS, Saffarini DA. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe (III) and Mn (IV) reduction. J Bacteriol 1998;180:6292–6297. [DOI] [PMC free article] [PubMed]
- 24.Shah HN, Seddon SV, Gharbia SE. Studies on the virulence properties and metabolism of pleiotropic mutants of Porphyromonas gingivalis (Bacteroides gingivalis) W50. Oral Microbiol Immunol 1989;4:19–23. [DOI] [PubMed]
- 25.Thomas CE, Olsen B, Elkins C. Cloning and characterization of tdhA, a locus encoding a TonB-dependent heme receptor from Haemophilus ducreyi. Infect Immun 1998;66:4254–4262. [DOI] [PMC free article] [PubMed]
- 26.Negrete-Abascal E, Tenorio VR, Serrano JJ, Garcia C, de la Garza M. Secret ed proteases from Actinobacillus pleuropneumoniae serotype 1 degrade porcine gelatin, hemoglobin and immunoglobulin A. Can J Vet Res 1994;58:83–86. [PMC free article] [PubMed]
- 27.Jarosik GP, Sanders JD, Cope LD, Muller-Eberhard U, Hansen EJ. A functional tonB gene is required for both utilization of haem and virulence expression by Haemophilus influenzae type B. Infect Immun 1994;62:2470–2477. [DOI] [PMC free article] [PubMed]